Piezoresistive Chemical Sensors Based on Functionalized Hydrogels
Abstract
:1. Introduction
 and
and  of ith mobile ions in the gel and in the surrounding solution, respectively (see Equation (A2) in Appendix A). This pressure, when present, leads to an additional water uptake by the hydrogel network. The values of
of ith mobile ions in the gel and in the surrounding solution, respectively (see Equation (A2) in Appendix A). This pressure, when present, leads to an additional water uptake by the hydrogel network. The values of  depend on the concentration of fixed charges in the gels, which depends in turn on the degree of substitution of ionizable groups, on the pH value, and on the ionic content of the external medium (values of
depend on the concentration of fixed charges in the gels, which depends in turn on the degree of substitution of ionizable groups, on the pH value, and on the ionic content of the external medium (values of  ).
).2. Experimental Section
2.1. Sensor Design

2.2. Materials and Methods
2.2.1. Glucose-Sensitive AAm/3-AAmPBA/BIS Hydrogels
2.2.2. pH-Sensitive HPMA/DMAEMA/TEGDMA Hydrogels
2.2.3. PNIPAAm-DMAAm-DMIAAm Terpolymer
2.2.4. Solution Characterization
3. Results and Discussion
3.1. Sensors for Glucose Concentration



 / cs
/ cs
- -
= 10− pH is the concentration of hydrogen ions related to the pH of the environment;
- -
, Ka, and α are the concentration, the acid dissociation constant, and the degree of ionization, respectively, of the ionizable polymer groups;
- -
and
are the concentration and dissociation constant, respectively, of the nth analyte species;
- -
- cs is the total ion concentration in the solution (see Equation (A4) in Appendix A).
 using the input data of the dried gel layer, and then the value of b using Equation (4).
using the input data of the dried gel layer, and then the value of b using Equation (4). of the ionizable PBA groups. Here, ρp and mp = ρpV0 are the density and the mass of the dried gel layer, respectively, M1 and N1 denote the molar mass and the number of the acrylamide (AAm, C3H5NO) monomer units, respectively, M2 and N2 denote the molar mass and the number of the 3-acrylamidophenylboronic acid (3-AAmPBA, C9H10BNO3) monomer units, respectively, M3 and N3 denote the molar mass and the number of N,N'-methylene-bisacrylamide (BIS, C7H10N2O2), respectively. Taking into account the polymer composition, we obtained for the mole numbers N1, N2 and N3 of AAm, 3-AAmPBA and BIS, respectively in the gel sample volume V0
of the ionizable PBA groups. Here, ρp and mp = ρpV0 are the density and the mass of the dried gel layer, respectively, M1 and N1 denote the molar mass and the number of the acrylamide (AAm, C3H5NO) monomer units, respectively, M2 and N2 denote the molar mass and the number of the 3-acrylamidophenylboronic acid (3-AAmPBA, C9H10BNO3) monomer units, respectively, M3 and N3 denote the molar mass and the number of N,N'-methylene-bisacrylamide (BIS, C7H10N2O2), respectively. Taking into account the polymer composition, we obtained for the mole numbers N1, N2 and N3 of AAm, 3-AAmPBA and BIS, respectively in the gel sample volume V0 = 2.23 M of the ionizable PBA groups concentration in the pre-swollen gel sample (thickness d1 = 400 μm, volume V1 = 3.6 μL) was calculated as:
= 2.23 M of the ionizable PBA groups concentration in the pre-swollen gel sample (thickness d1 = 400 μm, volume V1 = 3.6 μL) was calculated as:
 = N2 / V1
= N2 / V1
| d0, μm | S0, mm2 | V0, L | ρp, g/cm3 | mp, g |
|---|---|---|---|---|
| 330 | 9 | 3 × 10−6 | 1.29 [42] | 3.8 × 10−3 |
| Monomer | M, g/mol | N, mol | ξ, mol% |
|---|---|---|---|
| AAm (C3H5NO) | 71.08 | 3.2 × 10−5 | 80 |
| 3-AAmPBA (C9H10BNO3) | 190.99 | 8 × 10−6 | 20 |
| BIS (C7H10N2O2) | 154.17 | 1 × 10−7 | 0.25 |
| Transducer | Hydrogel | Sensitivity, kPa/mM | Dimensions of Cavity with Gel, mm3 | References |
|---|---|---|---|---|
| piezoresistive | AAm/3-AAmPBA/BIS | 3.7 | 3 × 3 × 0.37 | present work |
| capacitive | AAm/MAAmPBA/BIS | 0.15 | 2.8 × 2.8 × 0.2 | [1,2] |
| piezoresistive | AAm/3-AAmPBA/DMAPAAm/BIS | 0.005 | 1 × 1 × 0.4 | [43] |
3.1.1. Cross-Sensitivity to Interferents
3.1.2. Effects of pH and Ionic Strength Changes

3.1.3. Response Time
- (1)
- porous gels. A response time reduction of about 80% was observed compared to sensors with non-porous hydrogels [45]. However, the porous structure affects the mechanical stability of the gel and, consequently, the long-term stability of the sensor sensitivity, which needs to be improved.
- (2)
- composite as well as hybrid materials. A significant reduction (of 72%, compared to the homogeneous hydrogel) of the sensor response time was achieved for the hybrid hydrogel with incorporated hygroscopic fibers which accelerated the diffusion of the solution in the gel and, consequently, the gel swelling/deswelling [19]. The incorporated hydrophilic porous fibres led to a faster, and at the same time, increased solution uptake.
- (3)
- the method of initial rate determination of the solution uptake. It was found that the value v of this rate depends on the initial concentration gradient (
− cα0) of the analyte between the solution and the gel [10]. With the help of the values v1 and v2 for two solutions with a known concentration
and with an unknown one
, the value of
can be estimated (see Equation (B1) in Appendix B). By applying this method, the measuring time tm which is necessary to determine the glucose concentration in PBS solution was essentially shortened from the time to reach a full saturation of the solution uptake to the time which is necessary for the initial rate determination (tm ≤ 3 min, see Figure (B4) in Appendix B).
3.2. pH Sensors


 using the input data of the dried HPMA/DMAEMA/TEGDMA gel layer, and then the value of b using Equation (4).
using the input data of the dried HPMA/DMAEMA/TEGDMA gel layer, and then the value of b using Equation (4). of the ionizable DMAEMA groups. Similar, as in Section 3.1, the numbers of monomer units shown in Table 4 were calculated for a dried gel piece of the same size (3 mm × 3 mm × 0.33 mm). The value
of the ionizable DMAEMA groups. Similar, as in Section 3.1, the numbers of monomer units shown in Table 4 were calculated for a dried gel piece of the same size (3 mm × 3 mm × 0.33 mm). The value  = 2.06 M of the ionizable DMAEMA groups concentration in the pre-swollen gel sample (thickness d1 = 400 μm, volume V1 = 3.6 μL) was calculated as:
= 2.06 M of the ionizable DMAEMA groups concentration in the pre-swollen gel sample (thickness d1 = 400 μm, volume V1 = 3.6 μL) was calculated as:
 = NDMAEMA / V1
= NDMAEMA / V1
| Monomer | M, g/mol | N, mol | ξ, mol% |
|---|---|---|---|
| HPMA (C7H12O3) | 144.17 | 1.7 × 10−5 | 70 |
| DMAEMA (C8H15O2N) | 157.20 | 7.4 × 10−6 | 30 |
| TEGDMA (C16H26O7) | 330.37 | 4.9 × 10−7 | 2 |
| Transducer | Hydrogel | Sensitivity, kPa/0.1pH | Dimensions of cavity with gel, mm3 | References |
|---|---|---|---|---|
| piezoresistive | HPMA/DMAEMA/TEGDMA | 17.4 | 3 × 3 × 0.37 | present work |
| capacitive | MAA/AAm/EGDMA | 0.23 | 2.8 × 2.8 × 0.2 | [25] |
3.3. Sensors for Ionic Strength
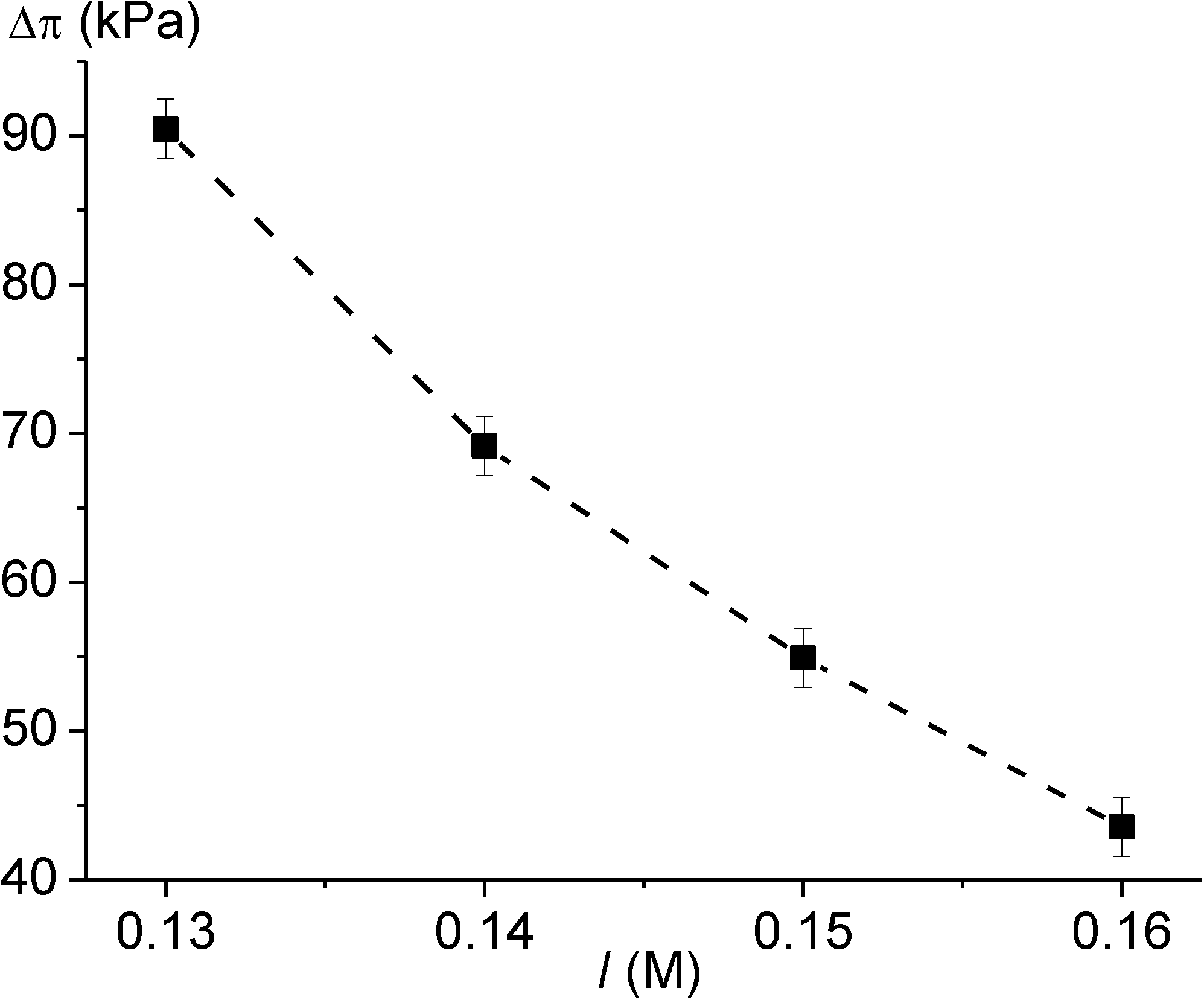
3.4. Simultaneous Monitoring of Analyte Concentration, pH Value and Ionic Strength
4. Conclusions
Acknowledgments
Conflicts of Interest
Appendix A. Ionic Osmotic Pressure of Polyelectrolyte Hydrogels

 and
and  are concentrations of ith mobile ions in the gel and in the surrounding solution, respectively, R the universal gas constant, and T the absolute temperature. The equilibrium state is characterized by the Donnan ratio λ, which describes the distribution of mobile ions between the gel and the solution. In the case of a uni-univalent electrolyte solution (the valence of ions zi = ±1) [23]:
are concentrations of ith mobile ions in the gel and in the surrounding solution, respectively, R the universal gas constant, and T the absolute temperature. The equilibrium state is characterized by the Donnan ratio λ, which describes the distribution of mobile ions between the gel and the solution. In the case of a uni-univalent electrolyte solution (the valence of ions zi = ±1) [23]:

 ,
,  ,
,  and
and  are the concentrations of monovalent mobile cations and anions in the gel and in the solution, respectively. For the polyelectrolyte gel with anionic side groups, λ > 1.
are the concentrations of monovalent mobile cations and anions in the gel and in the solution, respectively. For the polyelectrolyte gel with anionic side groups, λ > 1. +
+ 
 =
=  = cs
= cs
 .
. −
−  + zα·
+ zα·  = 0
= 0
 = λ · cs,
= λ · cs,  = cs / λ
= cs / λ
 is the concentration of the ionizable groups on the polymer backbone and α is the fraction of ionized polymer groups with the valence z.
is the concentration of the ionizable groups on the polymer backbone and α is the fraction of ionized polymer groups with the valence z.
 · cs
· cs

 = 10− pH is the concentration of hydrogen ions related to the pH of the environment, Ka the acid dissociation constant of the ionizable polymer groups,
= 10− pH is the concentration of hydrogen ions related to the pH of the environment, Ka the acid dissociation constant of the ionizable polymer groups,  and
and  the concentration and dissociation constant, respectively, of the nth analyte species.
the concentration and dissociation constant, respectively, of the nth analyte species.Appendix B. Piezoresistive Chemomechanical Sensors
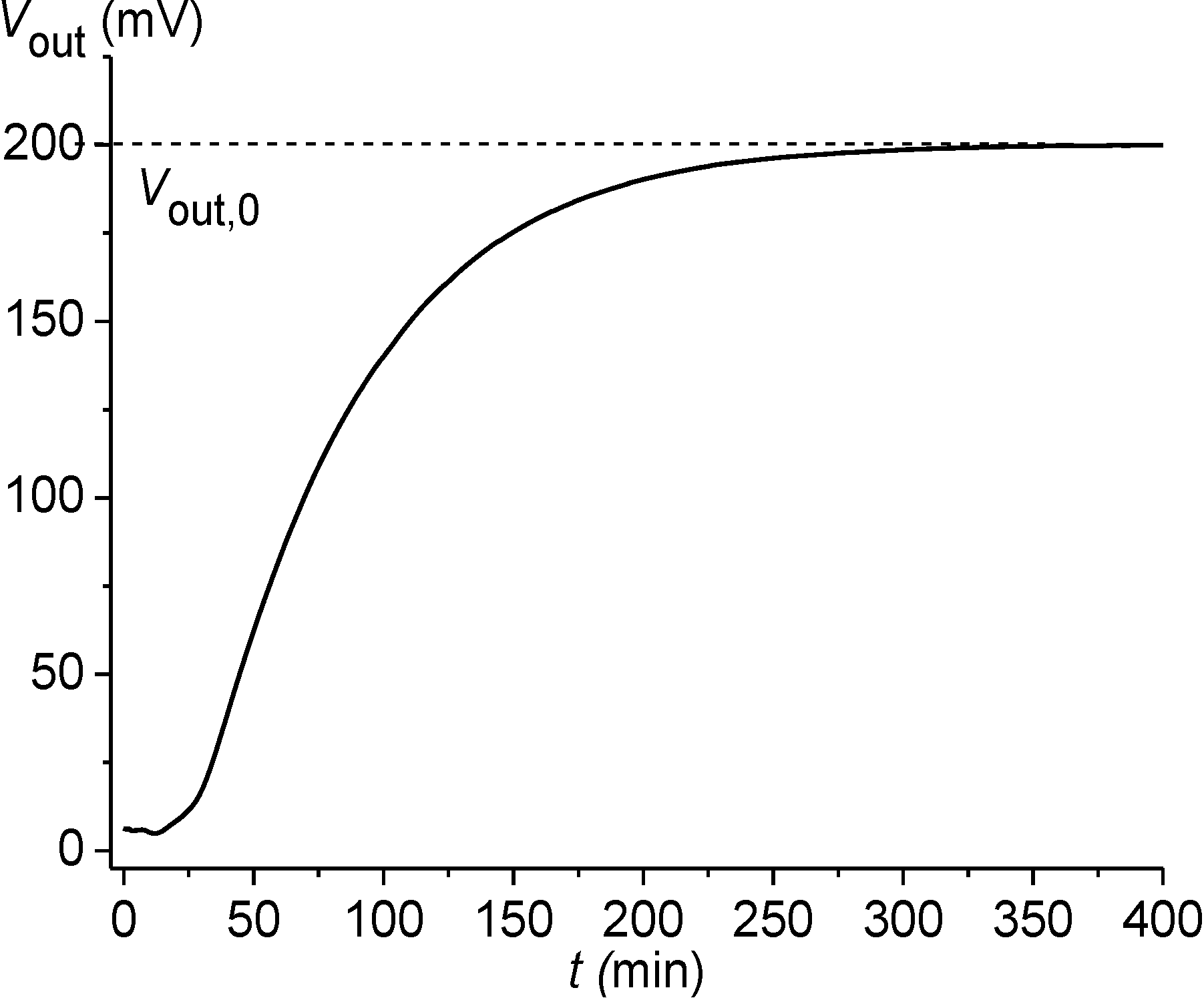


Method of Initial Rate Determination
 − cα0) of the analyte between the solution and the gel was used. With the help of the values v1 and v2 (see Figure B4) for two solutions with a known concentration
− cα0) of the analyte between the solution and the gel was used. With the help of the values v1 and v2 (see Figure B4) for two solutions with a known concentration  and with an unknown one
and with an unknown one  , the value of
, the value of  was estimated using Equation (B1).
was estimated using Equation (B1).
 − cα0,2) = (
− cα0,2) = (  − cα0,1)( v2 / v1)
− cα0,1)( v2 / v1)
 and from cG0,2 to
and from cG0,2 to  .
.
 and from cG0,2 to
and from cG0,2 to  .
.

Uncertainty of Measurements

Appendix C: AAm/3-AAmPBA/BIS Hydrogel
 = 2.23 M, cs = 0.15 M, b = 14.85, pKa = 8.86, and KG = 9.1 mM were used for the calculation of ∆πionic according to Equations (3)–(5) and (A9).
= 2.23 M, cs = 0.15 M, b = 14.85, pKa = 8.86, and KG = 9.1 mM were used for the calculation of ∆πionic according to Equations (3)–(5) and (A9).
| cG, mM | a1 | λ | ∆πionic, kPa |
|---|---|---|---|
| 0 | 28.84 | 1.23 | 16.60 |
| 1 | 25.98 | 1.25 | 19.16 |
| 2 | 23.64 | 1.27 | 22.60 |
| 2.5 | 22.62 | 1.28 | 23.64 |
| 3 | 21.69 | 1.29 | 25.16 |
| 4 | 20.03 | 1.31 | 28.32 |
| 5 | 18.61 | 1.33 | 31.77 |
| 6 | 17.38 | 1.36 | 36.08 |
| 7 | 16.30 | 1.38 | 39.66 |
| 8 | 15.35 | 1.40 | 43.36 |
| 9 | 14.50 | 1.41 | 46.60 |
| 10 | 13.74 | 1.43 | 49.71 |
| 11 | 13.06 | 1.45 | 53.10 |
| 12 | 12.44 | 1.46 | 56.56 |
| 13 | 11.88 | 1.49 | 62.20 |
| 14 | 11.36 | 1.51 | 66.49 |
| 15 | 10.89 | 1.53 | 69.76 |
| 16 | 10.46 | 1.54 | 73.09 |
| 17 | 10.06 | 1.55 | 76.01 |
| 18 | 9.68 | 1.57 | 79.42 |
| 19 | 9.34 | 1.58 | 82.18 |
| 20 | 9.02 | 1.60 | 86.85 |
 = 2.23 M, cs = 0.15 M, b = 14.85, pKa = 8.86, and KF = 0.23 mM were used for the calculation of ∆πionic according to Equations (3)–(5) and (A9).
= 2.23 M, cs = 0.15 M, b = 14.85, pKa = 8.86, and KF = 0.23 mM were used for the calculation of ∆πionic according to Equations (3)–(5) and (A9).
| cF, mM | a1 | λ | ∆πionic, kPa |
|---|---|---|---|
| 0 | 28.84 | 1.23 | 16.60 |
| 0.1 | 20.10 | 1.31 | 28.32 |
| 0.2 | 15.43 | 1.38 | 40.39 |
| 0.3 | 12.52 | 1.47 | 58.00 |
| 0.4 | 10.53 | 1.53 | 70.87 |
| 0.5 | 9.09 | 1.60 | 86.85 |
| 0.6 | 7.99 | 1.66 | 101.29 |
| 0.7 | 7.13 | 1.72 | 116.34 |
| 0.8 | 6.44 | 1.77 | 129.30 |
| 0.9 | 5.87 | 1.85 | 150.75 |
| 1.0 | 5.39 | 1.89 | 161.77 |
| 1.3 | 4.34 | 2.04 | 204.66 |
| 2.5 | 2.43 | 2.57 | 370.21 |
 = 2.23 M, b = 14.85).
= 2.23 M, b = 14.85).
| cG, mM | pH7.2 | pH7.3 | pH7.4 | pH7.5 | ||||
|---|---|---|---|---|---|---|---|---|
| a1 | λ | a1 | λ | a1 | λ | a1 | λ | |
| 0 | 45.71 | 1.15 | 36.31 | 1.19 | 28.84 | 1.23 | 22.91 | 1.28 |
| 1 | 41.18 | 1.17 | 32.71 | 1.20 | 25.98 | 1.25 | 20.64 | 1.30 |
| 2.5 | 35.86 | 1.19 | 28.48 | 1.23 | 22.62 | 1.28 | 17.97 | 1.34 |
| 5 | 29.50 | 1.22 | 23.43 | 1.28 | 18.61 | 1.33 | 14.79 | 1.40 |
| 10 | 21.78 | 1.29 | 17.30 | 1.36 | 13.74 | 1.43 | 10.91 | 1.51 |
| 15 | 17.26 | 1.36 | 13.71 | 1.42 | 10.89 | 1.53 | 8.65 | 1.60 |
| 20 | 14.29 | 1.42 | 11.35 | 1.51 | 9.02 | 1.60 | 7.16 | 1.73 |
 = 2.23 M).
= 2.23 M).
| cG, mM | a1 | λ
I = 0.13 M, b = 17.13 | λ
I = 0.14 M, b = 15.91 | λ
I = 0.15 M, b = 14.85 | λ
I = 0.16 M, b = 13.92 |
|---|---|---|---|---|---|
| 0 | 28.84 | 1.25 | 1.24 | 1.23 | 1.21 |
| 1 | 25.98 | 1.28 | 1.26 | 1.25 | 1.23 |
| 2.5 | 22.62 | 1.31 | 1.29 | 1.28 | 1.26 |
| 5 | 18.61 | 1.36 | 1.34 | 1.33 | 1.31 |
| 10 | 13.74 | 1.47 | 1.44 | 1.43 | 1.40 |
| 15 | 10.89 | 1.57 | 1.54 | 1.53 | 1.48 |
| 20 | 9.02 | 1.66 | 1.63 | 1.60 | 1.57 |
Appendix D: HPMA/DMAEMA/TEGDMA Hydrogel
 = 2.06 M).
= 2.06 M).
| pH | a2 | λ I = 0.13 M, b = 15.85 | λ I = 0.14 M, b = 14.72 | λ I = 0.15 M, b = 13.74 | λ I = 0.16 M, b = 12.88 |
|---|---|---|---|---|---|
| 7.2 | 10 | 0.63 | 0.64 | 0.66 | 0.67 |
| 7.3 | 12.59 | 0.68 | 0.69 | 0.71 | 0.72 |
| 7.4 | 15.85 | 0.71 | 0.72 | 0.74 | 0.75 |
| 7.5 | 19.95 | 0.75 | 0.76 | 0.77 | 0.78 |
Appendix E

References
- Lei, M.; Baldi, A.; Nuxoll, E.; Siegel, R.A.; Ziaie, B. A hydrogel-based implantable micromachined transponder for wireless glucose measurement. Diabetes Technol. Ther. 2006, 8, 112–122. [Google Scholar] [CrossRef]
- Siegel, R.A.; Gu, Y.; Lei, M.; Baldi, A.; Nuxoll, E.; Ziaie, B. Hard and soft micro- and nanofabrication: An integrated approach to hydrogel-based biosensing and drug delivery. J. Controll. Release 2010, 141, 303–313. [Google Scholar] [CrossRef]
- Lin, G.; Chang, S.; Hao, H.; Tathireddy, P.; Orthner, M.; Magda, J.; Solzbacher, F. Osmotic swelling pressure response of smart hydrogels suitable for chronically implantable glucose sensors. Sens. Actuat. B 2010, 144, 332–336. [Google Scholar] [CrossRef]
- Horkay, F.; Cho, S.H.; Tathireddy, P.; Rieth, L.; Solzbacher, F.; Magda, J. Thermodynamic analysis of the selectivity enhancement obtained by using smart hydrogels that are zwitterionic when detecting glucose with boronic acid moieties. Sens. Actuat. B 2011, 160, 1363–1371. [Google Scholar]
- Matsumoto, A.; Kurata, T.; Shiino, D.; Kataoka, K. Swelling and shrinking kinetics of totally synthetic, glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety. Macromolecules 2004, 37, 1502–1510. [Google Scholar] [CrossRef]
- Koudelka, M.; Rohner-Jeanrenaud, F.; Terrettaz, J.; Bobbioni-Harsch, E.; de Rooij, N.F.; Jeanrenaud, B. In-vivo behaviour of hypodermically implanted microfabricated glucose sensors. Biosens. Bioelectron. 1991, 6, 31–36. [Google Scholar] [CrossRef]
- Koschwanez, H.E.; Reichert, W.M. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: Current methods and recommendations. Biomaterials 2007, 28, 3687–3703. [Google Scholar] [CrossRef]
- Reach, G.; Wilson, G.S. Can continuous glucose monitoring be used for the treatment of diabetes. Anal. Chem. 1992, 64, A381–A386. [Google Scholar]
- Orthner, M.P.; Lin, G.; Avula, M.; Buetefisch, S.; Magda, J.; Rieth, L.W.; Solzbacher, F. Hydrogel based sensor arrays (2 × 2) with perforated piezoresistive diaphragms for metabolic monitoring (in-vitro). Sens. Actuat. B 2010, 145, 807–816. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G. Hydrogels for chemical sensors. In Hydrogel Sensors and Actuators; Gerlach, G., Arndt, K.-F., Eds.; Springer Series on Chemical Sensors and Biosensors, Springer: Berlin Heidelberg, Germany, 2009; Volume 6, pp. 165–195. [Google Scholar]
- Guenther, M.; Gerlach, G.; Wallmersperger, T.; Solzbacher, F.; Magda, J.J.; Lin, G.; Tathireddy, P.; Orthner, M.P. Biochemical microsensors on the basis of metabolically sensitive hydrogels. In Proc. SPIE 7976, Electroactive Polymer Actuators and Devices (EAPAD) 2011, 79762D. (March 28, 2011).
- Herber, S.; Bomer, J.; Olthuis, W.; Bergveld, P.; van den Berg, A. Miniaturized carbon dioxide gas sensor based on sensing of pH-sensitive hydrogel swelling with a pressure sensor. Biomed. Microdevices 2005, 7, 197–204. [Google Scholar] [CrossRef]
- Hilt, J.Z.; Gupta, A.K.; Bashir, R.; Peppas, N.A. Ultrasensitive biomems sensors based on microcantilevers patterned with environmentally responsive hydrogels. Biomed. Microdevices 2003, 5, 177–184. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef]
- Windisch, M.; Junghans, T. Hydrogel sensors for process monitoring. Adv. Sci. Technol. 2013, 77, 71–76. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Muscatello, M.M.W.; Stunja, L.E.; Asher, S.A. Polymerized crystalline colloidal array sensing of high glucose concentrations. Anal. Chem. 2009, 81, 4978–4986. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Wallmersperger, T.; Avula, M.N.; Cho, S.H.; Xie, X.; Devener, B.V.; Solzbacher, F.; Tathireddy, P.; Magda, J.J.; et al. Smart hydrogel-based biochemical microsensor array for medical diagnostics. Adv. Sci. Technol. 2013, 85, 47–52. [Google Scholar]
- Guenther, M.; Wallmersperger, T.; Keller, K.; Gerlach, G. Swelling behaviour of functionalized hydrogels for application in chemical sensors. In Intelligent Hydrogels; Sadowski, G., Richtering, W., Eds.; Springer Series on Progress in Colloid and Polymer Science, Springer International Publishing, 2013; Volume 140, pp. 265–273. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks i. rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks ii. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Donnan, F.G. The theory of membrane equilibria. Chem. Rev. 1924, 1, 73–90. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase transition in ionic gels. Phys. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar]
- Lei, M.; Baldi, A.; Nuxoll, E.; Siegel, R.A.; Ziaie, B. Hydrogel-based microsensors for wireless chemical monitoring. Biomed. Microdevices 2009, 11, 529–538. [Google Scholar] [CrossRef]
- Wallmersperger, T. Modelling and simulation of the chemo-electro-mechanical behaviour. In Hydrogel Sensors and Actuators; Gerlach, G., Arndt, K.-F., Eds.; Springer Series on Chemical Sensors and Biosensors, Springer: Berlin Heidelberg, Germany, 2009; Volume 6, pp. 137–163. [Google Scholar]
- Wallmersperger, T.; Ballhause, D.; Kröplin, B.; Günther, M.; Gerlach, G. Coupled multi-field formulation in space and time for the simulation of intelligent hydrogels. J. Intel. Mater. Syst. Struct. 2009, 20, 1483–1492. [Google Scholar] [CrossRef]
- Wallmersperger, T.; Keller, K.; Kröplin, B.; Guenther, M.; Gerlach, G. Modeling and simulation of pH-sensitive hydrogels. Colloid. Polym. Sci. 2011, 289, 535–544. [Google Scholar] [CrossRef]
- Wallmersperger, T.; Attaran, A.; Keller, K.; Brummund, J.; Guenther, M.; Gerlach, G. Modeling and simulation of hydrogels for the application as bending actuators. In Intelligent Hydrogels; Sadowski, G., Richtering, W., Eds.; Springer Series on Progress in Colloid and Polymer Science, Springer International Publishing, 2013; Volume 140, pp. 189–204. [Google Scholar]
- Keller, K.; Wallmersperger, T.; Kröplin, B.; Guenther, M.; Gerlach, G. Modelling of temperature-sensitive polyelectrolyte gels by the use of the coupled chemo-electro-mechanical formulation. Mech. Adv. Mater. Struct. 2011, 18, 511–523. [Google Scholar]
- 2014 General Electric Company doing business as GE Healthcare. Available online: http://www.gelifesciences.com/webapp/wcs/stores/servlet/catalog/en/GELifeSciences-de/products/AlternativeProductStructure_16220 (accessed on 29 May 2014).
- Vo, C.D.; Kuckling, D.; Adler, H.-J.P.; Schönhoff, M. Preparation of thermosensitive nanogels by photo-cross-linking. Colloid. Polym. Sci. 2002, 280, 400–409. [Google Scholar] [CrossRef]
- Kuckling, D.; Hoffmann, J.; Plötner, M.; Ferse, D.; Kretschmer, K.; Adler, H.-J.P.; Arndt, K.-F.; Reichelt, R. Photo cross-linkable poly(N-isopropylacrylamide) copolymers III: micro-fabricated temperature responsive hydrogels. Polymer 2003, 44, 4455–4462. [Google Scholar] [CrossRef]
- Jung, D.Y.; Magda, J.J.; Han, I.S. Catalase effects on glucose-sensitive hydrogels. Macromolecules 2000, 33, 3332–3336. [Google Scholar] [CrossRef]
- Schulz, V.; Gerlach, G.; Günther, M.; Magda, J.J.; Solzbacher, F. Piezoresistive pH microsensors based on stimuli-sensitive polyelectrolyte hydrogels. Techn. Messen 2010, 77, 179–186. [Google Scholar]
- Guenther, M.; Kuckling, D.; Corten, C.; Gerlach, G.; Sorber, J.; Suchaneck, G.; Arndt, K.-F. Chemical sensors based on multiresponsive block copolymer hydrogels. Sens. Actuat. B 2007, 126, 97–106. [Google Scholar] [CrossRef]
- Guide to the Expression of Uncertainty in Measurement, 1st ed.; International Organization for Standardization: Geneva, Switzerland, 1993.
- Hoa, P.L.P.; Gerlach, G.; Suchaneck, G. Uncertainty in measurement of semiconductor piezoresistive sensors. Microsyst. Technol. 2003, 9, 210–214. [Google Scholar] [CrossRef]
- Ivanov, A.E.; Galaev, I.Y.; Mattiasson, B. Smart boronate-containing copolymers and gels at solid-liquid interfaces, cell, membranes, and tissues. In Smart Polymers: Applications in Biotechnology and Biomedicine, 2nd ed.; Galaev, I., Mattiasson, B., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 299–329. [Google Scholar]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Arndt, K.-F.; Richter, A.; Ludwig, S.; Zimmermann, J.; Kressler, J.; Kuckling, D.; Adler, H.-J. Poly(vinyl alcohol)/poly(acrylic acid) hydrogels: FT-IR spectroscopic characterization of crosslinking reaction and work at transition point. Acta Polym. 1999, 50, 383–390. [Google Scholar]
- Tathireddy, P.; Avula, M.; Lin, G.; Cho, S.H.; Guenther, M.; Schulz, V.; Gerlach, G.; Magda, J.; Solzbacher, F. Smart hydrogel based microsensing platform for continuous glucose monitoring. In Merging Medical Humanism and Technology, Proceedings of the 32nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 677–679.
- Lee, M.-C.; Kabilan, S.; Hussan, A.; Yang, X.; Blyth, J.; Lowe, C.R. Clucose-sensitive holographic sensors for monitoring bacterial grown. Anal. Chem. 2004, 76, 5748–5755. [Google Scholar] [CrossRef]
- Schulz, V.; Zschoche, S.; Zhang, H.P.; Voit, B.; Gerlach, G. Macroporous smart hydrogels for fast-responsive piezoresistive chemical microsensors. Procedia Eng. 2011, 25, 1141–1144. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guenther, M.; Wallmersperger, T.; Gerlach, G. Piezoresistive Chemical Sensors Based on Functionalized Hydrogels. Chemosensors 2014, 2, 145-170. https://doi.org/10.3390/chemosensors2020145
Guenther M, Wallmersperger T, Gerlach G. Piezoresistive Chemical Sensors Based on Functionalized Hydrogels. Chemosensors. 2014; 2(2):145-170. https://doi.org/10.3390/chemosensors2020145
Chicago/Turabian StyleGuenther, Margarita, Thomas Wallmersperger, and Gerald Gerlach. 2014. "Piezoresistive Chemical Sensors Based on Functionalized Hydrogels" Chemosensors 2, no. 2: 145-170. https://doi.org/10.3390/chemosensors2020145
APA StyleGuenther, M., Wallmersperger, T., & Gerlach, G. (2014). Piezoresistive Chemical Sensors Based on Functionalized Hydrogels. Chemosensors, 2(2), 145-170. https://doi.org/10.3390/chemosensors2020145





