TiO2 Nanotubes Membrane Flexible Sensor for Low-Temperature H2S Detection
Abstract
:1. Introduction
2. Experimental Methods
2.1. TiO2 Membrane Preparation
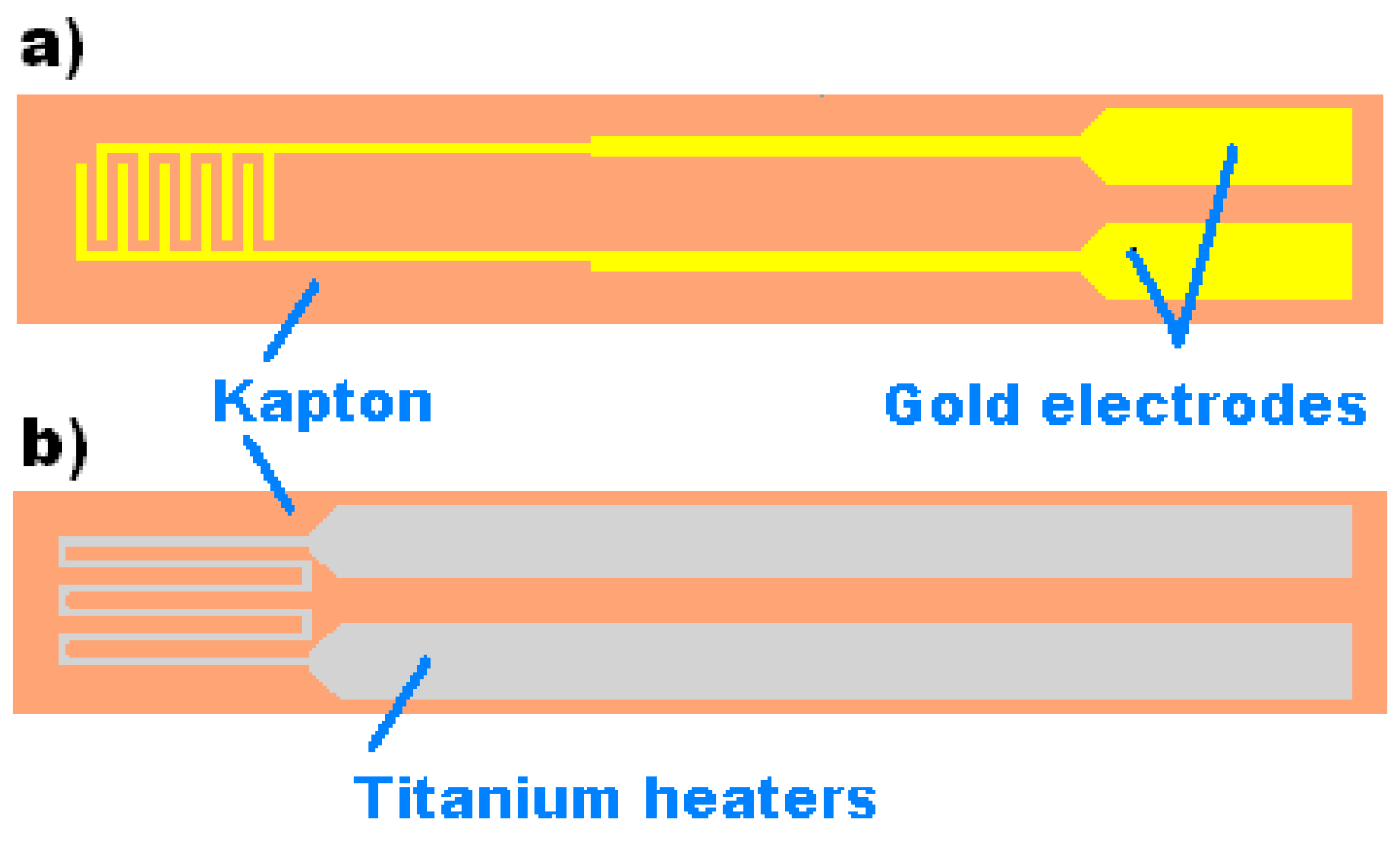
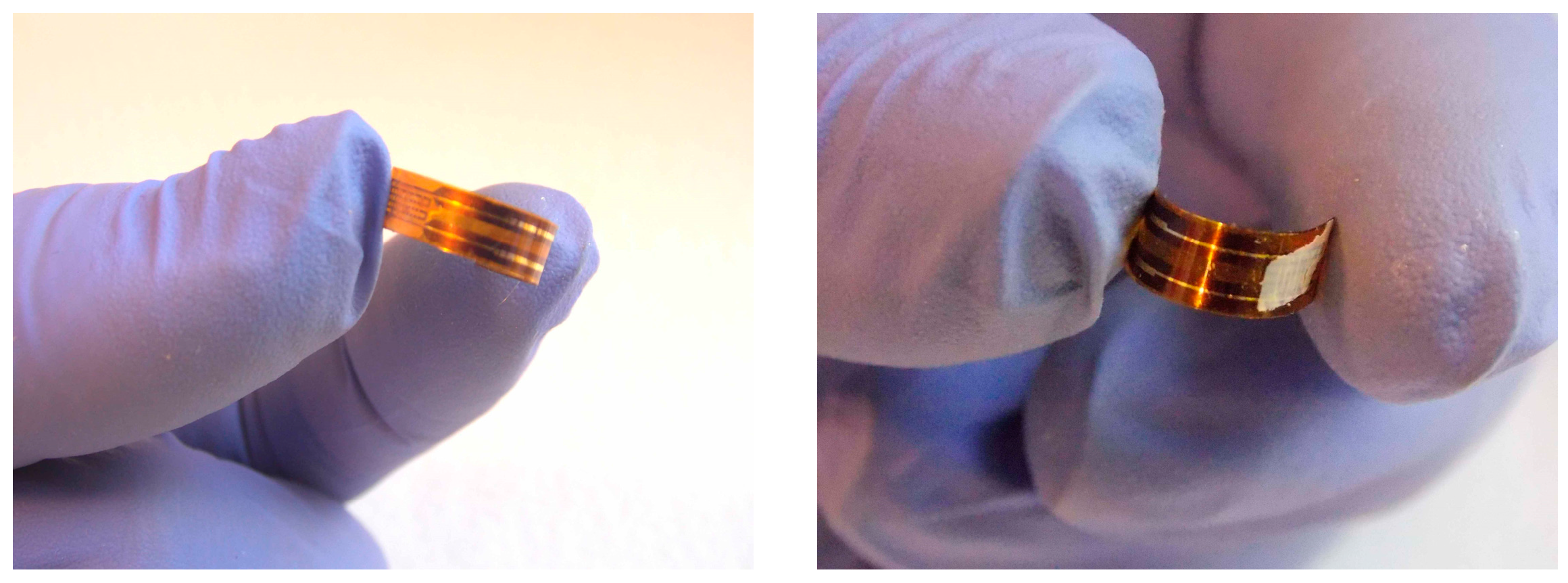
2.2. Sensor Fabrication
2.3. Sensor Characterization
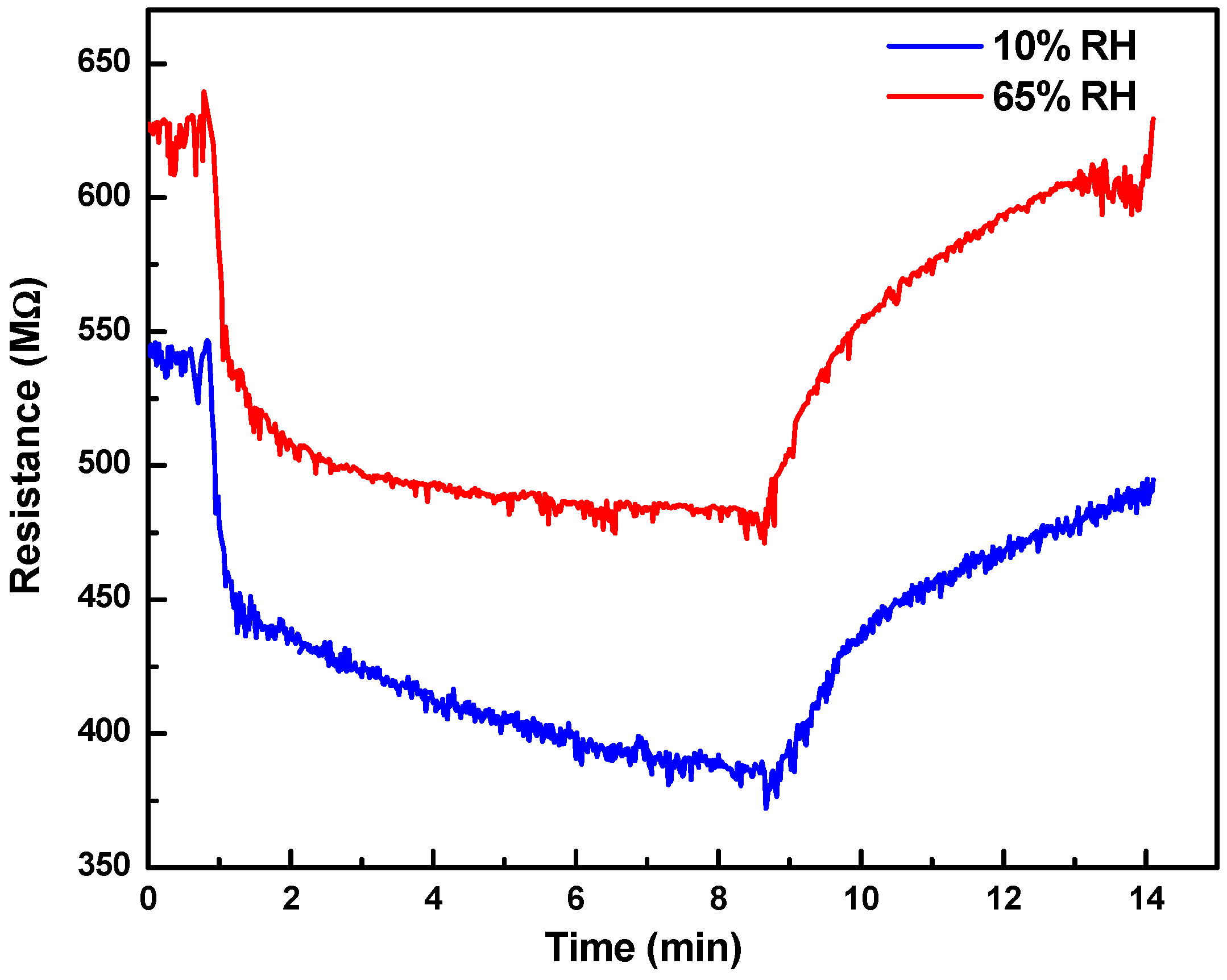
3. Results and Discussion
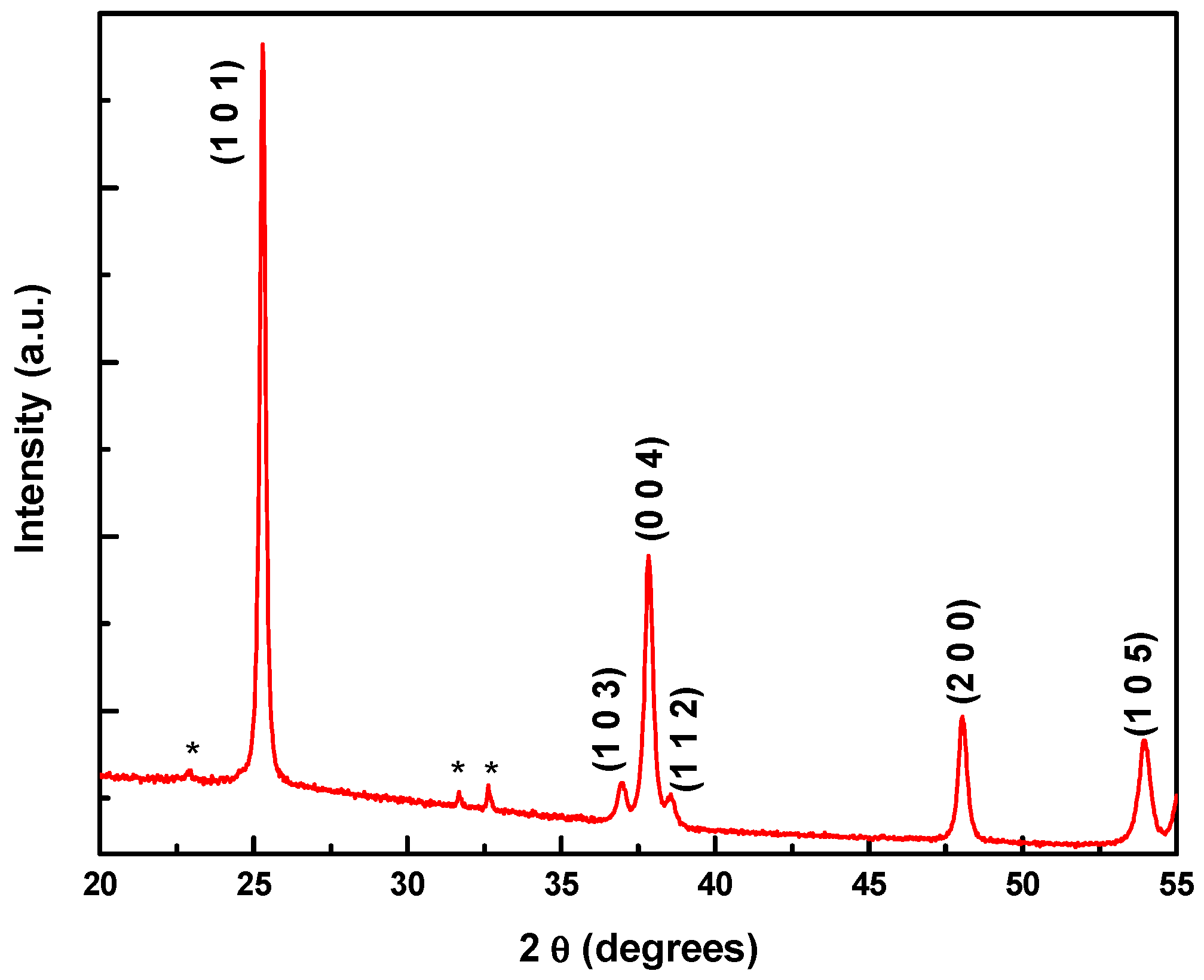
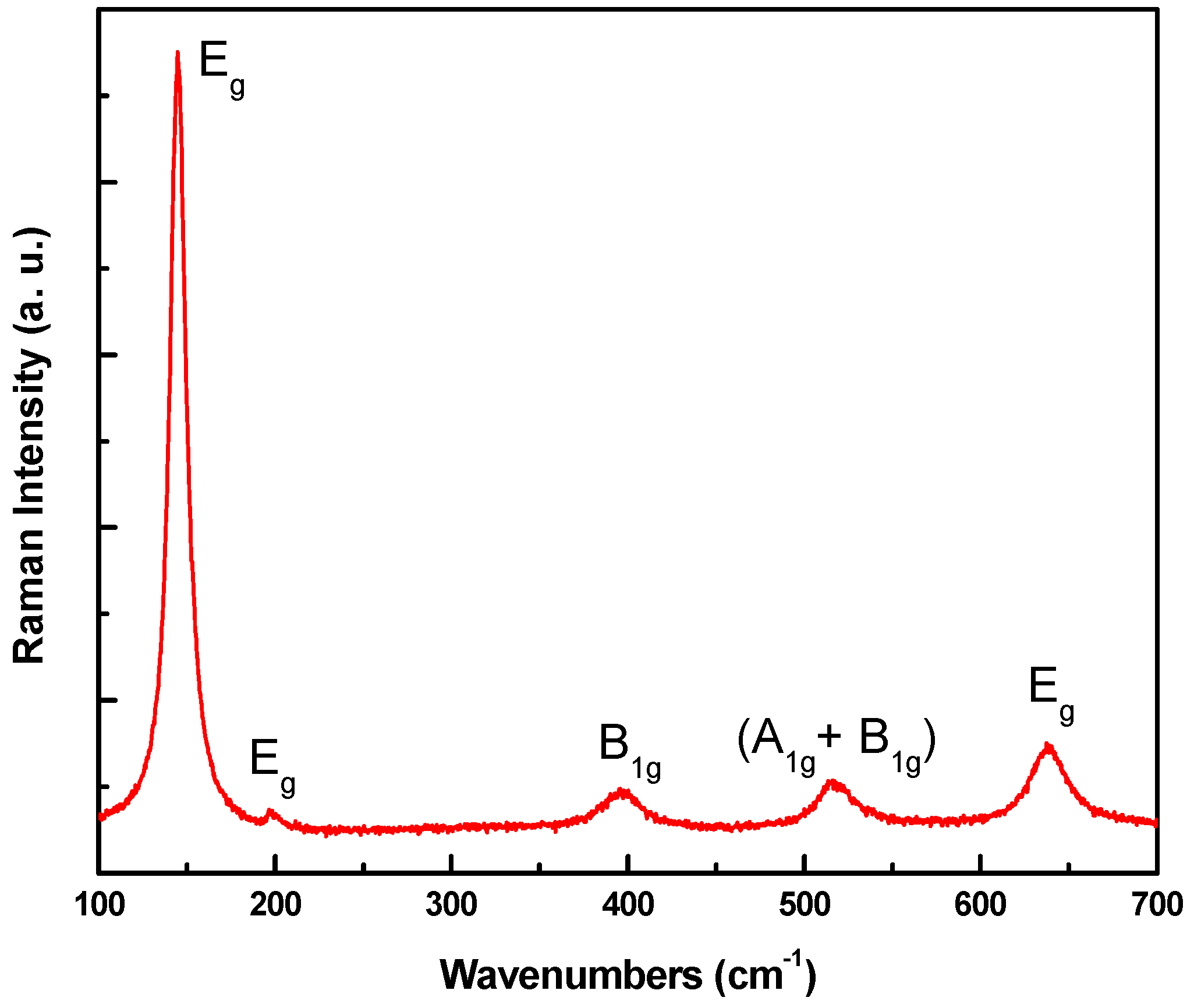
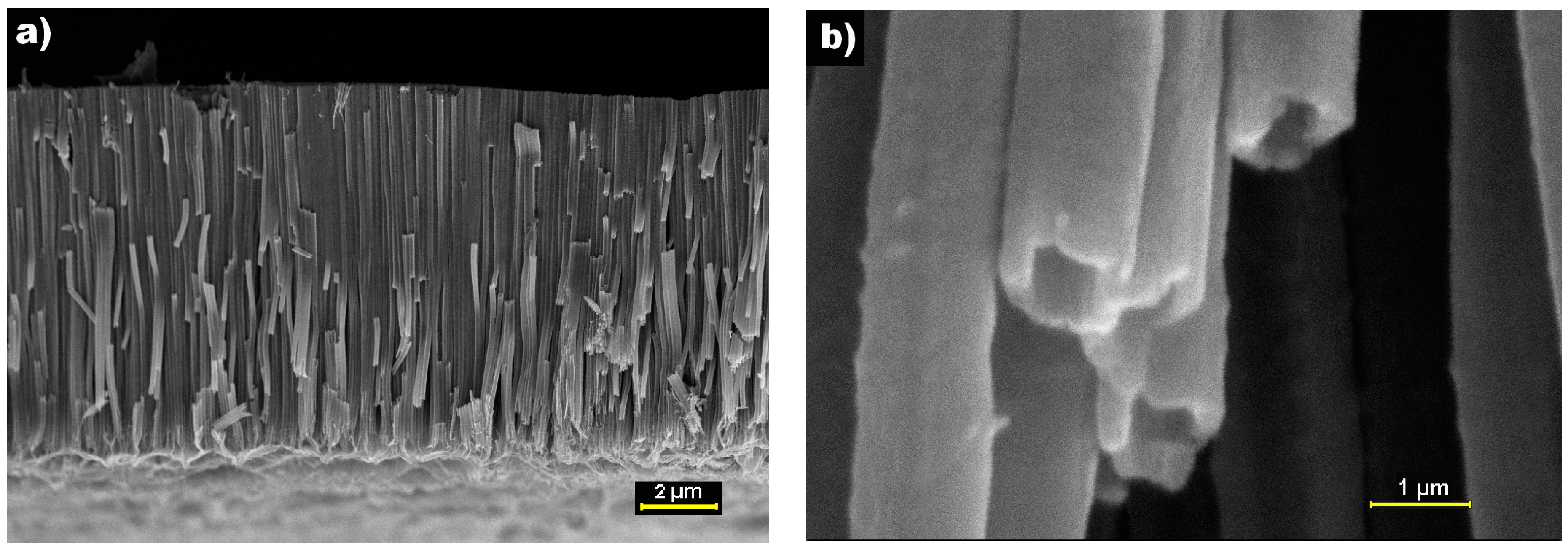
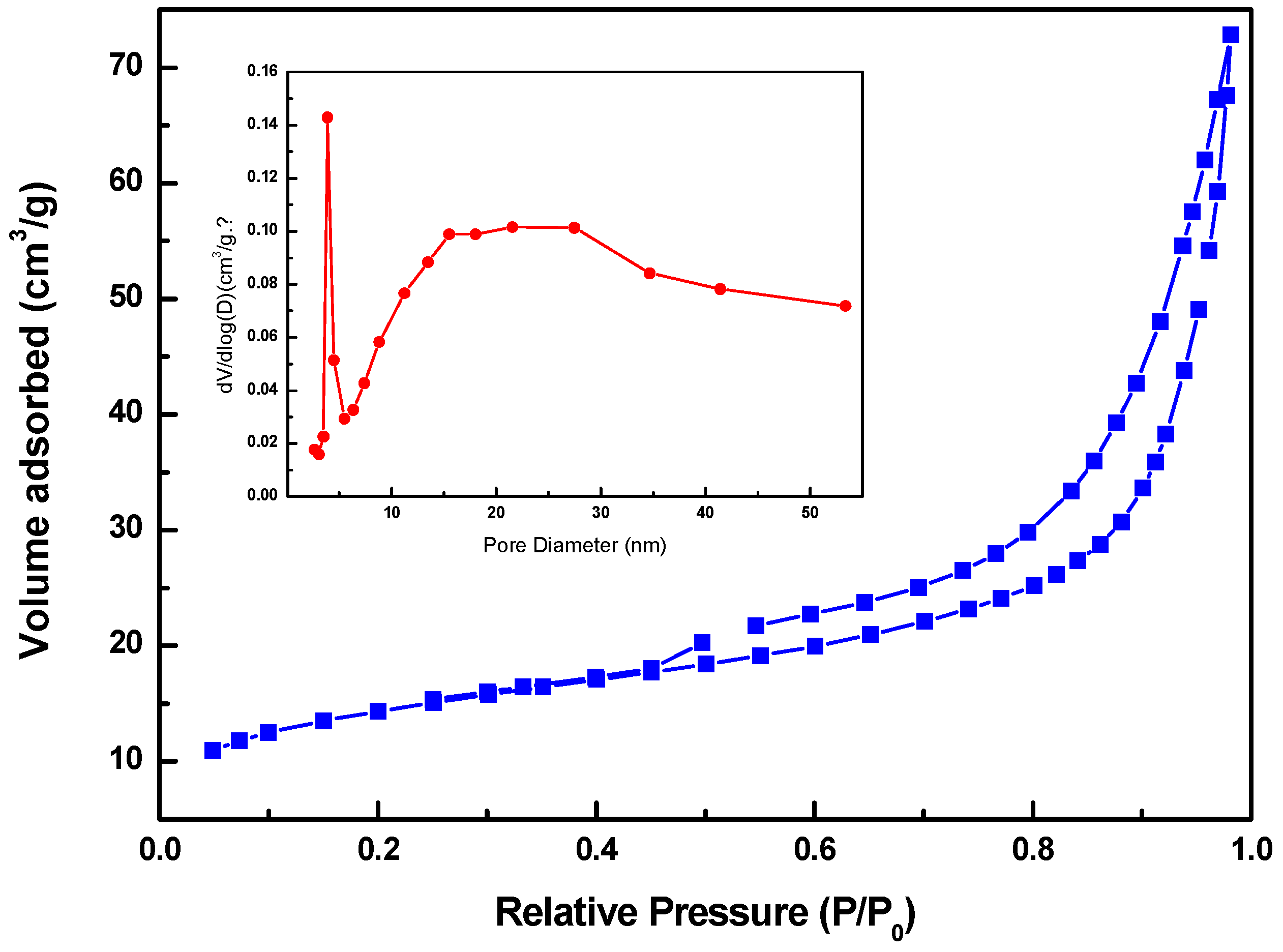
3.1. Microstructure Characterizations
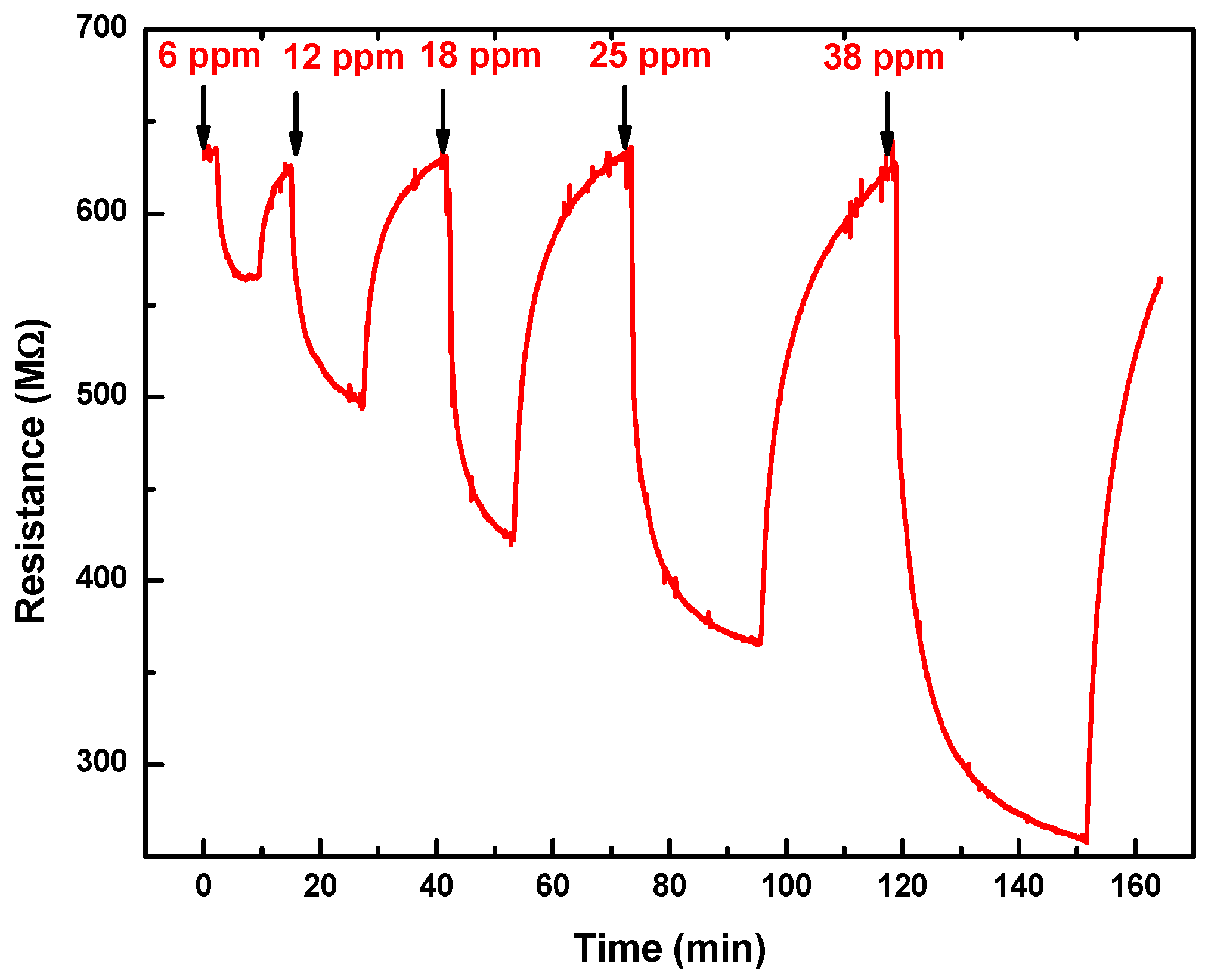
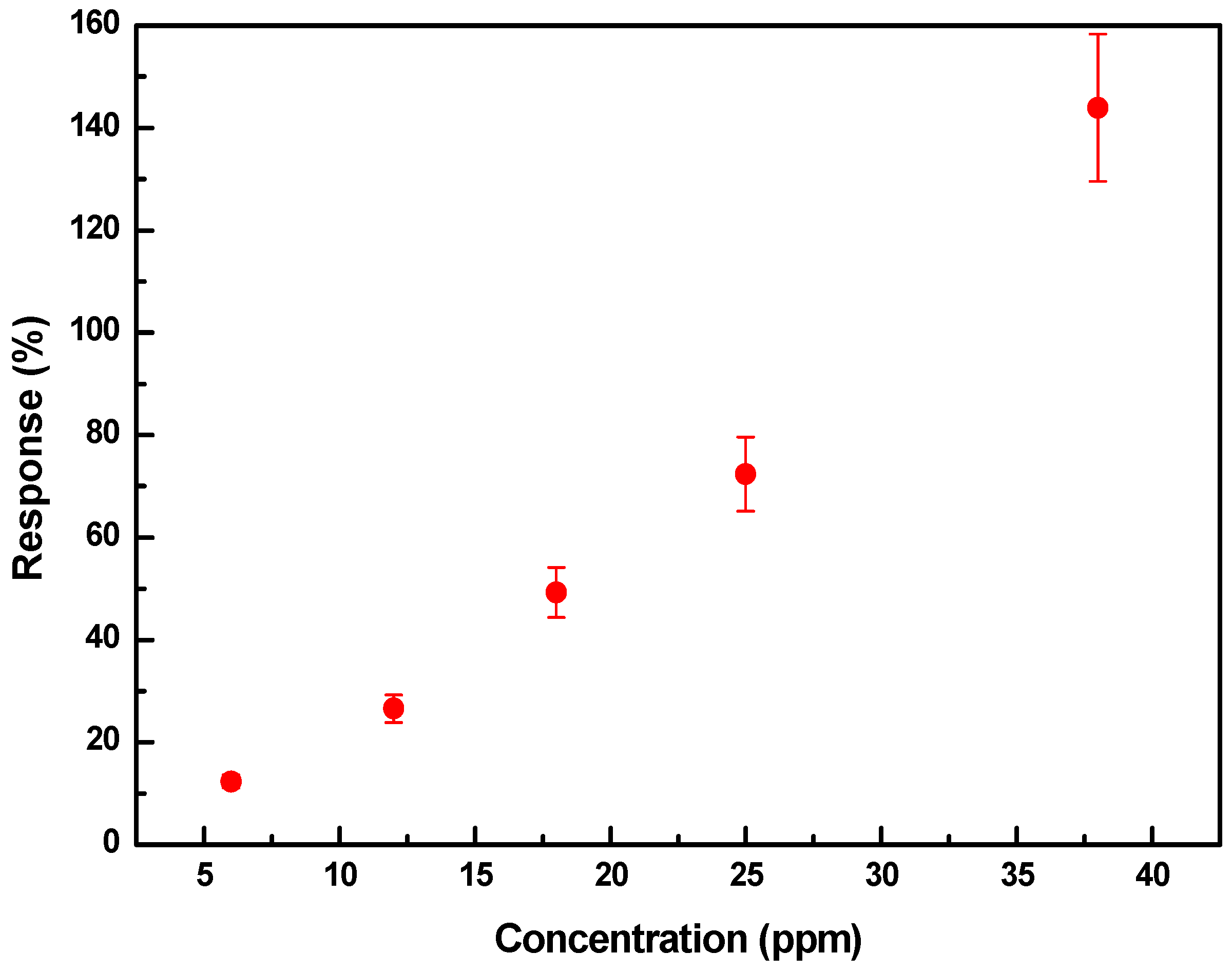
3.2. Gas Sensor Characteristics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beauchamp, R.O.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andgelkovich, D.A. A critical Review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Kelly, P.J.; Doukas, M.; Lopez, T.; Pfeffer, M.; McGimsey, R.; Neal, C. Degassing of CO2, SO2, and H2S associated with the 2009 eruption of Redoubt Volcano, Alaska. J. Volcanol. Geotherm. Res. 2013, 259, 270–284. [Google Scholar] [CrossRef]
- Hwanga, I.S.; Choi, J.K.; Kim, S.J.; Dong, K.Y.; Kwon, J.H.; Ju, B.K.; Lee, J.H. Enhanced H2S sensing characteristics of SnO2 nanowires functionalized with CuO. Sens. Actuators B 2009, 142, 105–110. [Google Scholar] [CrossRef]
- Peu, P.; Picard, S.; Diara, A.; Girault, R.; Béline, F.; Bridoux, G.; Dabert, P. Prediction of hydrogen sulphide production during anaerobic digestion of organic substrates. Bioresour. Technol. 2012, 121, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Bear, J.C.; McGettrick, J.D.; Parkin, I.P.; Dunnill, C.W.; Hasel, T. Porous carbons from inverse vulcanised polymers. Microporous Mesoporous Mater. 2016, 232, 189–195. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (US EPA). Toxicological Review of Hydrogen Sulfide; US EPA: Washington, DC, USA, 2003. Available online: http://www.epa.gov/IRIS/toxreviews/0061tr.pdf (accessed on 1 July 2016).
- Kaur, M.; Jain, N.; Sharma, K.; Bhattacharya, S.; Roy, M.; Tyagi, A.K.; Gupta, S.K.; Yakhmi, J.V. Room-temperature H2S gas sensing at ppb level by single crystal In2O3 whiskers. Sens. Actuators B 2008, 133, 456–461. [Google Scholar] [CrossRef]
- Guo, W.; Feng, Q.; Tao, Y.; Zheng, L.; Han, Z.; Ma, J. Systematic investigation on the gas-sensing performance of TiO2 nanoplate sensors for enhanced detection on toxic gases. Mater. Res. Bull. 2016, 73, 302–307. [Google Scholar] [CrossRef]
- Li, D.; Hu, J.; Fan, F.; Bai, S.; Luo, R.; Chen, A.; Liu, C.C. Quantum-sized ZnO nanoparticles synthesized in aqueous medium for toxic gases detection. J. Alloy. Compd. 2012, 539, 205–209. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene-metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Jia, Y.; Xiao, P.; Tang, J. TiO2 Nanotube Array Sensor for Detecting the SF6 Decomposition Product SO2. Sensors 2012, 12, 3302–3313. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas Sensing Properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, S.; Gràcia, I.; Figueras, E.; Sánchez, J.; Mas, R.; Beldarrain, O.; Cané, C. Microfabrication of flexible gas sensing devices based on nanostructured semiconducting metal oxides. Sens. Actuators A 2014, 219, 88–93. [Google Scholar] [CrossRef]
- McAlpine, M.C.; Ahmad, H.; Wang, D.; Heath, J.R. Highly ordered nanowirearrays on plastic substrates for ultrasensitive flexible chemical sensors. Nat. Mater. 2007, 6, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lee, J.M.W.; Park, I.I. Vertically aligned ZnO nanorods and graphenehybrid architectures for high-sensitive flexible gas sensors. Sens. Actuators B 2011, 155, 264–269. [Google Scholar] [CrossRef]
- Claramunt, S.; Monereo, O.; Boix, M.; Leghrib, R.; Prades, J.D.; Cornet, A.; Merino, P.; Merino, C.; Cirera, A. Flexible gas sensor array with an embedded heater basedon metal decorated carbon nanofibres. Sens. Actuators B 2013, 187, 401–406. [Google Scholar] [CrossRef]
- Walia, S.; Shah, C.M.; Gutruf, P.; Nili, H.; Chowdhury, D.R.; Withayachumnankul, W.; Bhaskaran, M.; Sriram, S. Flexible metasurfaces and metamaterials: A review of materials and fabrication processes at micro- and nano-scales. Appl. Phys. Rev. 2015, 2, 011303. [Google Scholar] [CrossRef]
- Perillo, P.M.; Rodríguez, D.F. Low temperature trimethylamine flexible gas sensor based on TiO2 membrane nanotubes. J. Alloy. Compd. 2016, 657, 765–769. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase TiO2. J. Raman Spectroc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Pongpaiboonkul, S.; Phokharatkul, D.; Hodak, J.H.; Wisitsoraat, A.; Hodak, S.K. Enhancement of H2S-sensing performances with Fe-doping in CaCu3Ti4O12 thin films prepared by a sol-gel method. Sens. Actuators B 2016, 224, 118–127. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Wang, Y.; Zhu, B.; Zhang, S.; Huang, W.; Wang, S. CuO nanoparticle decorated ZnO nanorod sensor for low-temperature H2S detection. Mater. Sci. Eng. C 2012, 32, 2079–2085. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Sharma, P.K.; Datta, N.; Kaur, M.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Room temperature H2S sensor based on Au modified ZnO nanowires. Sens. Actuators B 2013, 186, 718–726. [Google Scholar] [CrossRef]
- Moon, S.; Vuong, N.M.; Lee, D.; Kim, D.; Lee, H.; Kim, D.; Hong, S.; Yoon, S. Co3O4–SWCNT composites for H2S gas sensor application. Sens. Actuators B 2016, 222, 166–172. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Zhang, S.; Chen, W.; Kuang, Z.; Ao, D.; Liu, W.; Fu, Y. A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar] [PubMed]
- Asad, M.; Sheikhi, M.H.; Pourfath, M.; Moradi, M. High sensitive and selective flexible H2S gas sensors based on Cu nanoparticle decorated SWCNT. Sens. Actuators B 2015, 210, 1–8. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; Mortezaali, A.; Iraji zad, A.; Fardindoost, S. Sensitive and selective room temperature H2S gas sensor based on Au sensitized vertical ZnO nanorods with flower-like structures. J. Alloy. Compd. 2015, 628, 222–229. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Kohl, D. Surface processes in the detection of reducing gases with SnO2-based devices. Sens. Actuators B 1989, 18, 71–113. [Google Scholar] [CrossRef]

| Materials | Method | Temperature °C | Concentration (ppm) | H2S (Response) | Response Definition | Ref. |
|---|---|---|---|---|---|---|
| 9 wt% Fe-doped CaCu3Ti4O12 | sol-gel | 250 | 10 | 126 | Ra/Rg | [21] |
| CuO-ZnO | hydrothermal | 100 | 100 | 40 | Ra/Rg | [22] |
| ZnO nanowires | hydrothermal | 25 | 5 | 79 | Ig/Ia | [23] |
| Co3O4–SWCNT | arc-discharge | 250 | 100 | 500 | (Rg − Ra)/Ra × 100 | [24] |
| α-Fe2O3 nanopart | hydrothermal | 300 | 6 | 4 | Ra/Rg | [25] |
| Cu nanoparticles SWCNT | chemical route | 25 | 20 | 26 | (Rg − Ra)/Ra × 100 | [26] |
| ZnO nanorods | vapor phase transport | 25 | 3 | 475 | Ra/Rg | [27] |
| TiO2 nanotubes | anodization | 70 | 6–38 | 12–144 | (Ra − Rg)/Rg × 100 | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perillo, P.M.; Rodríguez, D.F. TiO2 Nanotubes Membrane Flexible Sensor for Low-Temperature H2S Detection. Chemosensors 2016, 4, 15. https://doi.org/10.3390/chemosensors4030015
Perillo PM, Rodríguez DF. TiO2 Nanotubes Membrane Flexible Sensor for Low-Temperature H2S Detection. Chemosensors. 2016; 4(3):15. https://doi.org/10.3390/chemosensors4030015
Chicago/Turabian StylePerillo, Patricia María, and Daniel Fabián Rodríguez. 2016. "TiO2 Nanotubes Membrane Flexible Sensor for Low-Temperature H2S Detection" Chemosensors 4, no. 3: 15. https://doi.org/10.3390/chemosensors4030015
APA StylePerillo, P. M., & Rodríguez, D. F. (2016). TiO2 Nanotubes Membrane Flexible Sensor for Low-Temperature H2S Detection. Chemosensors, 4(3), 15. https://doi.org/10.3390/chemosensors4030015







