Electrochemical Affinity Biosensors in Food Safety
Abstract
:1. Introduction
2. Electrochemical Affinity Biosensors for Toxins
3. Electrochemical Affinity Biosensors for Pesticides
4. Electrochemical Affinity Biosensors for Allergens
5. Electrochemical Affinity Biosensors for Microorganisms
6. Electrochemical Affinity Biosensors for Drug Residues
7. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Yáñez-Sedeño, P.; Pingarrón, J.M. Electroanalysis and food analysis. In Agricultural and Food Electroanalysis; González, A.E.C., López, M.A., Eds.; John Wiley and Sons: Chichester, UK, 2015; Chapter 1; pp. 1–20. [Google Scholar]
- García-Cañas, V.; Simó, C.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Present and future challenges in food analysis: Foodomics. Anal. Chem. 2012, 84, 10150–10159. [Google Scholar] [CrossRef] [PubMed]
- Pividori, M.I.; Alegret, S. Electrochemical Sensor Analysis, Wilson & Wilson’s Comprehensive Analytical Chemistry; Alegret, S., Merkoci, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 22; p. 467. [Google Scholar]
- Rooney, R.; Wall, P.G. Food safety. In Encyclopedia of Food Science and Nutrition; Caballero, B., Trugo, L., Finglas, P.M., Eds.; Academic Press: New York, NY, USA, 2003. [Google Scholar]
- Escarpa, A. Food electroanalysis: Sense and simplicity. Chem. Rec. 2012, 12, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.F.; Moore, E.J. Electrochemical immunosensors for food analysis: A review of recent developments. Anal. Lett. 2016. [Google Scholar] [CrossRef]
- Brahman, P.K.; Dar, R.A.; Pitre, K.S. DNA-functionalized electrochemical biosensor for detection of vitamin B1 using electrochemically treated multiwalled carbon nanotube paste electrode by voltammetric methods. Sens. Actuators B 2013, 177, 807–812. [Google Scholar] [CrossRef]
- Fotouhi, L.; Hashkavayi, A.B.; Heravi, M.M. Interaction of sulfadiazine with DNA on a MWCNT modified glassy carbon electrode: Determination of DNA. Int. J. Biol. Macromol. 2013, 53, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.-E.; Nassef, H.M.; Eissa, A. Voltammetric and ultraviolet–visible spectroscopic studies on the interaction of etoposide with deoxyribonucleic acid. Electrochim. Acta 2013, 113, 164–169. [Google Scholar] [CrossRef]
- Aydoğdu, G.; Günendi, G.; Zeybek, D.K.; Zeybek, B.; Pekyardımcı, S. A novel electrochemical DNA biosensor based onpoly-(5-amino-2-mercapto-1,3,4-thiadiazole) modified glassy carbon electrode for the determination of nitrofurantoin. Sens. Actuators B 2014, 197, 211–219. [Google Scholar] [CrossRef]
- Zeybek, D.K.; Demir, B.; Zeybek, B.; Pekyardımcı, Ş. A sensitive electrochemical DNA biosensor for antineoplastic drug 5-fluorouracil based on glassy carbon electrode modified with poly (bromocresolpurple). Talanta 2015, 144, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Taher, M.A.; Beitollahi, H.; Torkzadeh-Mahani, M. Electrochemical determination of the anticancer drug taxol at a ds-DNA modified pencil-graphite electrode and its application as a label-free electrochemical biosensor. Talanta 2015, 134, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Kurbanoglu, S.; Dogan-Topal, B.; Hlavata, L.; Labuda, J.; Ozkan, S.A.; Uslu, B. Electrochemical investigation of an interaction of the antidepressant drug aripiprazole with original and damaged calf thymus dsDNA. Electrochim. Acta 2015, 169, 233–240. [Google Scholar] [CrossRef]
- Daprà, J.; Lauridsen, L.H.; Nielsen, A.T.; Rozlosnik, N. Comparative study on aptamers as recognition elements for antibiotics in a label-free all-polymer biosensor. Biosens. Bioelectron. 2013, 43, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani, S.; Benvidi, A. Comparison of two fabricated aptasensors based on modified carbon paste/oleic acid and magnetic bar carbon paste/Fe3O4@oleic acid nanoparticle electrodes for tetracycline detection. Biosens. Bioelectron. 2016, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Amaya-González, S.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Aptamer-based analysis: A promising alternative for food safety control. Sensors 2013, 13, 16292–16311. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, M.; Zheng, N.; Xie, N.; Liu, D.; Xie, C.; Yao, D. A novel aptasensor for electrochemical detection of ractopamine, clenbuterol, salbutamol, phenylethanolamine and procaterol. Biosens. Bioelectron. 2016, 80, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Rahimian, A.; Son, K.; Shin, D.-S.; Patel, T.; Revzin, A. Development of an aptasensor for electrochemical detection of exosomes. Methods 2016, 97, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ragavan, K.V.; Thakur, M.S.; Raghavarao, K.S.M.S. Recent advances in nanoparticle based aptasensors for food contaminants. Biosens. Bioelectron. 2015, 74, 612–627. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, G.; Hayat, A.; Andreescu, S. Portable Nanoparticle-Based Sensors for Food Safety Assessment. Sensors 2015, 15, 30736–30758. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chen, B.H. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2016, 24, 15–28. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, Z.; Du, D.; Lin, Y. Nanomaterial-based electrochemical biosensors for food safety. J. Electroanal. Chem. 2016, 781, 147–154. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Reverte, L.; Prieto-Simón, B.; Campas, M. New advances in electrochemical biosensors for the detection of toxins: Nanomaterials, magnetic beads and microfluidics systems. A review. Anal.Chim. Acta 2016, 908, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Botana, L.M.; Alfonso, A.; Botana, A.; Vieytes, M.T.; Vale, C.; Vilariño, N.; Louzao, C. Functional assays for marine toxins as an alternative, high-throughput screening solution to animal tests. TrAC Trends Anal. Chem. 2009, 28, 603–611. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Agüí, L.; Pingarrón, J.M. Biosensors in Forensic Analysis. In Forensic Science: Chemistry, Physics, Biology and Engineering; Katz, E., Halámek, J., Eds.; Wiley: New York, NY, USA, 2016; pp. 215–262. [Google Scholar]
- Hou, L.; Jiang, L.; Song, Y.; Ding, Y.; Zhang, J.; Wu, X.; Tang, D. Amperometric aptasensor for saxitoxin using a gold electrode modified with carbon nanotubes on a self-assembled monolayer, and methylene blue as an electrochemical indicator probe. Microchim. Acta 2016, 183, 1971–1980. [Google Scholar] [CrossRef]
- Van Egmond, H. Marine Biotoxins: Food and Nutrition Paper; Food and Agricultural Organization of the United Nations (FAO): Rome, Italy, 2004. [Google Scholar]
- Flewelling, L.J.; Naar, J.P.; Abbott, J.P.; Baden, D.G.; Barros, N.B.; Bossart, G.D.; Bottein, M.Y.; Hammond, D.G.; Haubold, E.M.; Heil, C.A.; et al. Red tides and marine mammal mortalities. Nature 2005, 435, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, Q.; Lin, Y.; Lu, M.; Tang, D. Mesoporous carbon-enriched palladium nanostructures with redox activity for enzyme-free electrochemical immunoassay of brevetoxin B. Anal. Chim. Acta 2015, 887, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.; Tufts, N. Monitoring programs and epidemiology. In Toxic Dinoflagellate Blooms; Taylor, D., Seliger, H., Eds.; Elsevier Press: New York, NY, USA, 1979; pp. 489–492. [Google Scholar]
- Eissa, S.; Siaj, M.; Zourob, M. Aptamer-based competitive electrochemical biosensor for brevetoxin-2. Biosens. Bioelectron. 2015, 69, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.B.; Hayat, A.; Sassolas, A.; Alonso, G.A.; Muñoz, R.; Marty, J.L. Automated flow-through amperometric immunosensor for highly sensitive and on-line detection of okadaic acid in mussel sample. Talanta 2012, 99, 232–237. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Opinion of the scientific panel on contaminants in the food chain on a request from the European Comission on marine biotoxins in shelffish-okadaic acid and analogues. EFSA J. 2008, 1306, 1–62. [Google Scholar]
- Hayat, A.; Barthelmebs, L.; Sassolas, A.; Marty, J.L. Development of a novel label-free amperometric immunosensor for the detection of okadaic acid. Anal. Chim. Acta 2012, 724, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. A graphene-based electrochemical competitive immunosensor for the sensitive detection of okadaic acid in shellfish. Nanoscale 2012, 4, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hou, L.; Tang, D.; Liu, B.; Li, J.; Chen, G. Simultaneous multiplexed stripping voltammetric monitoring of marine toxins in seafood based on distinguishable metal nanocluster-labeled molecular tags. J. Agric. Food Chem. 2012, 60, 8974–8982. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins—Occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, Addendum to Volume 2: Health Criteria and Other Supporting Information; World Health Organization: Geneva, Switzerland, 1998; pp. 94–110. [Google Scholar]
- Hou, L.; Ding, Y.; Zhang, L.; Guo, Y.; Li, M.; Chen, Z.; Wu, X. An ultrasensitive competitive immunosensor for impedimetric detection of microcystin-LR via antibody-conjugated enzymaticbiocatalytic precipitation. Sens. Actuators B 2016, 233, 63–70. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, H.; Yuan, F.; Quan, X.; Chen, X.-N. Ultrasensitive immunoassay of microcystins-LR using G-quadruplex DNAzyme as an electrocatalyst. Int. J. Environ. Anal. Chem. 2014, 94, 988–1000. [Google Scholar] [CrossRef]
- Ge, S.; Liu, W.; Ge, L.; Yan, M.; Yan, J.; Huang, J.; Yu, J. In situ assembly of porous Au-paper electrode and functionalization of magnetic silica nanoparticles with HRP via click chemistry for microcystin-LR immunoassay. Biosens. Bioelectron. 2013, 49, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Queirós, R.B.; Guedes, A.; Marques, P.V.S.; Noronha, J.P.; Sales, M.G.F. Recycling old screen-printed electrodes with newly designed plastic antibodies on the wall of carbon nanotubes as sensory element for in situ detection of bacterial toxins in water. Sens. Actuators B 2013, 189, 21–29. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, J.; Quan, X. A graphene and multienzyme functionalized carbon nanosphere-based electrochemical immunosensor for microcystin-LR detection. Coll. Surf. B Biointerfaces 2013, 103, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Ling, L.; He, Z.; Lei, H.; Liu, Y. In-situ assembly of biocompatible core–shell hierarchical nanostructures sensitized immunosensor for microcystin-LR detection. Biosens. Bioelectron. 2016, 78, 381–389. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 2nd ed.; Addendum to Volume 1. Recommendations; World Health Organization: Geneva, Switzerland, 1998; p. 13. [Google Scholar]
- Han, C.; Doepke, A.; Cho, W.; Likodimos, V.; de la Cruz, A.A.; Back, T.; Heineman, W.R.; Halsall, H.B.; Shanov, V.N.; Schulz, M.J.; et al. A multiwalled carbon nanotube-based biosensor for monitoring microcystin-LR in sources of drinking water supplies. Adv. Funct. Mater. 2013, 23, 1807–1816. [Google Scholar] [CrossRef]
- Eissa, S.; Ng, A.; Siaj, M.; Zourob, M. Label-free voltammetric aptasensor for the sensitive detection of microcystin-LR using graphene-modified electrodes. Anal. Chem. 2014, 86, 7551–7557. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.O. Mycotoxins. Mycol. Res. 1996, 100, 513–523. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs, Official Journal of the European Union, 2006, L364/5–L364/24. Available online: https://www.fsai.ie/uploadedFiles/Regulation-EC-1881-2006.pdf (accessed on 19 February 2017).
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernández, S.; Bertolín, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Catanante, G.; Rhouati, A.; Hayat, A.; Marty, J.L. An overview of recent electrochemical immunosensing strategies for mycotoxins detection. Electroanalysis 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations FAO. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO: Rome, Italy, 2004; p. 9. [Google Scholar]
- Zheng, M.Z.; Richard, J.L.; Binder, J. A review of rapid methods for the analysisof mycotoxins. Mycopathologia 2006, 161, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Sun, J.; Zhang, Y.; Bian, C.; Xia, S.; Zhen, T. Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens. Bioelectron. 2016, 80, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, R.; Li, Z.; Xia, Q.; Fang, Y.; Liu, J. An immunosensor for ultrasensitive detection of aflatoxin B1 with an enhanced electrochemical performance based on graphene/conducting polymer/gold nanoparticles/the ionic liquid composite film on modified gold electrode with electrodeposition. Biochem. Eng. J. 2016, 115, 38–46. [Google Scholar]
- Zhang, X.; Li, C.R.; Wang, W.C.; Xue, J.; Huang, Y.L.; Yang, X.X.; Tan, B.; Zhou, X.P.; Shao, C.; Ding, S.J.; et al. A novel electrochemical immunosensor for highly sensitive detection of aflatoxin B1 in corn using single-walled carbon nanotubes/chitosan. Food Chem. 2016, 192, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Jodra, A.; Hervás, M.; López, M.Á.; Escarpa, A. Disposable electrochemical magneto immunosensor for simultaneous simplified calibration and determination of Ochratoxin A in coffee samples. Sens. Actuators B 2015, 221, 777–783. [Google Scholar] [CrossRef]
- Bulbul, G.; Hayat, A.; Andreescu, S. A generic amplification strategy for electrochemical aptasensors using a non-enzymatic nanoceria tag. Nanoscale 2015, 7, 13230–13238. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Huang, Y.; Zhang, C.; Liu, H.; Tang, D. Amplified impedimetric immunosensor based on instant catalyst for sensitive determination of ochratoxin A. Biosens. Bioelectron. 2016, 86, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Malvano, F.; Albanese, D.; Pilloton, R.; Di Matteo, M. A highly sensitive impedimetric label free immunosensor for Ochratoxin measurement in cocoa beans. Food Chem. 2016, 212, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Mayorga-Martinez, C.; Quesada-Gonzalez, D.; Zamora-Galvez, A.; Escosura-Muñiz, A.; Merkoci, A. Label-free impedimetric aptasensor for ochratoxin-A detection using iridium oxide nanoparticles. Anal. Chem. 2015, 87, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.-L.; Zhang, Y.-F.; Sun, G.-P.; Wang, X.-N.; Tang, D. Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer–graphene oxide nanosheets and DNaseI-based target recycling reaction. Biosens. Bioelectron. 2017, 89, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Dietrich, R.; Didier, A.; Doyscher, D.; Märtlbauer, E. Recent Developments in antibody-based assays for the detection of bacterial toxins. Toxins 2014, 6, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tang, J.; Su, B.; Chen, G. Ultrasensitive electrochemical immunoassay of Staphylococcal Enterotoxin B in food using enzyme-nanosilica-doped carbon nanotubes for signal amplification. J. Agric. Food Chem. 2010, 58, 10824–10830. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Pimenta-Martins, M.G.; Ferro Furtado, R.; Dias Heneine, L.G.; Souza Dias, R.; Borges, M.F.; Alves, C.R. Development of an amperometric immunosensor for detection of staphylococcal enterotoxin type A in cheese. J. Microb. Meth. 2012, 91, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, B.; Zhang, F.; Sun, X.; Zhang, Y.; Li, Z. A novel electrochemical immunosensor based on magnetosomes for detection of staphylococcal enterotoxin B in milk. Talanta 2013, 106, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in electrochemical biosensors for pesticide detection: Advances and challenges in food analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- European Union Introduction to EC Pesticides Residues Legislation. European Union: Brussels. Available online: http://europa.eu.int/comm/food/plant/protection/resources/intro_en.pdf (accessed on 29 January 2017).
- JRC Foresight Study of European Commission, Joint Research Centre Tomorrow’s Healthy Society Research Priorities for Foods and Diets 2014 Final Report. Available online: https://ec. europa.eu/jrc/sites/default/files/jrc-study-tomorrow-healthlysociety.pdf (accessed on 31 January 2016).
- Wei, W.; Zong, X.; Wang, X.; Yin, L.; Pu, Y.; Liu, S. A disposable amperometric immunosensor for chlorpyrifos-methyl based on immunogen/platinum doped silica sol–gel film modified screenprinted carbon electrode. Food Chem. 2012, 135, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Guo, Y.; Sun, X.; Wang, X. An Electrochemical Immunosensor Based on Microfluidic Chip for Detection of Chlorpyrifos. Int. J. Electrochem. Sci. 2015, 10, 8750–8758. [Google Scholar]
- Dai, Z.; Liu, H.; Shen, Y.; Su, X.; Xu, Z.; Sun, Y.; Zou, X. Attomolar determination of coumaphos by electrochemical displacement immunoassay coupled with oligonucleotide sensing. Anal. Chem. 2012, 84, 8157–8163. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, W.; Song, D. A multianalyte electrochemical immunosensor based on patterned carbon nanotubes modified substrates for detection of pesticides. Biosens. Bioelectron. 2014, 52, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, Y.; Wang, X. Amperometric immunosensor based on deposited gold nanocrystals/4, 4′-thiobisbenzenethiol for determination of carbofuran. Food Control 2012, 28, 184–191. [Google Scholar] [CrossRef]
- Liu, G.; Wang, S.; Liu, J.; Song, D. An electrochemical immunosensor based on chemical assembly of vertically aligned carbon nanotubes on carbon substrates for direct detection of the pesticide endosulfan in environmental water. Anal. Chem. 2012, 84, 3921–3928. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Li, Z. A highly selective electrochemical impedance spectroscopy-based aptasensor for sensitive detection of acetamiprid. Biosens. Bioelectron. 2013, 43, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Fei, A.; Liu, Q.; Huan, J.; Qian, J.; Dong, X.; Qiu, B.; Mao, H.; Wang, K. Label-free impedimetric aptasensor for detection of femtomole level acetamiprid using gold nanoparticles decorated multiwalled carbon nanotube-reduced graphene oxide nanoribbon composites. Biosens. Bioelectron. 2015, 70, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, W.J.; Li, L.; Yang, Y.; Mao, L.G.; Peng, Z. A label-free electrochemical immunosensor based on gold nanoparticles for direct detection of atrazine. Sens. Actuators B 2014, 191, 408–414. [Google Scholar] [CrossRef]
- Wang, M.; Kang, H.; Xu, D.; Wang, C.; Liu, S.; Hu, X. Label-free impedimetric immunosensor for sensitive detection of fenvalerate in tea. Food Chem. 2013, 141, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Valera, E.; García-Febrero, R.; Pividori, I.; Sánchez-Baeza, F.; Marco, M.P. Coulombimetric immunosensor for paraquat based on electrochemical nanoprobes. Sens. Actuators B 2014, 194, 353–360. [Google Scholar] [CrossRef]
- Vasilescu, A.; Nunes, G.; Hayat, A.; Latif, U.; Marty, J.L. Electrochemical affinity biosensors based on disposable screen-printed electrodes for detection of food allergens. Sensors 2016, 16, 1863. [Google Scholar] [CrossRef] [PubMed]
- Pilolli, R.; Monaci, L.; Visconti, A. Advances in biosensor development based on integrating nanotechnology and applied to food-allergen management. TrAC Trends Anal. Chem. 2013, 47, 12–26. [Google Scholar] [CrossRef]
- Poms, R.E.; Agazzi, M.E.; Bau, A.; Brohee, M.; Capelletti, C.; Nørgaard, J.V.; Anklam, E. Inter-laboratory validation study of five commercial ELISA test kitsfor the determination of peanut proteins in biscuits and dark chocolate. Food Addit. Contam. 2005, 22, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Monaci, L.; Visconti, A. Immunochemical and DNA-based methods in food allergen analysis and quality assurance perspectives. Trends Food Sci. Technol. 2010, 21, 272–283. [Google Scholar] [CrossRef]
- Holzhauser, T.; Kleiner, K.; Janise, A.; Röder, M. Matrix-normalised quantification of species by threshold-calibrated competitive real-time PCR: Allergenic peanut in food as one example. Food Chem. 2014, 163, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Nordlee, J.A.; Niemann, L.M.; Lambrecht, D.M. Allergen immunoassays—Considerations for use of naturally incurred standards. Anal. Bioanal. Chem. 2009, 395, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, S.; Fourdrilis, S.; Dobson, R.; Scippo, M.L.; Maghuin-Rogister, G.; De Pauw, E. Quantitative methods for food allergens: A review. Anal. Bioanal. Chem. 2009, 395, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkan, E.; Saber, R.; Karimi, Z.; Shamsipur, M. A novel antibody–antigen based impedimetricimmunosensor for low level detection of HER2 in serum samples of breast cancer patients via modification of a gold nanoparticles decorated multiwall carbon nanotube-ionic liquid electrode. Anal. Chim. Acta 2015, 874, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhao, H.; Yang, Y.; He, Y.; Ding, N.; Wang, J.; Wu, Z.; Xiang, K.; Wang, G. Electrochemical immunosensor for casein based on gold nanoparticles and poly(l-Arginine)/multi-walled carbon nanotubes composite film functionalized interface. Biosens. Bioelectron. 2011, 26, 3469–3474. [Google Scholar] [CrossRef] [PubMed]
- Laube, T.; Kergaravat, S.V.; Fabiano, S.N.; Hernandez, S.R.; Alegret, S.; Pividori, M.I. Magneto immunosensor for gliadin detection in gluten-free foodstuff: Towards food safety for celiac patients. Biosens. Bioelectron. 2011, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Tlili, C.; L’Hocine, L.; Zourob, M. Electrochemical immunosensor for the milk allergen b-lactoglobulin based on electrografting of organic film on graphene modified screen-printed carbon electrodes. Biosens. Bioelectron. 2012, 38, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; L’Hocine, L.; Siaj, M.; Zourob, M. A graphene-based label-free voltammetric immunosensor for sensitive detection of the egg allergen ovalbumin. Analyst 2013, 138, 4378–4384. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas Montiel, V.; Campuzano, S.; Conzuelo, F.; Torrente-Rodríguez, R.M.; Gamella, M.; Reviejo, A.J.; Pingarrón, J.M. Electrochemical magnetoimmunosensing platform for determination of the milk allergen β-lactoglobulin. Talanta 2015, 131, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Cadková, M.; Metelka, R.; Holubová, L.; Horák, D.; Dvoráková, V.; Bílková, Z.; Korecká, L. Magnetic beads-based electrochemical immunosensor for monitoring allergenic food proteins. Anal. Biochem. 2015, 484, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas Montiel, V.; Campuzano, S.; Pellicanò, A.; Torrente-Rodríguez, R.M.; Reviejo, A.J.; Cosio, M.S.; Pingarrón, J.M. Sensitive and selective magneto-immunosensing platform for determination of the food allergen Ara h 1. Anal. Chim. Acta 2015, 880, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Marques, R.C.B.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Correr, W.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of the peanut allergen Ara h 6 in foodstuffs using a voltammetricbiosensing approach. Anal. Bioanal. Chem. 2015, 497, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Molinari, J.; Moina, C.; Ybarra, G. Electrochemical immunosensor for the determination of β-casein. J. Electrochem. Sci. Eng. 2015, 5, 9–16. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Torrente-Rodríguez, R.M.; Campuzano, S.; Pellicanò, A.; Reviejo, A.J.; Cosio, M.S.; Pingarrón, J.M. Simultaneous determination of the main peanut allergens in foods using disposable amperometric magnetic beads-based immunosensing platforms. Chemosensors 2016, 4, 11. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Campuzano, S.; Torrente-Rodríguez, R.M.; Reviejo, A.J.; Pingarrón, J.M. Electrochemical magnetic beads-based immunosensing platform for the determination of α-lactalbumin in milk. Food Chem. 2016, 213, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guan, L.; Shan, X.; Zhang, Y.; Li, Z. Electrochemical detection of peanut allergen Ara h 1 using a sensitive DNA biosensor based on stem–loop probe. J. Agric. Food Chem. 2012, 60, 10979–10984. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jia, M.; Guan, L.; Ji, J.; Zhang, Y.; Tang, L.; Li, Z. Multilayer graphene–gold nanocomposite modified stem-loop DNA biosensor for peanut allergen-Ara h 1 detection. Food Chem. 2015, 172, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Paniagua López, M.; Frutos Cabanillas, G.; Lobo Castañón, M.J.; López-Ruiz, B. Development of a genosensor for peanut allergen ARA h 2 detection and its optimization by surface response methodology. Biosens. Bioelectron. 2014, 62, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, B.; de-los-Santos-Álvarez, N.; Martín-Clemente, J.P.; Lobo-Castañón, M.J.; López-Ruiz, B. Challenging genosensors in food samples: The case of gluten determination in highly processed samples. Talanta 2016, 146, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Gonzalez, S.; de-los-Santos-Alvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Aptamer binding to celiac disease-triggering hydrophobic proteins: A sensitive gluten detection approach. Anal. Chem. 2014, 86, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Codex Alimentarius Alinorm 08/31/REP; Codex Alimentarius: Geneva, Switzerland, 2008. [Google Scholar]
- Trashin, S.; de Jong, M.; Breugelmans, T.; Pilehvar, S.; De Wael, K. Label-free impedance aptasensor for major peanut allergen Ara h 1. Electroanalysis 2015, 27, 32–37. [Google Scholar] [CrossRef]
- Ocaña, C.; Hayat, A.; Mishra, R.K.; Vasilescu, A.; del Valle, M.; Marty, J.L. Label free aptasensor for Lysozyme detection: A comparison of the analytical performance of two aptamers. Bioelectrochemistry 2015, 105, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, C.; Hayat, A.; Mishra, R.K.; Vasilescu, A.; del Valle, M.; Marty, J.L. A novel electrochemical aptamer–antibody sandwich assay for lysozyme detection. Analyst 2015, 140, 4148–4153. [Google Scholar] [CrossRef] [PubMed]
- International Code of Oenological Practices. Available online: http://www.oiv.int/oiv/info/enplubicationoiv#code 2013 (accessed on 29 January 2017).
- Yang, C.; Gu, B.; Xu, C.; Xu, X. Self-assembled ZnO quantum dot bioconjugates for direct electrochemical determination of allergen. J. Electroanal. Chem. 2011, 660, 97–100. [Google Scholar] [CrossRef]
- Sugawara, K.; Kadoya, T.; Kuramitz, H. Construction of a peptide with an electroactive daunomycin like a pendant arm to detect ovalbumin. Anal. Chim. Acta 2015, 857, 71–78. [Google Scholar] [CrossRef]
- Cucu, T.; Jacxsens, L.; De Meulenaer, B. Analysis to support allergen risk management: Which way to go? J. Agric. Food Chem. 2013, 61, 5624–5633. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lei, Y.; Yan, L.; Yu, T.; Li, Q.; Zhang, D.; Ding, S.; Ju, H. A rapid and sensitive aptamer-based electrochemical biosensor for direct detection of Escherichia coli O111. Electroanalysis 2012, 24, 1186–1191. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, P.; Gong, J.; Fang, L.; Deng, J.; Liang, W.; Zheng, J. Amperometric immunosensor for the detection of Escherichia coli O157:H7 in food specimens. Anal. Biochem. 2012, 421, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.A.; de la Escosura-Muñiz, A.; Merkoçi, A. Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags. Biosens. Bioelectron. 2015, 67, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, R.; Li, Y. Rapid detection of Escherichia coli O157:H7 and Salmonella Typhimurium in foods using an electrochemical immunosensor based on screen-printed interdigitated microelectrode and immunomagnetic separation. Talanta 2016, 148, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhao, H.; Xu, M.; Ma, Q.; Ai, S. A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem. 2013, 141, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Bagheryan, Z.; Raoof, J.-B.; Golabi, M.; Turner, A.P.F.; Beni, V. Diazonium-based impedimetric aptasensor for the rapid label-free detection of Salmonella typhimurium in food sample. Biosens. Bioelectron. 2016, 80, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Mutreja, R.; Jariyal, M.; Pathania, P.; Sharma, A.; Sahoo, D.K.; Raman Suri, C. Novel surface antigen based impedimetric immunosensor for detection of Salmonella typhimurium in water and juice samples. Biosens. Bioelectron. 2016, 85, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Peng, Y.; Bai, J.; Zhang, X.; Liu, Y.; Fan, X.; Ning, B.; Gao, Z. Rapid detection of Listeria monocytogenes in milk by self-assembled electrochemical immunosensor. Sens. Actuators B 2014, 190, 900–906. [Google Scholar] [CrossRef]
- Hu, X.; Dou, W.; Zhao, G. Electrochemical immunosensor for Enterobacter sakazakii detection based on electrochemically reduced graphene oxide–gold nanoparticle/ionic liquid modified electrode. J. Electroanal. Chem. 2015, 756, 43–48. [Google Scholar] [CrossRef]
- Wu, J.; Campuzano, S.; Halford, C.; Haake, D.A.; Wang, J. Ternary surface monolayers for ultrasensitive (zeptomole) amperometric detection of nucleic acid hybridization without signal amplification. Anal. Chem. 2010, 82, 8830–8837. [Google Scholar] [CrossRef] [PubMed]
- Campanhã, M.T.N.; Hoshino-Shimizu, S.; Baquerizo Martinez, M. Urinary tract infection: Detection of Escherichia coli antigens in human urine with an ELIEDA immunoenzymatic assay. Rev. Panam. Salud Publica/Pan Am. J. Public Health 1999, 6, 89–94. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Li, Y. Electrochemical biosensors for rapid detection of Escherichia coli O157:H7. Talanta 2017, 162, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Liébana, S.; Brandao, D.; Alegret, S.; Pividori, M.I. Electrochemical immunosensors, genosensors and phagosensors for Salmonella detection. Anal. Meth. 2014, 22, 8858–8873. [Google Scholar] [CrossRef]
- Melo, A.M.A.; Alexandre, D.L.; Furtado, R.F.; Borges, M.F.; Figueiredo, E.A.T.; Biswas, A.; Cheng, H.N.; Alves, C.R. Electrochemical immunosensors for Salmonella detection in food. Appl. Microbiol. Biotechnol. 2016, 100, 5301–5312. [Google Scholar] [CrossRef] [PubMed]
- Zelada-Guillén, G.A.; Bhosale, S.V.; Riu, J.; Rius, F.X. Real-Time potentiometric detection of bacteria in complex samples. Anal. Chem. 2010, 82, 9254–9260. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—A comprehensive review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Du, D.; Lin, Y. Recent progress on nanomaterial-based biosensors for veterinary drug residues in animal-derived food. TrAC Trends Anal. Chem. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Chen, D.; Yao, D.; Xie, C.; Liu, D. Development of an aptasensor for electrochemical detection of tetracycline. Food Control 2014, 42, 109–115. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No. 37/2010. Off. J. Eur. Union L 2009. Available online: http://data.europa.eu/eli/reg/2010/37(1)/oj (accessed on 19 February 2017).
- Que, X.; Chen, X.; Fu, L.; Lai, W.; Zhuang, J.; Chen, G.; Tang, D. Platinum-catalyzed hydrogen evolution reaction for sensitive electrochemical immunoassay of tetracycline residues. J. Electroanal. Chem. 2013, 704, 111–117. [Google Scholar] [CrossRef]
- Conzuelo, F.; Gamella, M.; Campuzano, S.; Reviejo, A.J.; Pingarrón, J.M. Disposable amperometric magneto-immunosensor for direct detection of tetracyclines antibiotics residues in milk. Anal. Chim. Acta 2012, 737, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Guo, Y.; Sun, X.; Wang, X. Electrochemical aptasensor based on Prussian blue-chitosan-glutaraldehyde for the sensitive determination of tetracycline. Nano-Micro Lett. 2014, 6, 143–152. [Google Scholar] [CrossRef]
- Zhou, L.; Li, D.J.; Gai, L.; Wang, J.P.; Li, Y.B. Electrochemical aptasensor for the detection of tetracycline with multi-walled carbon nanotubes amplification. Sens. Actuators B 2012, 162, 201–208. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Khani, H. Determination of tetracycline at a UV-irradiated DNA film modified glassy carbon electrode. Electroanalysis 2013, 25, 461–467. [Google Scholar] [CrossRef]
- Conzuelo, F.; Gamella, M.; Campuzano, S.; Pinacho, D.G.; Reviejo, A.J.; Marco, M.P.; Pingarrón, J.M. Disposable and integrated amperometric immunosensor for direct determination of sulfonamide antibiotics in milk. Biosens. Bioelectron. 2012, 36, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Conzuelo, F.; Campuzano, S.; Gamella, M.; Pinacho, D.G.; Reviejo, A.J.; Marco, M.P.; Pingarrón, J.M. Integrated disposable electrochemical immunosensors for the simultaneous determination of sulfonamide and tetracycline antibiotics residues in milk. Biosens. Bioelectron. 2013, 50, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Granja, R.H.; Niño, A.M.; Zucchetti, R.A.; Niño, R.E.; Patel, R.; Salerno, A.G. Determination of streptomycin residues in honey by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2009, 637, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Abnous, K.; Taghdisi, S.M. A novel electrochemical aptasensor based on arch-shape structure of aptamer-complimentary strand conjugate and exonuclease I for sensitive detection of streptomycin. Biosens. Bioelectron. 2016, 75, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Liu, Y.; Lin, M.; Kang, J.; Sun, Y.; Lei, H. A dual amplified electrochemical immunosensor for ofloxacin: Polypyrrole film-Au nanocluster as the matrix and multi-enzyme-antibody functionalized gold nanorod as the label. Electrochim. Acta 2013, 90, 246–253. [Google Scholar] [CrossRef]
- Todd, P.; Faulds, D. Ofloxacin a reappraisal of its antimicrobial activity, pharmacology and therapeutic use. Drugs 1991, 42, 825–876. [Google Scholar] [CrossRef] [PubMed]
- Megoulas, N.C.; Koupparis, M.A. Direct determination of kanamycin in raw materials, veterinary formulation and culture media using a novel liquid chromatography-evaporative light scattering method. Anal. Chim. Acta 2005, 547, 64–72. [Google Scholar] [CrossRef]
- EMEA/MRL/886/03—FINAL. 2003. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014538.pdf (accessed on 29 January 2017).
- Wei, Q.; Zhao, Y.; Du, B.; Wu, D.; Li, H.; Yang, M. Ultrasensitive detection of kanamycin in animal derived foods by label-free electrochemical immunosensor. Food Chem. 2012, 134, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chandra, P.; Song, K.M.; Ban, C.; Shim, Y.B. Label-free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite. Biosens. Bioelectron. 2012, 36, 29–34. [Google Scholar] [CrossRef]
- Yan, L.; Luo, C.; Cheng, W.; Mao, W.; Zhang, D.; Ding, S. A simple and sensitive electrochemical aptasensor for determination of chloramphenicol in honey based on target-induced strand release. J. Electroanal. Chem. 2012, 687, 89–94. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, H.; Asghar, W.; Inci, F.; Yuksekkaya, M.; Jahangir, M.; Zhang, M.H.; Durmus, N.G.; Gurkan, U.A.; Kuritzkes, D.R.; Demirci, U. Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Sci. Rep. 2015, 5, 8719. [Google Scholar] [CrossRef] [PubMed]
- Busa, L.S.A.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in Microfluidic Paper-Based Analytical Devices for Food and Water Analysis. Micromachines 2016, 7, 86. [Google Scholar] [CrossRef]
- Desmet, C.; Marquette, C.A.; Blum, L.J.; Doumèche, B. Paper electrodes for bioelectrochemistry: Biosensors and biofuel cell. Biosens. Bioelectron. 2016, 76, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.M.; Monteiro, T.; Almeida, M.G. Biosensing with paper-based miniaturized printed electrodes—A modern trend. Biosensors 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]



| Electrode | Type of Biosensor/Format | Analyte/Sample | Electrochemical Technique | L.R. | LOD | Reference |
|---|---|---|---|---|---|---|
| MWCNTs/ODT/AuE | Aptasensor/covalent immobilization of aptamer; direct detection of target-induced conformational change with methylene blue as indicator | saxitoxin/mussels | DPV | 0.9–30 nM | 0.38 nM | [28] |
| Cys-PDIC/AuE | Aptasensor/ competitive indirect with BTX-2 immobilized and BTX-10 aptamer mixed with BTX-2 analyte | BTX-2/shellfish extracts | EIS | 0.1–100 ng·mL−1 | 106 pg·mL−1 | [33] |
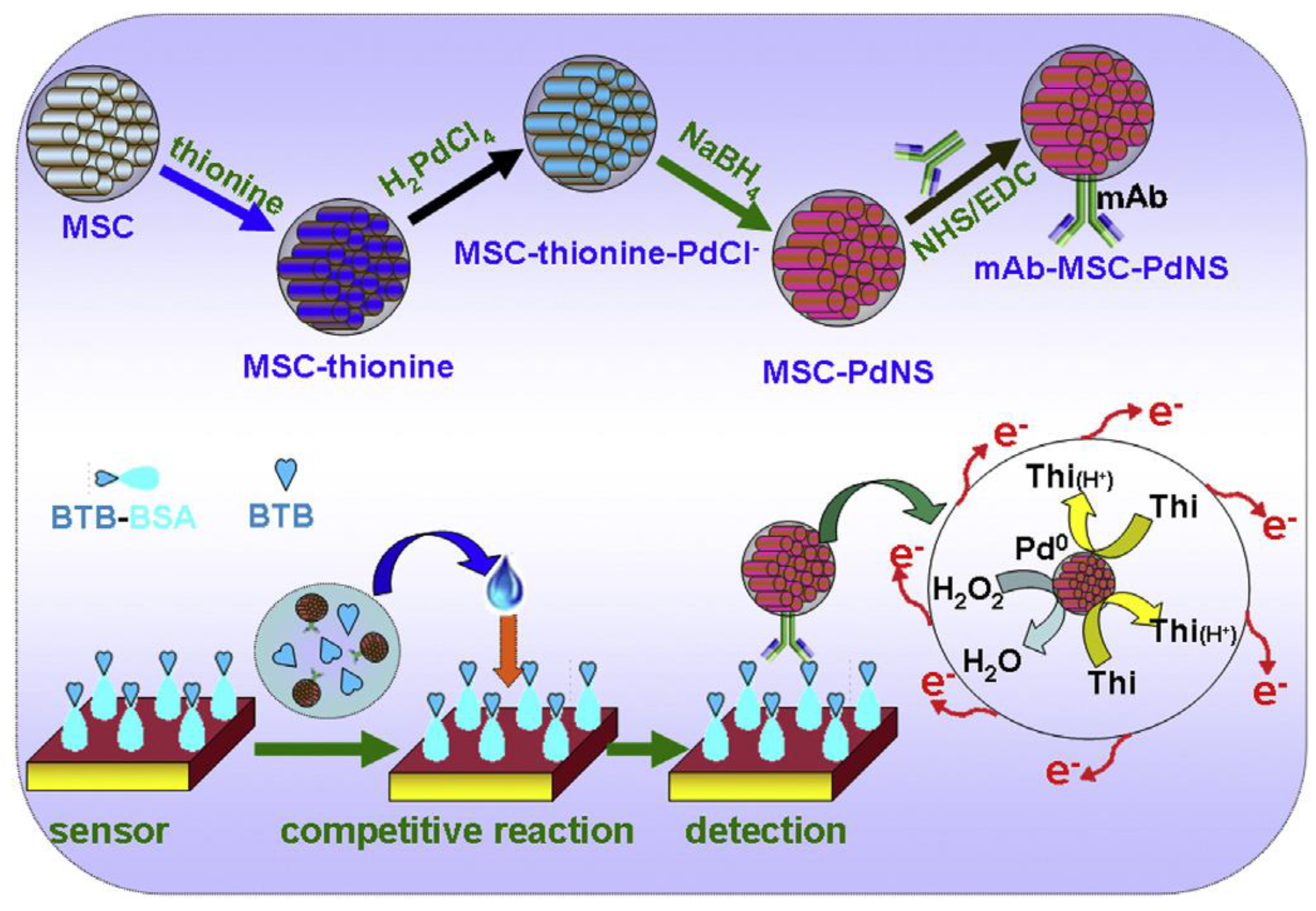
| AuNPs/GCE | Immunosensor/competitive indirect/immobilized BSA-BTB; anti-BTB/MSC/THI/PdNS as signal tag | BTB/asian mussel | DPV | 0.01–10 ng·mL−1 | 5.0 pg·mL−1 | [31] |
| SPCE | Immunosensor/competitive indirect/automated flow; OA-MBs/SPCE and anti-OA mixed with OA analyte; addition of AP-anti-OA/1-NPP | okadaic acid/mussels | FI-amperometry | 0.19–25 µg·L−1 | 0.15 µg·L−1 | [34] |
| AuE | Immunosensor/ direct with anti-OA-MBs and [Fe(CN)6]3−/4− as the redox probe | okadaic acid/mussel | DPV | 10–100 ng·mL−1 | 0.5 µg·L−1 | [36] |
| graphene/SPCE | Immunosensor/covalent immobilization of anti-OA; competitive assay between OA and OA-OVA; [Fe(CN)6]3−/4− as the redox probe | okadaic acid/spiked shellfish tissue, mussel | SWV | up to 5000 ng·L−1 | 19 ng·L−1 | [37] |
| ITO | Multiplex immunosensor/ anti-BTX-2 and anti-DTX-1 co-immob. on MB; competitive assay CdNC/BSA/BTX-2 and CuNC/BSA/DTX-1 | BTX-2 and DTX-1/seafood | SWASV | 0.005–5.0 ng·mL−1 | 1.8 pg·mL−1 (BTX-2) 2.2 pg·mL−1 (DTX-1) | [38] |
| graphene/Chit/GCE | Immunosensor/competitive between immobilized MC-LR and MC-LR conjugated to anti-MCLR- HRP-CNS-Ab2 | MC-LR/water | DPV | 0.05–15 µg·L−1 | 0.016 µg·L−1 | [45] |
| CNT@Co/ITO | Immunosensor/ competitive indirect/ immobilized MC-LR and signal amplification with Ab2-HRP/Fe3O4@PDA-AuNPs | MC-LR/water | CV | 0.05–50 µg·L−1 | 0.004 µg·L−1 | [46] |
| MWCNTs/Si | Immunosensor/ competitive indirect/ immobilized MC-LR and MC-LR conjugated to anti-MCLR | MC-LR/water | EIS | 0.05–20 µg·L−1 | 0.04 µg·L−1 | [48] |
| graphene/SPCE | Aptasensor/immobilization of aptamer; direct detection with [Fe(CN)6]3−/4− as the redox probe | MC-LR/fish extracts, water | SWV | 1 nM–10 nM | 1.9 pM | [49] |
| Chit/AuNPs/AuE | Immunosensor/ immobilization of anti-AFB; direct detection with [Fe(CN)6]3−/4− as the redox probe | AFB1/wheat | CV | 0.2–2 ng·mL−12–30 ng·mL−1 | 0.12 ng·mL−1 | [57] |
| AuNPs/DPB/GO/AuE | Immunosensor/ anti-AFB immobilization, addition of IL and Chit | AFB1/peanut, rice, soybean, milk, flour | EIS | 3.2 fM–0.32 pM | 1 fM | [58] |
| SWCNTs/Chit/GCE | Immunosensor/competitive indirect between free AFB1 and AFB1-BSA. Addition of AP-IgG and 1-NPP substrate | AFB1/corn powder | DPV | 0.01–100 ng·mL−1 | 3.5 pg·mL−1 | [59] |
| MWCNTs/IL | Immunosensor/ Anti-AFB1 immobilization. Direct detection with [Fe(CN)6]3−/4− as the redox probe | AFB1/oil | EIS | 0.1–10 ng·mL−1 | 0.03 ng·mL−1 | [60] |
| GO-modified electrode | Aptameric sensor/direct competitive between OTA and OTA labeled with a nanoceria (nCe) tag | OTA/Spiked corn samples | CV (H2O2 oxidation) | 0.15–180 nM | 0.1 nM | [62] |
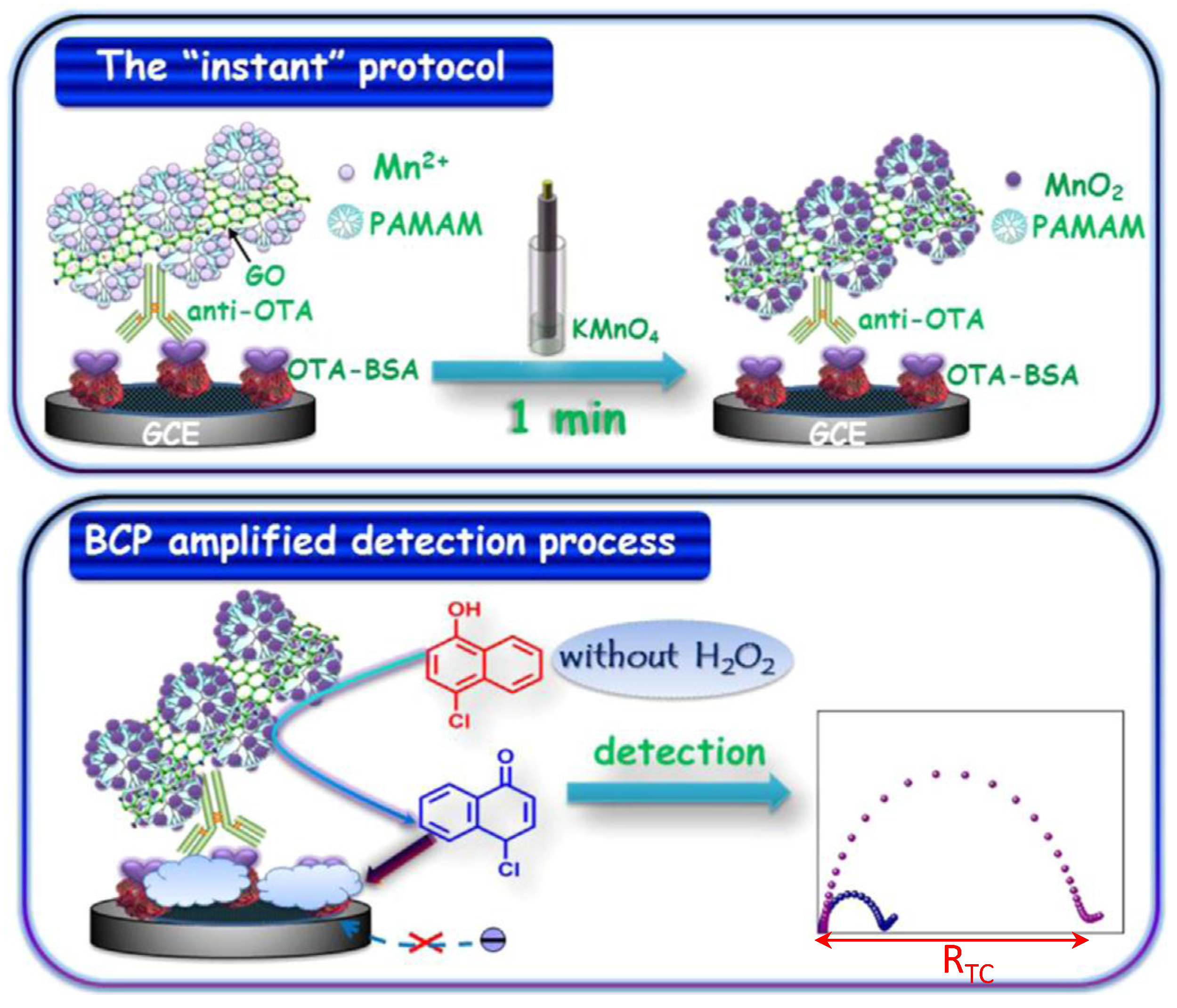
| GCE | Immunosensor/competitive. OTA-BSA immobilized; anti-OTA/GO/PAMAM/Mn2+ as signal label. MnO2 generation and 4-CN precipitation | OTA/wine | EIS | 0.1 pg·mL−1–30 ng·mL−1 | 0.55 pg·mL−1 | [63] |
| SPCEs | Immunosensor/immobilization of anti-OTA onto protein G-MBs and competitive assay between OTA and HRP-OTA | OTA/coffee | amperometry | 1.3–153.8 µg·mL−1 | 0.32 µg·mL−1 | [61] |
| Protein A/G-MBA-AuE | Immunosensor/immobilization of anti-OTA. Direct detection with [Fe(CN)6]3−/4− as the redox probe | OTA/cocoa beans | EIS | 0.01–5 ng·mL−1 | 0.01 ng·mL−1 | [64] |
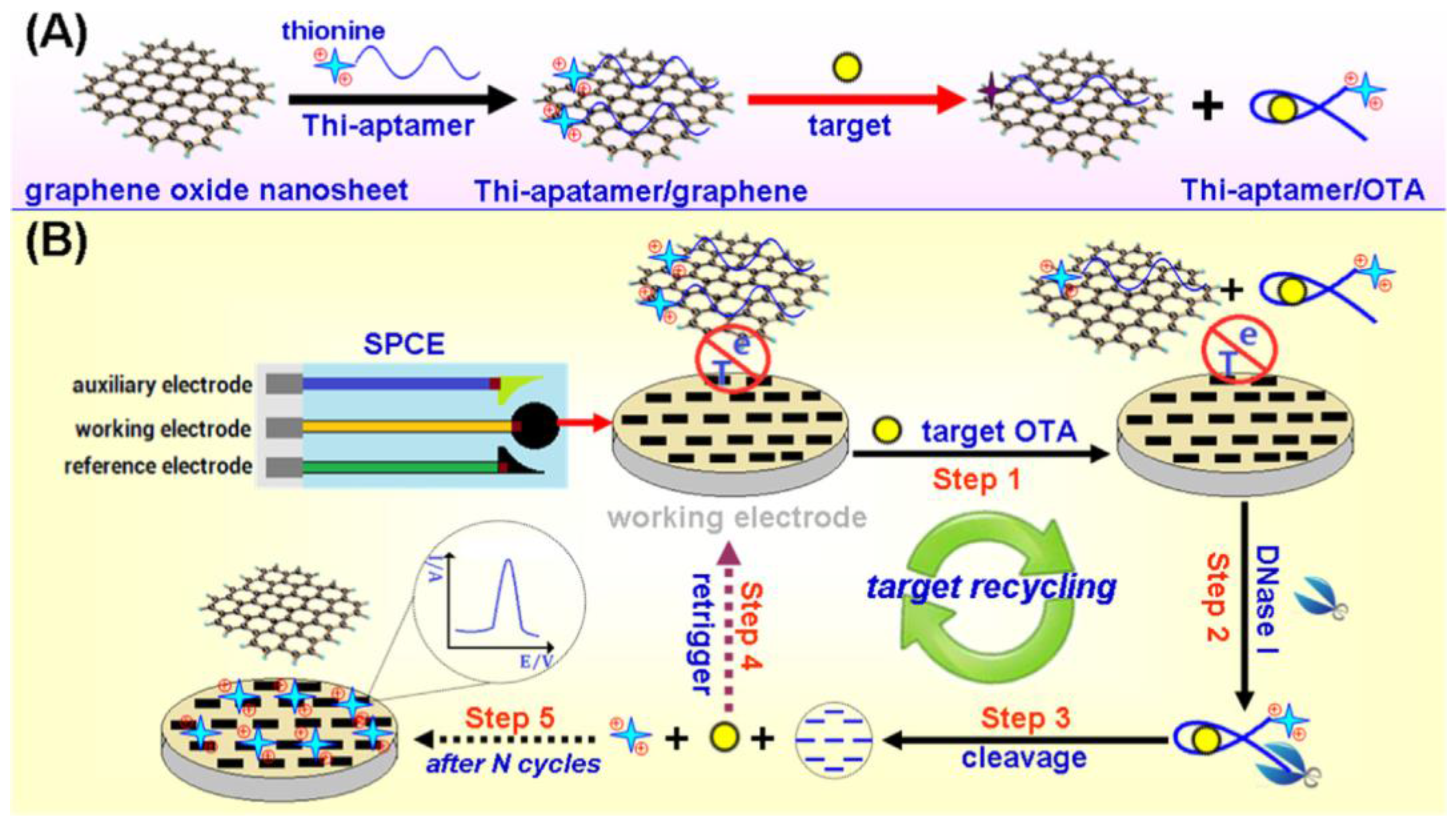
| SPCE | Aptasensor/dissociation of THI-OTA aptamer-GO in the presence of OTA; cleavage by DNaseI of THI-aptamer OTA; free THI detection | OTA/wheat | DPV | 0.01–50 ng·mL−1 | 5.6 pg·mL−1 | [65] |
| SPCE | Immunosensor/sandwich type using antibody conjugated with HRP-nanosilica-doped MWCNTs (HRPSiCNTs) as advanced labels | SEB/watermelon juice, soymilk, apple juice, and pork food | DPV | 0.05–15 ng·mL−1 | 10 pg·mL−1 | [68] |
| ProteinA/Cyst/AuE | Immunosensor/sandwich type immunoassay by immobilization of capture antibody and conjugation with HRP-anti-SEA | SEA/cheese | Amperometry | 16–150 µg·mL−1 | 33.9 ng·mL−1 | [69] |
| [D(n-C4)Im][PF6]/(PANI/Au)-AuE | Immunosensor/direct immunoassay by immobilization of SEB immuno-magnetosomes | SEB/milk | EIS | 0.05 to 5 ng·mL−1 | 0.017 ng·mL−1 | [70] |
| Electrode | Type of Biosensor/Format | Analyte/Sample | Electrochemical Technique | L.R. | LOD | Reference |
|---|---|---|---|---|---|---|
| PtNPs/SiO2 SPCE | Immunosensor/competitive indirect; BSA-CM immobilization and capture of unbound anti-CM-HRP | chlorpyrifos-methyl (CM) /grapes | DPV | 0.4–20 ng·mL−1 | 22.6 ng·L−1 | [74] |
| Protein A/AuNPs /PDDA/IDAM | Immunosensor/microfluidic chip. Immobilization of capture antibody. Direct detection with [Fe(CN)6]3−/4− as the redox probe | chlorpyrifos/ cucumber, lettuce | EIS | 0.5–500 ng·mL−1 | 0.5 ng·mL−1 | [75] |
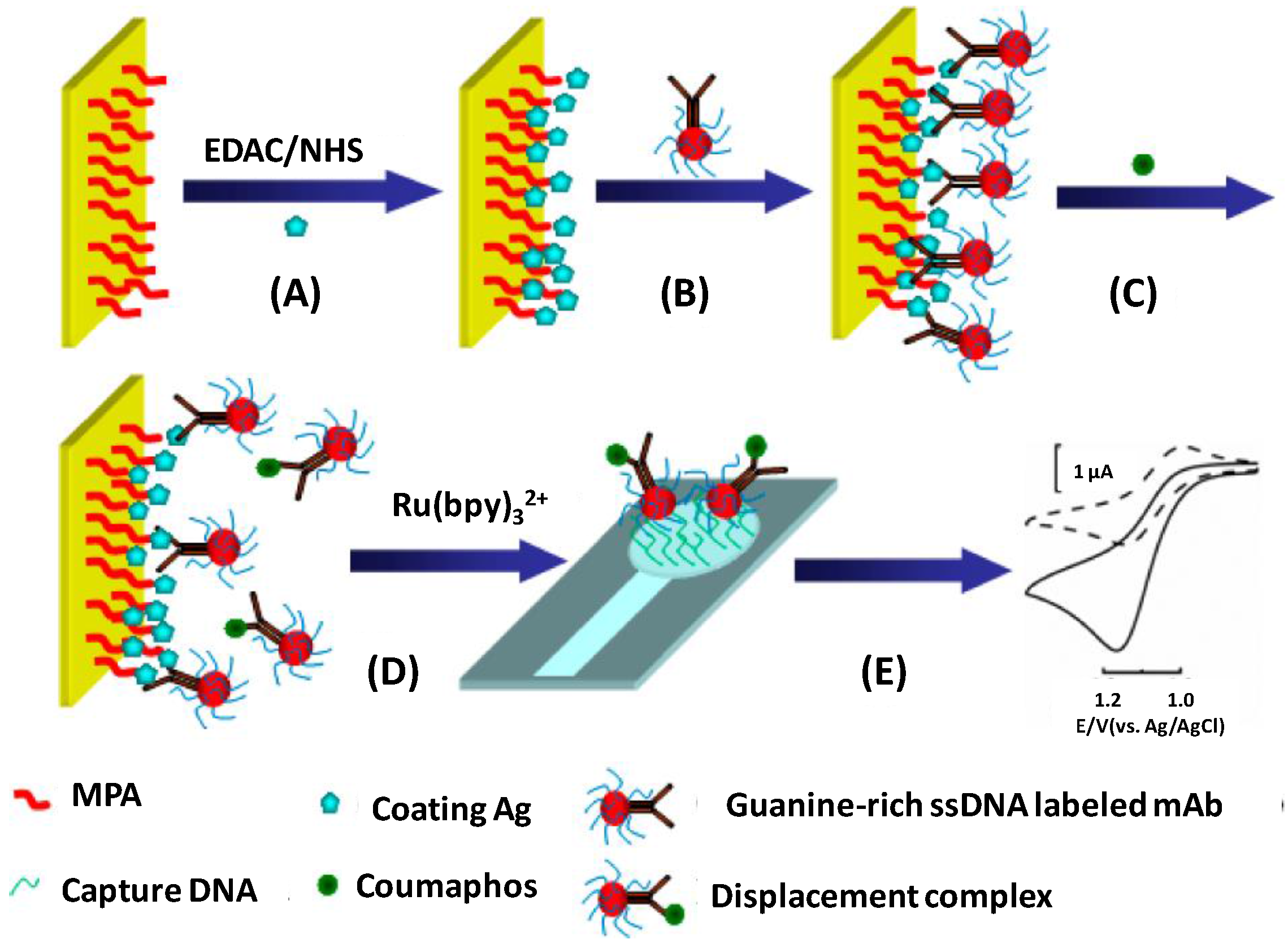
| MPA/ITO chip | Immunosensor/electrochemical displacement immunoassay and oligonucleotide sensing; antigen immobilization + ssDNA-Ab + antigen + displacement + Ru(bpy)32+ and hybridization | coumaphos/milk | amperometry | 0.5 to 80 ng·L−1 | <0.18 ng·L−1 | [76] |
| FDMA- or PQQ-SWCNTs/GCE | Immunosensor/immobilization of the respective antigens and antibodies. Direct detection | endosulfan/water paraoxon/water | SWV | 0.05–100 ng·mL−1 2–2500 ng·mL−1 | 0.05 ng·mL−1 2 ng·mL−1 | [77] |
| (DpAu/ DMDPSE)n/AuE | Immunosensor/anti-carbofuran immobilization and direct detection with [Fe(CN)6]3−/4− as the redox probe | carbofuran/lettuce, strawberries, tomatoes | DPV | 0.1–106 ng·mL−1 | 0.06 ng·mL−1 | [78] |
| FDMA-SWCNTs/GCE | Immunosensor/ immobilization of endosulfan and antibody. Direct detection | endosulfan/water | SWV | 0.01–20 ng·mL−1 | 0.01 ng·mL−1 | [79] |
| AuNPs/AuE | Aptasensor/ immobilization of aptamer and direct detection with [Fe(CN)6]3−/4− as the redox probe | acetamiprid/tomatoes | EIS | 5–600 nM | 1 nM | [80] |
| AuNPs/MWCNT/rGONR | Aptasensor/ immobilization of aptamer and direct detection with [Fe(CN)6]3−/4− as the redox probe | acetamiprid/water | EIS | 5 × 10−10–10−5 M | 7 × 10−14 M | [81] |
| AuNPs/AuE | Immunosensor/immobilization of antibody and direct detection with [Fe(CN)6]3−/4− as the redox probe | atrazine/maize | DPV | 0.05–0.5 ng·mL−1 | 0.016 ng·mL−1 | [82] |
| GA/Chit/GCE | Immunosensor/immobilization of antibody and direct detection with [Fe(CN)6]3−/4− as the redox probe | fenvalerate/tea | EIS | 1.0–104 µg·L−1 | 0.80 µg·L−1 | [83] |
| GEC | Immunosensor/immobilization of CdS-labeled-anti-PQ and PQ-MBs. Cd dissolution and detection of Cd2+ by current reduction | paraquat (PQ)/potatoes | Culombimetry | 3.08–67.76 µg·kg −1 | 1.4 µg·kg −1 | [84] |
| Electrode | Type of Biosensor/Format | Analyte/Sample | Electrochemical Technique | L.R. | LOD | Reference |
|---|---|---|---|---|---|---|
| GCE functionalized with (AuNPs) and (P-L-Arg/MWCNT) composite | Immunosensor/direct detection with [Fe(CN)6]3−/4− as the redox probe | Casein/Cheese | DPV | 1 × 10−7–1 × 10−5 g·mL−1 | 5 × 10−8 g·mL−1 | [93] |
| m-GEC | Tosyl-MBs-based immunosensor/indirect competitive with H2O2 in the presence of HQ | gliadin or small gliadin fragments/spiked gluten-free foodstuffs (skimmed milk and beer) | Amperometry | 12.5–329.3 ng·mL−1 | 12.5–329.3 ng·mL−1 | [94] |
| Aryl diazonium salt organic film electrographted on graphene modified-SPCE | Immunosensor/direct detection with [Fe(CN)6]3−/4− as the redox probe | β-lactoglobulin/several milk-containing food products (cake, cheese, snacks and biscuits) | DPV | 1 pg·mL−1–100 ng·mL−1 | 0.85 pg·mL−1 | [95] |
| Aryl diazonium salt organic film electrographted on graphene modified-SPCE | Immunosensor/direct detection with [Fe(CN)6]3−/4− as the redox probe | OVA/spiked cake extracts | DPV | 1pg·mL−1–0.5 μg·mL−1 | 0.83 pg·mL−1 | [96] |
| Pt-SPE | Immunosensor/sandwich configuration onto HOOC-MBs; addition of H2O2 with THI as the redox mediatorruiz | Ovalbumin/° | LSV | 11–222 nM | 5 nM | [98] |
| SPCEs | Immunosensor/sandwich configuration onto HOOC-MBs; addition of H2O2 with HQ as the redox mediator | β-lactoglobulin/Milk samples | Amperometry | 2.8–100 ng·mL−1 | 0.8 ng·mL−1 | [97] |
| SPCEs | Immunosensor/sandwich configuration onto HOOC-MBs; addition of H2O2 with HQ as the redox mediator | Ara h 1/Food extracts and saliva samples | Amperometry | 20.8–1000.0 ng·mL−1 | 6.3 ng·mL−1 | [99] |
| AuNPs-SPCEs | Immunosensor/sandwich; addition of 3-IP and Ag+; Ag° stripping | Ara h 1/food matrices | ASV | 12.6–2000 ng·mL−1 | 3.8 ng·mL−1 | [100] |
| AuNPs-SPCEs | Immunosensor/sandwich; addition of 3-IP and Ag+; Ag° stripping | Ara h 6/food matrices | ASV | 1–100 ng·mL−1 | 0.27 ng·mL−1 | [101] |
| 8 carbon working electrodes screen printed on alumina | Immunosensor/indirect competitive; addition of H2O2 with HQ as the redox mediator | β-casein | Amperometry | 0–10 ppm | — | [102] |
| SPdCEs | Immunosensors/sandwich configurations onto HOOC-MBs; addition of H2O2 with HQ as the redox mediator | Ara h 1 and Ara h 2/food extracts | Amperometry | 60–1000 ng·mL−1 (Ara h 1); 0.25–5 (Ara h 2) | 18.0 ng·mL−1 (Ara h 1); 0.07 ng·mL−1 (Ara h 2) | [103] |
| SPCEs | Immunosensor/sandwich configuration onto HOOC-MBs; addition of H2O2 with HQ as the redox mediator | α-lactalbumin/milk samples and infant formulations | Amperometry | 37.0–5000 pg·mL−1 | 11.0 pg·mL−1 | [104] |
| Gold electrode | DNA/direct, dually-labeled stem-loop probe (thiol and biotin tags) | Ara h 1/peanut-milk beverage | EIS | 10−15–10−10 M | 0.35 fM | [105] |
| multilayer graphene–gold nanocomposite prepared on a GCE | DNA/direct dually-labeled stem-loop probe (thiol and biotin tags); Direct detection with [Fe(CN)6]3−/4− as the redox probe | Ara h 1/peanut-milk beverage | DPV | 10−16 to 10−13 M | 0.041 fM | [106] |
| Au-SPCEs | DNA sensor/sandwich hybridization; addition of 1-NPP in the presence of AP-Strept | Ara h 2/— | DPV | 5 × 10−11–5 × 10−8 M | 10 pM | [107] |
| Au-SPCEs | DNA sensor/sandwich hybridization; addition of anti-FITC and TMB/H2O2 | α2-gliadin/commercial food products (gluten-containing and gluten-free) | Chronoamperometry | — | 0.001% (w/w) of wheat flour in rice flour | [108] |
| SPCE | Aptameric sensor/competitive assay between Biotin-peptide immobilized on Strep-MBs and free biotin-aptamer for gliadin | Gliadin/heated and hydrolyzed foods | Chronoamperometry | — | 0.5 ppm | [109] |
| Gold disk electrode | Aptameric sensor/direct assay using athiolated aptamer | Ara h 1 | EIE | — | — | [111] |
| SPCE | Aptameric sensor/direct assay by aptamer immobilization on a SPCE by covalent binding via diazonium salt chemistry | Lysozyme/spiked wine samples | EIE | 0.025 to 0.8 μM | 25 nM | [112] |
| SPCE | Aptameric sensor/sandwich assay by aptamer immobilization on a SPCE by covalent binding via diazonium salt chemistry | Lysozyme | DPV | 5 fM to 5 nM | 4.3 fM | [113] |
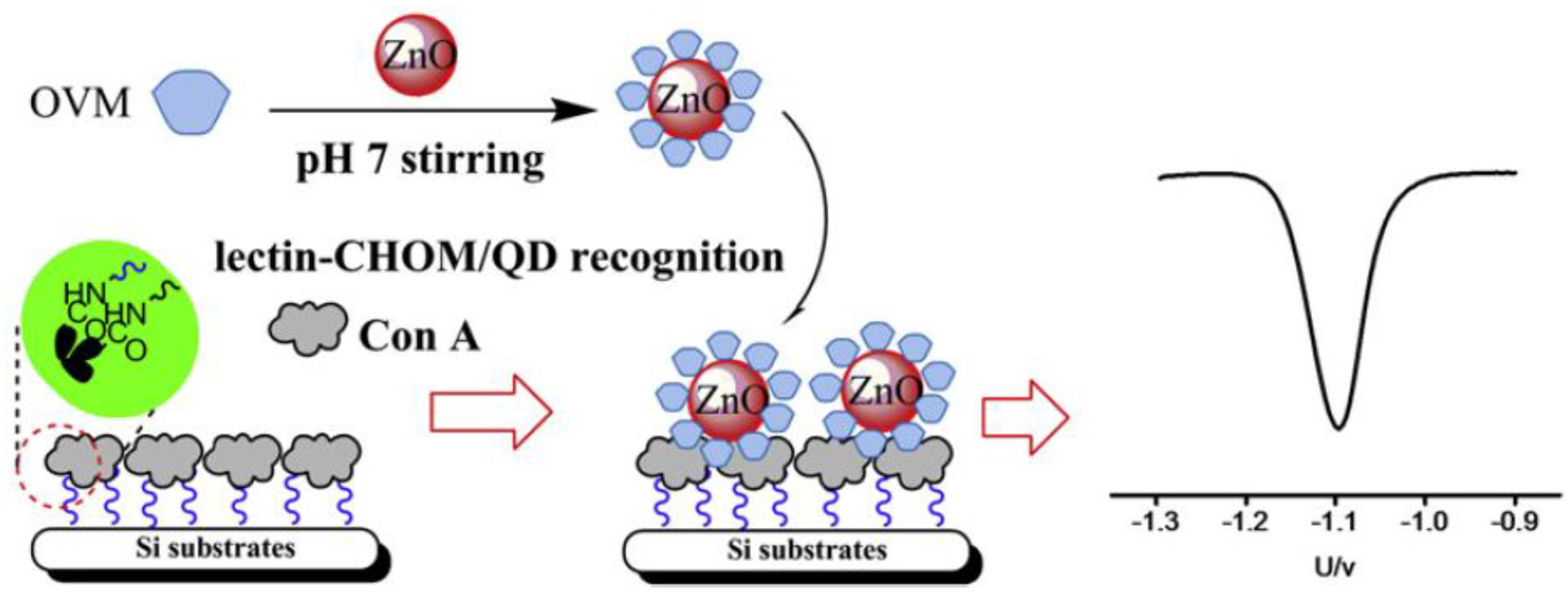
| GCE | Lectins-based sensors/direct assay using ZnO QDs/CHOM bio-conjugatesand Con A-modified Si substrates | CHOM/— | SWV | 1–140 ng·mL−1 | 0.1 ng·mL−1 | [115] |
| GCE | Peptide-based sensors/direct assay specific peptide probe (peptide-1) conjugated with daunomycin adsorbed on a GCE | OVA/chickens and quail eggs and spiked fetal bovine serum | DPV | 1.5 × 10−11–3.0 × 10−10 M | — | [116] |
| Electrode | Type of Biosensor/Format | Analyte/Sample | Electrochemical Technique | L.R. | LOD | Reference |
|---|---|---|---|---|---|---|
| AuE | Aptasensor/aptamer immob; dissociation by E. coli; hybridization with biotin-detection probe, tagging with Strept-AP and 1-NPP | E. coli O111/milk | DPV | 2 × 102–2 × 106 CFU·mL−1 | 112 CFU·mL−1 | [118] |
| (Chit-MWCNTs-SiO2@THI)n/AuNPs/AUT/AuE | Immunosensor/antibody immobilization. Direct detection of electrochemical response from THI | E. coli O157:H7/milk, water | CV | 4.12 × 102–4.12 × 105 CFU·mL−1 | 250 CFU·mL−1 | [119] |
| SPCEs | Immunosensor/sandwich-type immunoassay with Ab1-MBs and AuNPs-Ab2 | E. coli O157:H7/meat, water | amperometry | 103–104 CFU·mL−1 | 148 CFU·mL−1 | [120] |
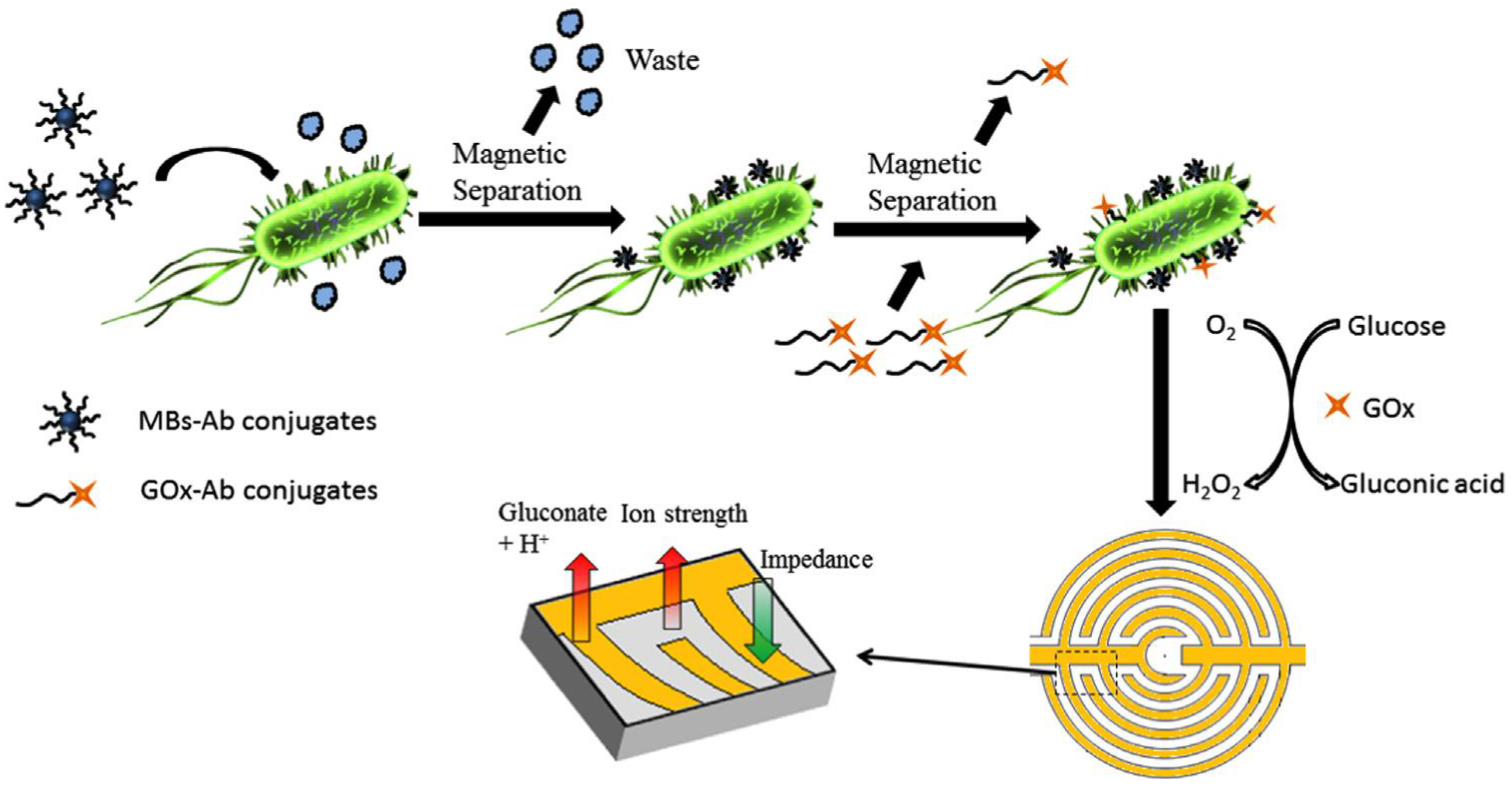
| SPIDMEs | Immunosensor/sandwich; immobilization of antibodies onto Strept-MBs and conjugation with GOs-Ab2. Addition of glucose triggers the enzymatic reaction | E. coli O157:H7/chicken rinse water; S. typhimurium/ground beef | EIS | 102–106 CFU·mL−1 | 2.41 × 103CFU·mL−1 1.04 × 103CFU·mL−1 | [121] |
| AuNPs/PAMAM/MWCNTs/Chit/GCE | Immunosensor/immobilization of capture antibody and direct detection with [Fe(CN)6]3−/4− as the redox probe | S. typhimurium/milk | EIS | 1.0 × 103–1.0 × 107 CFU·mL−1 | 5 × 102 CFU·mL−1 | [122] |
| SPCEs | Aptasensor/immobilization of aminated aptamer bydiazonium-grafting; direct detection with [Fe(CN)6]3−/4− as the redox probe | S. typhimurium/ apple juice | EIS | 10–108 CFU·mL−1 | 6 CFU·mL−1 | [123] |
| graphene/GO/SPCEs | Immunosensor/Immobilization of capture antibody and direct detection with [Fe(CN)6]4− as the redox probe | S. typhimurium (OmpD) water, apple juice | EIS | 10–105 CFU·mL−1 | 10 CFU·mL−1 | [124] |
| MPA/AuE | Immunosensor/sandwich type immunoassay by immobilization of capture antibody and conjugation with HRP-anti-LM | Listeria monocytogenes (LM)/milk | amperometry | 102–106 CFU·mL−1 | - | [125] |
| AuNPs/[BMIM]PF6/ERGO/SPCEs | Immunosensor/immobilization of HRP-anti-E. sakazakii; direct detection with THIas the redox probe | Enterobacter sakazakii | CV | 103–109 CFU·mL−1 | 1.19 × 102 CFU·mL−1 | [126] |
| Electrode | Type of Biosensor/Format | Analyte/Sample | Electrochemical Technique | L.R. | LOD | Reference |
|---|---|---|---|---|---|---|
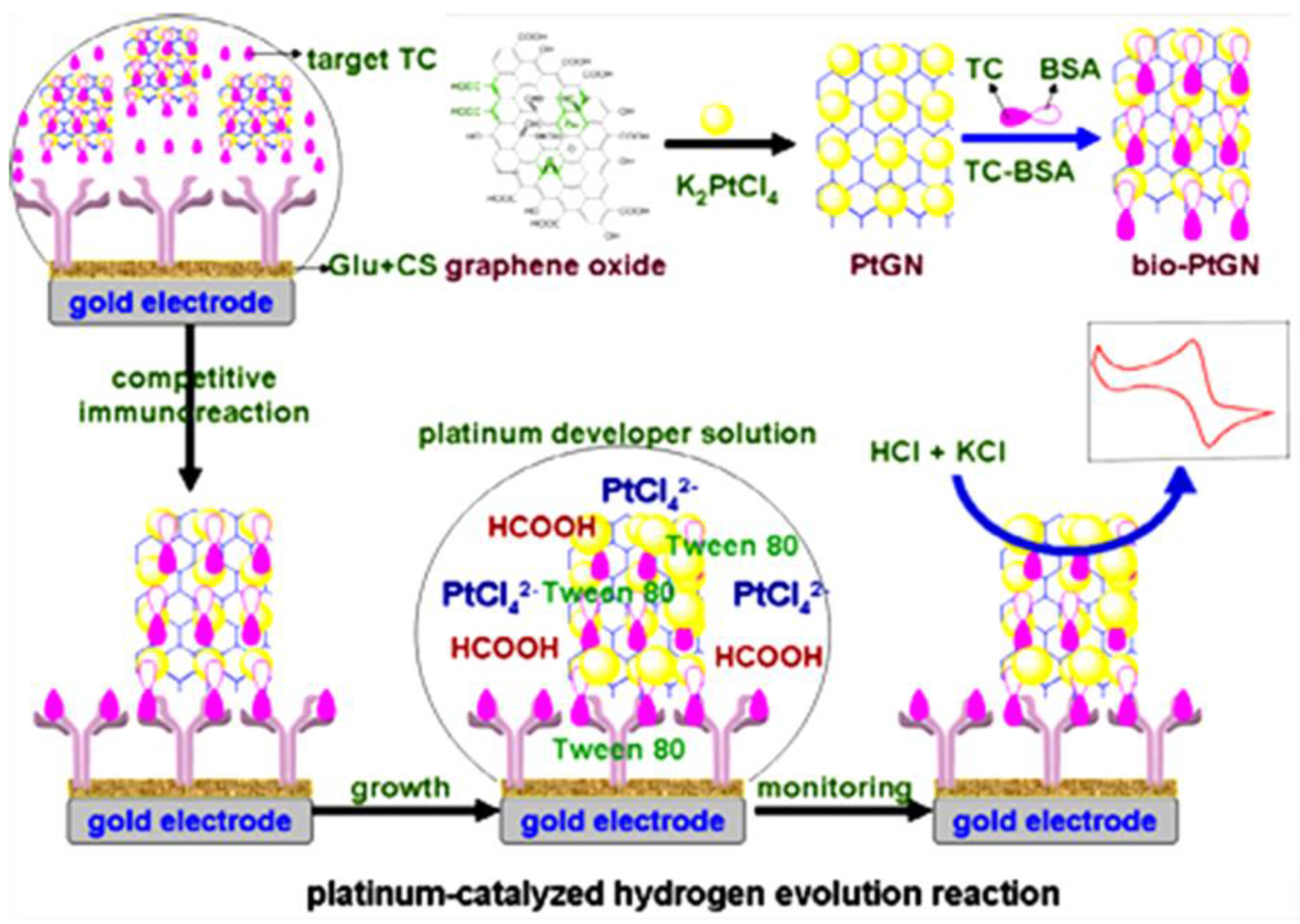
| Chit/GA/AuE | Immunosensor/competitive; immobilization of anti-TC. Signal amplification based on HER with PtNPs/graphene-TC-BSA | tetracycline (TC)/ honey, milk, peanut | LSV | 0.05–100 ng·mL−1 | 6 pg·mL−1 | [137] |
| SPCE | Immunosensor/competitive; immobilization of anti-TC onto protein G-MBs and use of HRP-TC; addition of H2O2 in the presence of HQ | oxytetracycliine/milk | amperometry | 12.5–676.2 ng·mL−1 | 3.9 ng·mL−1 | [138] |
| AuNPs/GA/Chit/PB/GCE | Aptasensor/immobilization of TC-aptamer; direct detection with PB as the redox probe | TCs/milk | DPV | 10−9–10−5 M | 3.2 × 10−10 M | [139] |
| MWCNTs/GCE | Aptasensor/immobilization of aptamer; direct detection with [Fe(CN)6]3−/4− as the redox probe | tetracycline/milk | DPV | 10−8–5 × 10−5 M | 5 × 10−9 M | [140] |
| AuE | Aptasensor/immobilization of an amino-modified aptamer; direct detection | tetracycline/milk | EIS | 5.0–5.0 × 103 ng·mL−1 | 1.0 ng·mL−1 | [135] |
| GCE | Interaction of TC with UV-ct-dsDNA-GCE; direct electrochemical detection of TC | TC/milk | DPV | 0.30–90.00 µM | 0.27 µM | [141] |
| SPCE | Immunosensor/competitive; covalent immobilization of capture antibody and use of Ag-HRP conjugate; addition of H2O2 in the presence of HQ | sulfonamides/milk | amperometry | 0.6–64.2 ng·mL−1 | 0.15 ng·mL−1 | [142] |
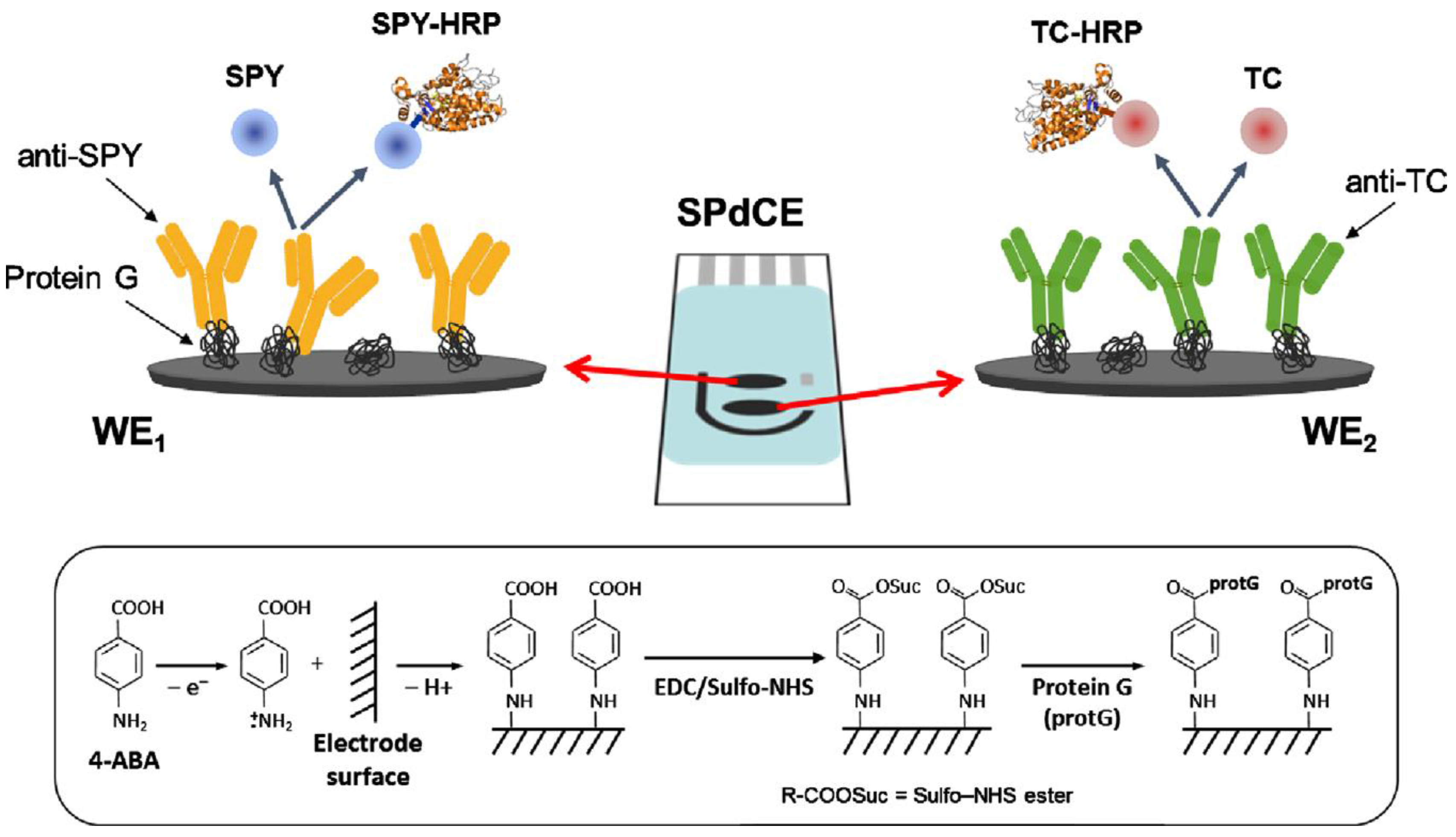
| ProteinG-dual SPCE | Immunosensor; competitive; immobilization of capture antibodies and use of Ag-HRP conjugates; addition of H2O2 in the presence of HQ | sulfonamides tetracyclines | amperometry | 1.92–454 nM; 6.40–385 nM | 0.39 ± 0.01 nM 1.9 ± 0.01 nM | [143] |
| AuE | Aptasensor/immobilization of Apt-CS conjugate; addition of Exo I and streptomycin. Direct detection with [Fe(CN)6]3−/4− as redox probe | streptomycin/milk | DPV | 30 –1500 nM | 11.4 nM | [145] |
| pPy/AuNC/GCE | Immunosensor/immobilization of OFL-OVA conjugate; competitive immunoassay with gold nanorod-HRP and HRP-Ab2using H2O2 in the presence of HQ | ofloxacin (OFL) | CV | 0.08–410 ng·mL−1 | 0.03 ng·mL−1 | [146] |
| PtNPs/THI/Nf/GS/GCE | Immunosensor/immobilization of anti-kanamycin; direct detection with THI as the redox probe | kanamycin/chicken liver | amperometry | 0.01–12.0 ng·mL−1 | 5.74 pg·mL−1 | [150] |
| AuNPs/poly-DBP/SPE | Aptasensor/immobilization of aptamer; direct detection of kanamycin | kanamycin/milk | LSV | 0.05–9.0 µM | 9.4 ± 0.4 nM | [151] |
| PEDOT-TsO chip | Microfluidic aptasensor/ immobilization of aptamers; direct detection | kanamycin ampicillin/milk | EIS | 10 nM–1 µM 100 pM–1 µM | − | [14] |
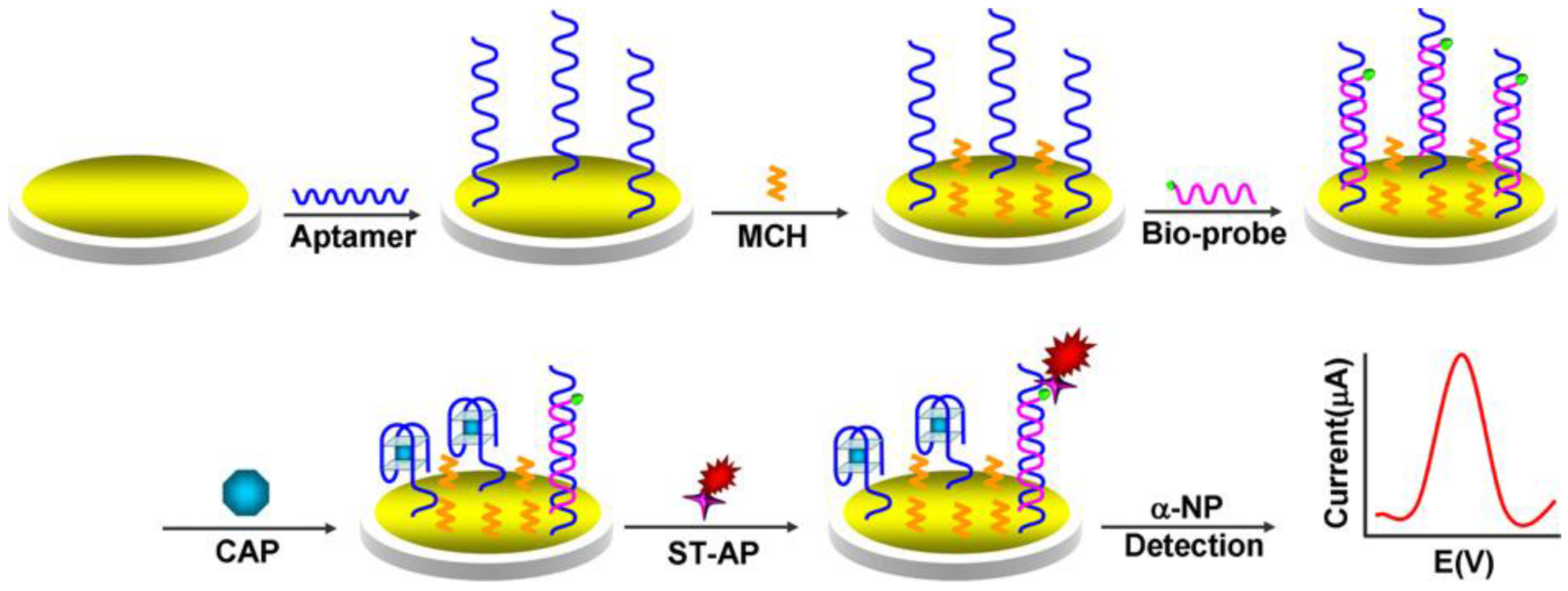
| AuE | Aptasensor/aptamer immobilization; formation of aptamer/DNA duplex; TISR and binding to Strept-AP; addition of αNP | chloramphenicol/honey | SWV | 1–1000 nM | 0.29 nM | [152] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campuzano, S.; Yáez-Sedeño, P.; Pingarrón, J.M. Electrochemical Affinity Biosensors in Food Safety. Chemosensors 2017, 5, 8. https://doi.org/10.3390/chemosensors5010008
Campuzano S, Yáez-Sedeño P, Pingarrón JM. Electrochemical Affinity Biosensors in Food Safety. Chemosensors. 2017; 5(1):8. https://doi.org/10.3390/chemosensors5010008
Chicago/Turabian StyleCampuzano, Susana, Paloma Yáez-Sedeño, and José Manuel Pingarrón. 2017. "Electrochemical Affinity Biosensors in Food Safety" Chemosensors 5, no. 1: 8. https://doi.org/10.3390/chemosensors5010008






