Nitric Oxide Sensors for Biological Applications
Abstract
:1. Introduction
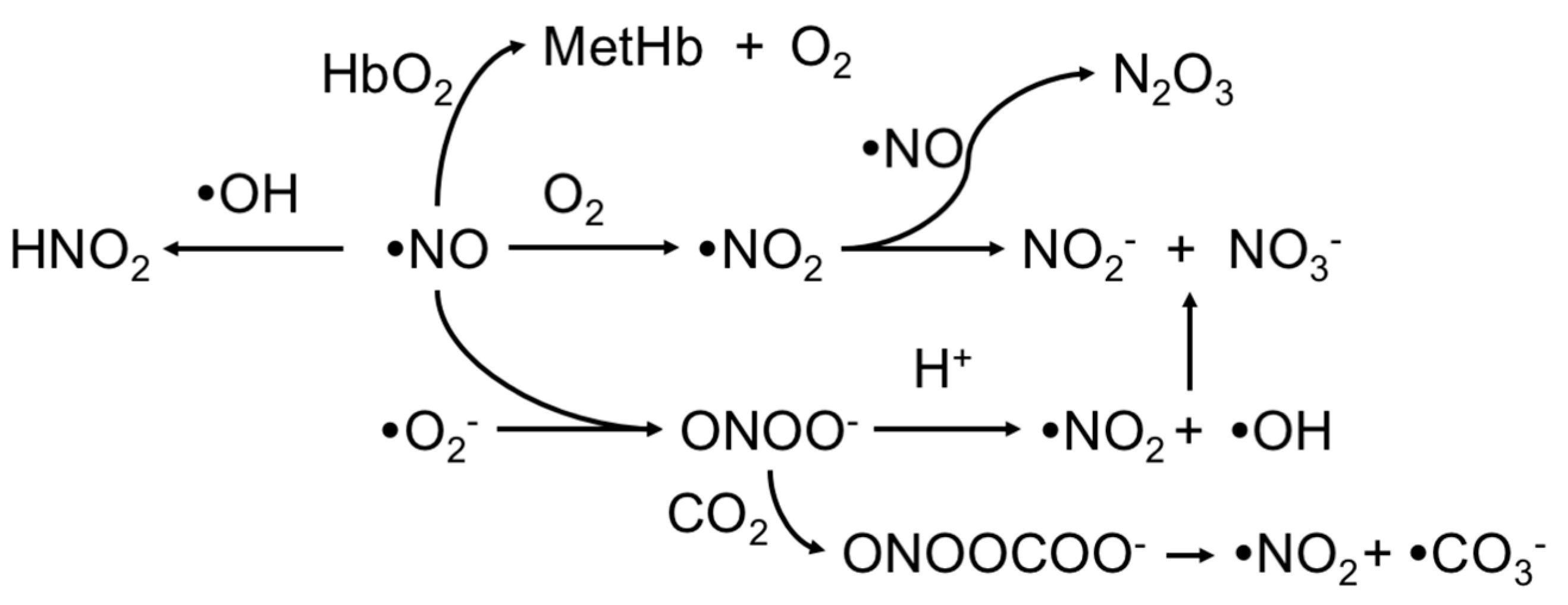
2. Upstream and Downstream Measurements
3. Electrochemical Detection
4. Chemiluminescent Probes
5. Fluorescence Probes
6. Genetic Biosensors
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yetik-Anacak, G.; Catravas, J.D. Nitric oxide and the endothelium: History and impact on cardiovascular disease. Vasc. Pharmacol. 2006, 45, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J.; Welch, G. Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 1995, 38, 87–104. [Google Scholar] [CrossRef]
- Lanas, A. Role of nitric oxide in the gastrointestinal tract. Arthritis Res. Ther. 2008, 10, S4. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Mount, P.F.; Power, D.A. Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiol. 2006, 187, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shimoyama, N.; Mizuguchi, T. Nitric oxide synthase inhibitor blocks spinal sensitization induced by formalin injection into the rat paw. Anesth. Analg. 1993, 77, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Farr, S.A.; Sell, R.L.; Hileman, S.M.; Banks, W.A. Nitric oxide is a central component in neuropeptide regulation of appetite. Peptides 2011, 32, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Jantos, H. Effects of L-arginine and SIN-1 on sleep and waking in the rat during both phases of the light-dark cycle. Life Sci. 2004, 75, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.C.R.; Marubayashi, U.; Coimbra, C.C. Nitric oxide pathway is an important modulator of heat loss in rats during exercise. Brain Res. Bull. 2005, 67, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bon, C.L.M.; Garthwaite, J. On the Role of Nitric Oxide in Hippocampal Long-Term Potentiation. J. Neurosci. 2003, 23, 1941–1948. [Google Scholar] [PubMed]
- Dinerman, J.L.; Dawson, T.M.; Schell, M.J.; Snowman, A.; Snyder, S.H. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: Implications for synaptic plasticity. Proc. Natl. Acad. Sci. USA 1994, 91, 4214–4218. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.E. Nitric oxide and homeostatic control: An intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog. Biophys. Mol. Biol. 2004, 84, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Contestabile, A.; Ciani, E. Role of nitric oxide in the regulation of neuronal proliferation, survival and differentiation. Neurochem. Int. 2004, 45, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Alvania, R.S.; Lonze, B.E.; Ramanan, N.; Kim, T.; Huang, Y.; Dawson, T.M.; Snyder, S.H.; Ginty, D.D. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell 2006, 21, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-R.; Zhu, Y.; Halushka, P.V.; Lincoln, T.M.; Mendelsohn, M.E. Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 4888–4893. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.C.; Hassid, A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Investig. 1989, 83, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Mehta, P.; Mehta, J.L. Oxidized LDL decreases L-arginine uptake and nitric oxide synthase protein expression in human platelets: Relevance of the effect of oxidized LDL on platelet function. Circulation 1996, 93, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.F.; Keates, A.C.; Hanson, P.J.; Whittle, B.J. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 1993, 265, G418–G422. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Tigley, A.W. Review article: New insights into prostaglandins and mucosal defence. Aliment. Pharmacol. Ther. 1995, 9, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 1991, 88, 4651–4655. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Redeen, S.; Grenegard, M.; Ericson, A.C.; Sjostrand, S.E. Nitric oxide inhibits gastric acid secretion by increasing intraparietal cell levels of cGMP in isolated human gastric glands. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y.; Han, M.K.; Choi, K.S.; Park, I.H.; Park, S.Y.; Sohn, M.H.; Kim, U.H.; McGregor, J.R.; Samlowski, W.E.; Yim, C.Y. Cytokines secreted by lymphokine-activated killer cells induce endogenous nitric oxide synthesis and apoptosis in DLD-1 colon cancer cells. Cell. Immunol. 2000, 203, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. The Function of Nitric Oxide in the Immune System in Nitric Oxide; Springer: Heidelberg, Germany, 2000. [Google Scholar]
- Xu, W.; Liu, L.; Smith, G.C.M.; Charles, l.G. Nitric oxide upregulates expression of DNA-PKcs to protect cells from DNA-damaging anti-tumour agents. Nat. Cell Biol. 2000, 2, 339. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Ganopolsky, J.G.; Labbe, A.; Wahl, C.; Prakash, S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010, 88, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Majid, D.S.A.; Navar, L.G. Nitric oxide in the control of renal hemodynamics and excretory function. Am. J. Hypertens. 2001, 14, 74S–82S. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Nitric Oxide: Biology and Pathobiology; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric Oxide in Health and Disease of the Respiratory System. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. The Multiplex Function of Nitric Oxide in (Auto)immunity. J. Exp. Med. 1998, 187, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Huang, S.; Dong, Z.; Juang, S.H.; Gutman, M.; Xie, Q.W.; Nathan, C.; Fidler, I.J. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J. Exp. Med. 1995, 181, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Massi, D.; Franchi, A.; Sardi, I.; Magnelli, L.; Paglierani, M.; Borgognoni, L.; Maria Reali, U.; Santucci, M. Inducible nitric oxide synthase expression in benign and malignant cutaneous melanocytic lesions. J. Pathol. 2001, 194, 194–200. [Google Scholar] [CrossRef]
- Liu, L.; Stamler, J.S. NO: An inhibitor of cell death. Cell Death Differ. 1999, 6, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Brune, B.; von Knethen, A.; Sandau, K.B. Nitric oxide (NO): An effector of apoptosis. Cell Death Differ. 1999, 6, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Frostell, C.; Fratacci, M.D.; Wain, J.C.; Jones, R.; Zapol, W.M. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991, 83, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Rothrock, A.R.; Schoenfisch, M.H. Nitric oxide-releasing sol-gels as antibacterial coatings for orthopedic implants. Biomaterials 2005, 26, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Colletta, A.; Koley, D.; Wu, J.; Xi, C.; Major, T.C.; Bartlett, R.H.; Meyerhoff, M.E. Thromboresistant/anti-biofilm catheters via electrochemically modulated nitric oxide release. Bioelectrochemistry 2015, 104, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.R.; Flitney, F.W.; Williams, D.L.H. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: A chemist’s perspective. Trends Pharmacol. Sci. 1995, 16, 18–22. [Google Scholar] [CrossRef]
- Gaston, B. Nitric oxide and thiol groups. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 323–333. [Google Scholar] [CrossRef]
- Grube, R.; Kelm, M.; Motz, W.; Strauer, B. The biology of nitric oxide. Enzymol. Biochem. Immunol. 1994, 4, 201–204. [Google Scholar]
- Liu, X.; Miller, M.J.; Joshi, M.S.; Thomas, D.D.; Lancaster, J.R. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA 1998, 95, 2175–2179. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Borland, C. Endothelium in control. Br. Heart J. 1991, 66, 405. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.; Feelisch, M.; Grube, R.; Motz, W.; Strauer, B.E. The Biology of Nitric Oxide, Physiological and Clinical Aspects; Portland Press: London, UK, 1992. [Google Scholar]
- Stamler, J.S.; Jaraki, O.; Osborne, J.; Simon, D.I.; Keaney, J.; Vita, J.; Singel, D.; Valeri, C.R.; Loscalzo, J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. USA 1992, 89, 7674–7677. [Google Scholar] [CrossRef] [PubMed]
- Malinski, T.; Taha, Z.; Grunfeld, S.; Patton, S.; Kapturczak, M.; Tomboulian, P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem. Biophys. Res. Commun. 1993, 193, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M.; Yoshida, K. Metabolic Fate of Nitric Oxide and Related N-Oxides; John Wiley and Sons: Chichester, UK, 1996. [Google Scholar]
- Kelm, M. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta 1999, 1411, 273–289. [Google Scholar] [CrossRef]
- Liu, X.; Miller, M.J.; Joshi, M.S.; Sadowska-Krowicka, H.; Clark, D.A.; Lancaster, J.R., Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 1998, 273, 18709–18713. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. The importance of radiation chemistry to radiation and free radical biology (The 2008 Silvanus Thompson Memorial Lecture). Br. J. Radiol. 2009, 82, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt Ueber einige Azoverbindungen. Ber. Deutsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Promega. Griess Reagent System, Instructions for Use of Product G2930; Promega: Madison, WI, USA, 2009. [Google Scholar]
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512. [Google Scholar] [CrossRef] [PubMed]
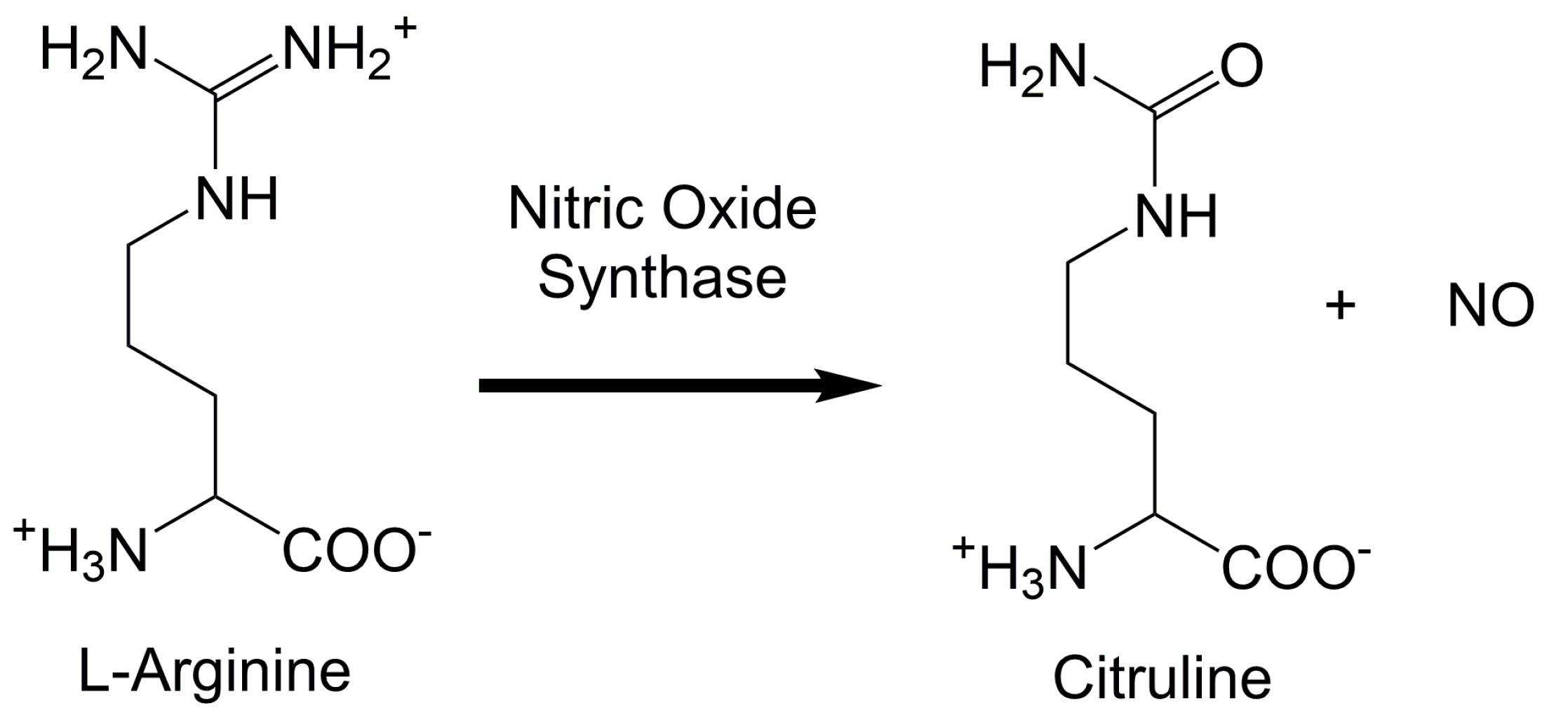
- Knowles, R.G.; Palacios, M.; Palmer, R.M.; Moncada, S. Formation of nitric oxide from L-arginine in the central nervous system: A transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 1989, 86, 5159–5162. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, J.S.; Martasek, P.; McMillan, K.; Salerno, J.; Liu, Q.; Gross, S.S.; Masters, B.S. Modular structure of neuronal nitric oxide synthase: Localization of the arginine binding site and modulation by pterin. Biochem. Biophys. Res. Commun. 1995, 210, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Mellouk, S.; Hoffman, S.L.; Meltzer, M.S.; Nacy, C.A. Cellular mechanisms of nonspecific immunity to intracellular infection: Cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol. Lett. 1990, 25, 15–19. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. The anti-aggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987, 92, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Sessa, W.C.; Harris, H.J.; Anggard, E.E.; Vane, J.R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: Cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. USA 1990, 87, 8612–8616. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Scharfstein, J.S.; Keaney, J.F.; Slivka, A.; Welch, G.N.; Vita, J.A.; Stamler, J.S.; Loscalzo, J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J. Clin. Investig. 1994, 94, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Kleinbongard, P.; Preik, M.; Dejam, A.; Gharini, P.; Lauer, T.; Erckenbrecht, J.; Duschin, A.; Schulz, R.; Heusch, G.; et al. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO: Experimental and clinical Study on the fate of NO in human blood. Circ. Res. 2002, 91, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Cha, W.; Meyerhoff, M.E. S-Nitrosothiol Detection via Amperometric Nitric Oxide Sensor with Surface Modified Hydrogel Layer Containing Immobilized Organoselenium Catalyst. Langmuir 2006, 22, 10830–10836. [Google Scholar] [CrossRef] [PubMed]
- Malinski, T.; Taha, Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature 1992, 358, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Bhagat, K.; MacAllister, R.; Patton, S.; Malinski, T.; Radomski, M.; Moncada, S. Direct measurement of nitric oxide in human beings. Lancet 1995, 346, 153–154. [Google Scholar] [CrossRef]
- Innovative Instruments, Inc. All-Plastic Leak-Free Reference Electrode: Handles over 5 M Hydroxide and Hydrofluoric Acid. Available online: http://www.2in.com/index.html (accessed on 27 November 2017).
- Dunham, A.J.; Barkley, R.M.; Sievers, R.E. Aqueous nitrite ion determination by selective reduction and gas phase nitric oxide chemiluminescence. Anal. Chem. 1995, 67, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Archer, S.L. The measurement of NO in biological systems using chemiluminescence. Meth. Mol. Biol. 1998, 100, 111–127. [Google Scholar]
- Woldman, Y.Y.; Eubank, T.D.; Mock, A.J.; Stevens, N.C.; Varadharaj, S.; Turco, J.; Gavrilin, M.A.; Branchini, B.R.; Khramtsov, V.V. Detection of nitric oxide production in cell cultures by luciferin–luciferase chemiluminescence. Biochem. Biophys. Res. Commun. 2015, 465, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Woldman, Y.Y.; Sun, J.; Zweier, J.L.; Khramtsov, V.V. Direct chemiluminescence detection of nitric oxide in aqueous solutions using the natural nitric oxide target soluble guanylyl cyclase. Free Radic. Biol. Med. 2009, 47, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Ulissi, Z.W.; Sen, F.; Gong, X.; Sen, S.; Iverson, N.; Boghossian, A.A.; Godoy, L.C.; Wogan, G.N.; Mukhopadhyay, D.; Strano, M.S. Spatiotemporal Intracellular Nitric Oxide Signaling Captured Using Internalized, Near-Infrared Fluorescent Carbon Nanotube Nanosensors. Nano Lett. 2014, 14, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Kasim, N.; Branton, R.L.; Clarke, D.J. Neuronal nitric oxide synthase immunohistochemistry and 4,5-diaminofluorescein diacetate: Tools for nitric oxide research. J. Neurosci. Methods 2001, 112, 1–8. [Google Scholar] [CrossRef]
- Rathel, T.R.; Leikert, J.J.; Vollmar, A.M.; Dirsch, V.M. Application of 4,5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol. Proced. Online 2003, 5, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leikert, J.F.; Rathel, T.R.; Muller, C.; Vollmar, A.M.; Dirsch, V.M. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett. 2001, 506, 131–134. [Google Scholar] [CrossRef]
- Strijdom, H.; Muller, C.; Lochner, A. Direct intracellular nitric oxide detection in isolated adult cardiomyocytes: Flow cytometric analysis using the fluorescent probe, diaminofluorescein. J. Mol. Cell. Cardiol. 2004, 37, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Hirotani, M.; Nakatsubo, N.; Kikuchi, K.; Urano, Y.; Higuchi, T.; Hirata, Y.; Nagano, T. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal. Chem. 2001, 73, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
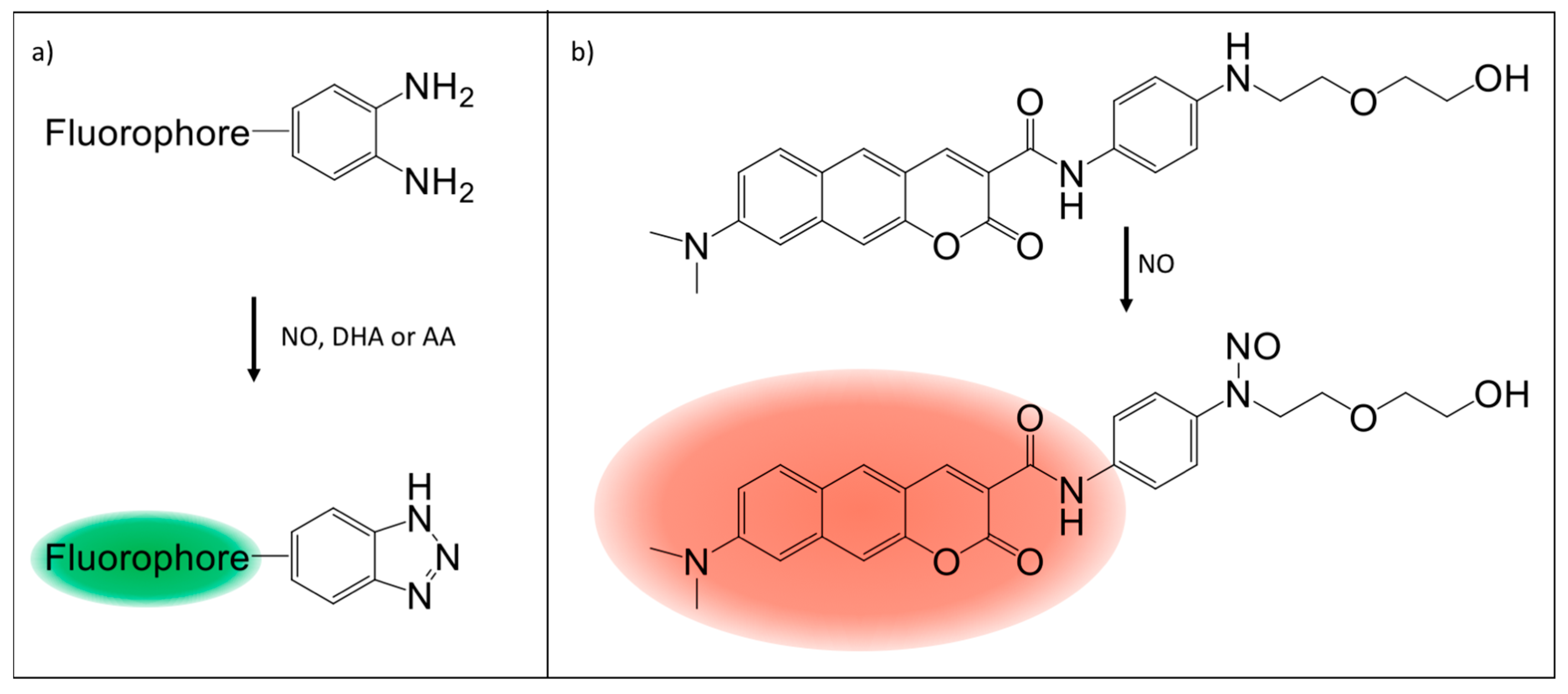
- Zhang, X.; Kim, W.S.; Hatcher, N.; Potgieter, K.; Moroz, L.L.; Gillette, R.; Sweedler, J.V. Interfering with nitric oxide measurements. 4,5-diaminofluorescein reacts with dehydroascorbic acid and ascorbic acid. J. Biol. Chem. 2002, 277, 48472–48478. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Rubakhin, S.S.; Sweedler, J.V. Simultaneous Nitric Oxide and Dehydroascorbic Acid Imaging by Combining Diaminofluoresceins and Diaminorhodamines. J. Neurosci. Methods 2008, 168, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Johannes, L.; Goud, B.; Antony, C.; Lingwood, C.A.; Daneman, R.; Grinstein, S. Noninvasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc. Natl. Acad. Sci. USA 1998, 95, 2997–3002. [Google Scholar] [CrossRef] [PubMed]
- Llopis, J.; McCaffery, J.M.; Miyawaki, A.; Farquhar, M.G.; Tsien, R.Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 6803–6808. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Seidlits, S.K.; Adams, M.M.; Lynch, V.M.; Schmidt, C.E.; Anslyn, E.V.; Shear, J.B. A Highly Selective Low-Background Fluorescent Imaging Agent for Nitric Oxide. J. Am. Chem. Soc. 2010, 132, 13114–13116. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Lim, M.H.; Lippard, S.J. Dirhodium tetracarboxylate scaffolds as reversible fluorescence-based nitric oxide sensors. J. Am. Chem. Soc. 2004, 126, 4972–4978. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Tennyson, A.G.; Lim, M.H.; Lippard, S.J. Conjugated Polymer-Based Fluorescence Turn-On Sensor for Nitric Oxide. Org. Lett. 2005, 7, 3573–3575. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tian, X.; Shin, I.; Yoon, J. Fluorescent and luminescent probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2011, 40, 4783–4804. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Urano, Y.; Izumi, S.; Kojima, H.; Nagano, T. A practical strategy to create near-infrared luminescent probes: Conversion from fluorescein-based sensors. Chem. Commun. 2012, 48, 2840–2842. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Jiang, H.; Li, Z.; Zhong, C.; Zhang, W.; Liu, Z. An N-nitrosation reactivity-based two-photon fluorescent probe for the specific in situ detection of nitric oxide. Chem. Sci. 2017, 8, 4533–4538. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.R.A.; Oliveira, A.D.P.R.; Firmino, T.V.C.; Tenório, D.P.L.A.; Pereira, G.; Carvalho, L.B.; Santos, B.S.; Correia, M.T.S.; Fontes, A. Biomedical applications of glyconanoparticles based on quantum dots. Biochim. Biophys. Acta Gen. Subj. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, M.-Y.; Huang, D. Nitric Oxide Switches on the Photoluminescence of Molecularly Engineered Quantum Dots. J. Am. Chem. Soc. 2009, 131, 11692–11694. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Heller, D.A.; Kalbacova, M.; Kim, J.-H.; Zhang, J.; Boghossian, A.A.; Maheshri, N.; Strano, M.S. Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 2010, 5, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.A.; Jin, H.; Martinez, B.M.; Patel, D.; Miller, B.M.; Yeung, T.K.; Jena, P.V.; Hobartner, C.; Ha, T.; Silverman, S.K.; et al. Multimodal optical sensing and analyte specificity using single-walled carbon nanotubes. Nat. Nanotechnol. 2009, 4, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Heller, D.A.; Kim, J.H.; Strano, M.S. Stochastic Analysis of Stepwise Fluorescence Quenching Reactions on Single-Walled Carbon Nanotubes: Single Molecule Sensors. Nano Lett. 2008, 8, 4299–4304. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, CA, USA, 1996. [Google Scholar] [CrossRef]
- Bachilo, S.M.; Strano, M.S.; Kittrell, C.; Hauge, R.H.; Smalley, R.E.; Weisman, R.B. Structure-Assigned Optical Spectra of Single-Walled Carbon Nanotubes. Science 2002, 298, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Heller, D.A.; Jin, H.; Barone, P.W.; Song, C.; Zhang, J.; Trudel, L.J.; Wogan, G.N.; Tannenbaum, S.R.; Strano, M.S. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat. Chem. 2009, 1, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Boghossian, A.A.; Barone, P.W.; Rwei, A.; Kim, J.H.; Lin, D.H.; Heller, D.A.; Hilmer, A.J.; Nair, N.; Reuel, N.F.; et al. Single Molecule Detection of Nitric Oxide Enabled by d(AT)(15) DNA Adsorbed to Near Infrared Fluorescent Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2011, 133, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Iverson, N.M.; Barone, P.W.; Shandell, M.; Trudel, L.J.; Sen, S.; Sen, F.; Ivanov, V.; Atolia, E.; Farias, E.; McNicholas, T.P.; et al. In vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 2013, 8, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.A.; Baik, S.; Eurell, T.E.; Strano, M.S. Single-walled carbon nanotube spectroscopy in live cells: Towards long-term labels and optical sensors. Adv. Mater. 2005, 17, 2793–2799. [Google Scholar] [CrossRef]
- Cherukuri, P.; Bachilo, S.M.; Litovsky, S.H.; Weisman, R.B. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 2004, 126, 15638–15639. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Nakayama-Ratchford, N.; Davis, C.R.; Kam, N.W.; Chu, P.; Liu, Z.; Sun, X.; Dai, H.; Gambhir, S.S. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008, 3, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Graff, R.A.; Swanson, J.P.; Barone, P.W.; Baik, S.; Heller, D.A.; Strano, S.M. Achieving individual-nanotube dispersion at high loading in single-walled carbon nanotube composites. Adv. Mater. 2005, 17, 980–984. [Google Scholar] [CrossRef]
- Eroglu, E.; Gottschalk, B.; Charoensin, S.; Blass, S.; Bischof, H.; Rost, R.; Madreiter-Sokolowski, C.T.; Pelzmann, B.; Bernhart, E.; Sattler, W. Development of novel FP-based probes for live-cell imaging of nitric oxide dynamics. Nat. Commun. 2016, 7, 10623. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.; Ghosh, T.; Tucker, N.; Zhang, X.; Dixon, R. Transcriptional Regulation by the Dedicated Nitric Oxide Sensor, NorR: A Route towards NO Detoxification; Portland Press Limited: London, UK, 2011. [Google Scholar]
- D’autréaux, B.; Tucker, N.P.; Dixon, R.; Spiro, S. A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature 2005, 437, 769–772. [Google Scholar] [CrossRef] [PubMed]

| Detection Method | Sensitivity | Molecule Detected | Rate of Detection | Scale | In Vivo, In Vitro, Both | Strengths | Drawbacks |
|---|---|---|---|---|---|---|---|
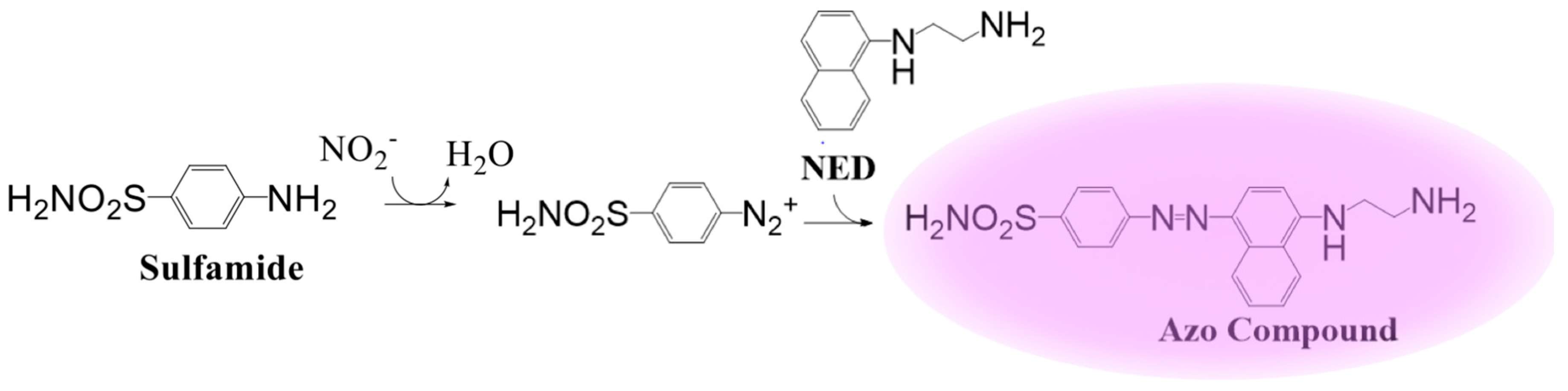
| Griess Assay [58,59,60] | 1.0 µM | NO2 | End-Point | System | In Vitro | Availability, NO2 is stable and provides an estimate of NO in the system | Does not detect NO directly, inconsistent results from system to system |
| NOS Activity Assay [61,67] | 5.0 µU | L-Citrulline | End-Point | System | In Vitro | Availability, L-citrulline is stable and estimates NO generated by NOS | Does not detect NO directly, natural L-arginine sources will interfere with readings |
| Electrochemical Probe [71,72,73] | 1.0 nM | NO | Real-Time | System | Both | Availability, Real-time detection, High sensitivity | Detects on a system level, cannot detect over long time intervals |
| Chemiluminescent Probes [76,77] | 50 pM | NO | End-Point | System | In Vitro | High sensitivity to NO | Detects on a system level, Cannot detect in vivo |
| o-diamino Aromatic Compounds [83,86,88] | 5.0 µM | NO | Real-Time | Single Cell | In Vitro | Real-time detection of NO at a cellular level | Limited aqueous solubility, false positives with DHA and AA |
| Luminescent Lanthanide Complexes [92] | ~0.5 µM | NO, N-Nitrosation | Real-Time | Single Cell | Both | Real-time detection at a cellular level | Does not detect NO directly |
| Transition-Metal Complexes [90,91] | 4.0 µM | NO | Real-Time | System | In Vitro | Sensitivity, real-time detection of NO | Limited aqueous solubility |
| Quantum Dots [95] | 3.3 µM | NO | Real-Time | Single Cell | Both | Real-time detection of NO at a cellular level | Irreversibly altered by NO |
| Carbon Nanotubes [103,104,105] | 1.0 µM | NO | Real-Time | Single Cell | Both | Real-time detection of NO at a cellular level | Turn off sensing of NO |
| Genetic Biosensors [110] | 50–94 nM | NO | Real-Time | Single Cell | In Vitro | Real-time detection of NO at a cellular level | Fluorescence emission is not detectable through tissue |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iverson, N.M.; Hofferber, E.M.; Stapleton, J.A. Nitric Oxide Sensors for Biological Applications. Chemosensors 2018, 6, 8. https://doi.org/10.3390/chemosensors6010008
Iverson NM, Hofferber EM, Stapleton JA. Nitric Oxide Sensors for Biological Applications. Chemosensors. 2018; 6(1):8. https://doi.org/10.3390/chemosensors6010008
Chicago/Turabian StyleIverson, Nicole M., Eric M. Hofferber, and Joseph A. Stapleton. 2018. "Nitric Oxide Sensors for Biological Applications" Chemosensors 6, no. 1: 8. https://doi.org/10.3390/chemosensors6010008




