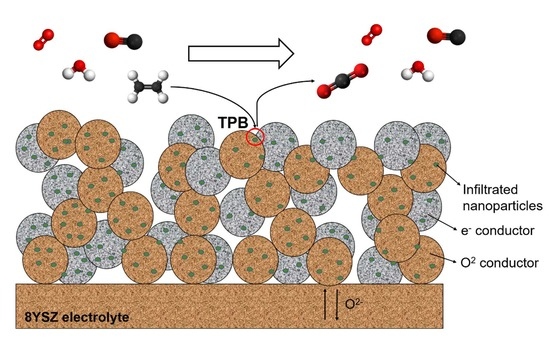
Potentiometric C2H4-Selective Detection on Solid-State Sensors Activated with Bifunctional Catalytic Nanoparticles
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Fabrication of the Sensor Device
3. Sample Characterization
4. Results and Discussion
4.1. Microstructural Characterization
4.2. Electrochemical Characterization
4.2.1. Potentiometric Characterization
4.2.2. Electrochemical Impedance Spectroscopy Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; de Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air pollution and noncommunicable diseases: A review by the Forum of International Respiratory Societies’ Environmental Committee, part 2: Air pollution and organ systems. Chest 2019, 155, 417–426. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; de Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air pollution and noncommunicable diseases. Chest 2019, 155, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Krzyzanowski, M.; Kuna-Dibbert, B.; Schneider, J. (Eds.) Health Effects of Transport-Related Air Pollution; World Health Organization (WHO): Copenhagen, Denmark, 2005. [Google Scholar]
- Horizon Europe. Work Programme 2021–2022: Climate, Energy and Mobility. Call: HORIZON-CL5-2021-D5-01. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/docs/2021-2027/horizon/wp-call/2021-2022/wp-8-climate-energy-and-mobility_horizon-2021-2022_en.pdf (accessed on 15 September 2021).
- Fu, S.; Gu, Y. Highway toll and air pollution: Evidence from Chinese cities. J. Environ. Econ. Manag. 2017, 83, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Corvec, S.S.-L.; Mercier, A.; Ovtracht, N.; Chevallier, A. Urban toll and electric vehicles: The winning ticket for Lyon Metropolitan Area (France). Res. Transp. Econ. 2019, 73, 17–33. [Google Scholar] [CrossRef]
- Croci, E. Urban road pricing: A comparative study on the experiences of London, Stockholm and Milan. Transp. Res. Procedia 2016, 14, 253–262. [Google Scholar] [CrossRef] [Green Version]
- H2020 Work Programme 2018–2020: Smart, Green and Integrated Transport. Call: H2020-MG-2018-2019-2020. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/lc-mg-1-1-2018 (accessed on 25 April 2019).
- H2020 Work Programme 2018–2020: Smart, Green and Integrated Transport. Call: H2020-MG-2018-2019-2020. Available online: https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/lc-mg-1-9-2019 (accessed on 25 April 2019).
- Yu, J.; Deng, S.; Jin, H.; Huang, M.; Wang, S.; Zhang, X. Gas phase reaction combined light-regulated electrochemical sensing technique for improved response selectivity and sensitivity to hydrocarbons. Ionics 2020, 26, 6351–6357. [Google Scholar] [CrossRef]
- Ikeda, H.; Iio, A.; Anggraini, S.A.; Miura, N. Impedancemetric YSZ-based oxygen sensor using BaFeO3 sensing-electrode. Sens. Actuators B Chem. 2016, 243, 279–282. [Google Scholar] [CrossRef]
- Macam, E.R.; White, B.M.; Blackburn, B.M.; di Bartolomeo, E.; Traversa, E.; Wachsman, E.D. La2CuO4 sensing electrode configuration influence on sensitivity and selectivity for a multifunctional potentiometric gas sensor. Sens. Actuators B Chem. 2011, 160, 957–963. [Google Scholar] [CrossRef] [Green Version]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef] [Green Version]
- Striker, T.; Ramaswamy, V.; Armstrong, E.N.; Willson, P.D.; Wachsman, E.D.; Ruud, J.A. Effect of nanocomposite Au–YSZ electrodes on potentiometric sensor response to NOx and CO. Sens. Actuators B Chem. 2013, 181, 312–318. [Google Scholar] [CrossRef]
- Ueda, T.; Abe, H.; Kamada, K.; Bishop, S.R.; Tuller, H.L.; Hyodo, T.; Shimizu, Y. Enhanced sensing response of solid-electrolyte gas sensors to toluene: Role of composite Au/metal oxide sensing electrode. Sens. Actuators B Chem. 2017, 252, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Guth, U.; Wiemhöfer, H.-D. Gas sensors based on oxygen ion conducting metal oxides. In Gas Sensors Based on Conducting Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–60. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, Y.; Yu, J.; Zhang, X.; Zhang, X.; Jin, H.; Jin, Q.; Shen, W.; Zou, J.; Deng, S.; et al. Compact yttria-stabilized zirconia based total NOx sensor with a dual functional Co3O4/NiO sensing electrode. ACS Sens. 2019, 4, 2150–2155. [Google Scholar] [CrossRef]
- Iio, A.; Ikeda, H.; Anggraini, S.A.; Miura, N. Potentiometric YSZ-Based Oxygen Sensor Using BaFeO3 Sensing-Electrode. Electrochem. Commun. 2014, 48, 134–137. [Google Scholar] [CrossRef]
- Anggraini, S.A.; Fujio, Y.; Ikeda, H.; Miura, N. YSZ-based sensor using Cr-Fe-based spinel-oxide electrodes for selective detection of CO. Anal. Chim. Acta 2017, 982, 176–184. [Google Scholar] [CrossRef]
- Zou, J.; Zheng, Y.; Li, J.; Zhan, Z.; Jian, J. Potentiometric NO2 sensors based on thin stabilized zirconia electrolytes and asymmetric (La0. 8Sr0. 2) 0.95 MnO3 electrodes. Sensors 2015, 15, 17558–17571. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, S.; Kusnezoff, M.; Michaelis, A. Studies of indium tin oxide-based sensing electrodes in potentiometric zirconia solid electrolyte gas sensors. Sensors 2021, 21, 2345. [Google Scholar] [CrossRef]
- Chevallier, L.; Traversa, E.; di Bartolomeo, E. Propene detection at high temperatures using highly sensitive non-nernstian electrochemical sensors based on Nb and Ta oxides. J. Electrochem. Soc. 2010, 157, J386–D581. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Moore, Z.; Aravamudhan, S.; Khosla, A. A new low-temperature electrochemical hydrocarbon and NOx sensor. Sensors 2017, 17, 2759. [Google Scholar] [CrossRef] [Green Version]
- Fujio, Y.; Plashnitsa, V.V.; Elumalai, P.; Miura, N. Stabilization of sensing performance for mixed-potential-type zirconia-based hydrocarbon sensor. Talanta 2011, 85, 575–581. [Google Scholar] [CrossRef]
- Jin, H.; Plashnitsa, V.V.; Breedon, M.; Miura, N. Compact YSZ-rod-based hydrocarbon sensor utilizing metal-oxide sensing-electrode and Mn-based reference-electrode combination. Electrochem. Solid-State Lett. 2011, 14, J23–J25. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Ludwig, T.; Wilhelm, M.; Graf, D.; Riheen, M.A.; Mathur, S. Potentiometric ethene sensor for postharvest detection applications. J. Electrochem. Soc. 2019, 166, B1477–B1482. [Google Scholar] [CrossRef]
- Saberi, M.; Mortazavi, Y.; Khodadadi, A. Dual selective Pt/SnO2 sensor to CO and propane in exhaust gases of gasoline engines using Pt/LaFeO3 filter. Sens. Actuators B Chem. 2015, 206, 617–623. [Google Scholar] [CrossRef]
- Plashnitsa, V.V.; Anggraini, S.A.; Miura, N. CO sensing characteristics of YSZ-based planar sensor using Rh-sensing electrode composed of tetrahedral sub-micron particles. Electrochem. Commun. 2011, 13, 444–446. [Google Scholar] [CrossRef]
- Yang, B.; Wang, C.; Xiao, R.; Yu, H.; Wang, J.; Xu, J.; Liu, H.; Xia, F.; Xiao, J. CO response characteristics of NiFe2O4 sensing material at elevated temperature. J. Electrochem. Soc. 2019, 166, B956–B960. [Google Scholar] [CrossRef]
- Yang, B.; Wang, C.; Xiao, R.; Yu, H.; Huang, C.; Wang, J.; Xu, J.; Liu, H.; Xia, F.; Xiao, J. High NH3 selectivity of NiFe2O4 sensing electrode for potentiometric sensor at elevated temperature. Anal. Chim. Acta 2019, 1089, 165–173. [Google Scholar] [CrossRef]
- Schönauer-Kamin, D.; Fleischer, M.; Moos, R. Influence of the V2O5 content of the catalyst layer of a non-Nernstian NH3 sensor. Solid State Ion. 2014, 262, 270–273. [Google Scholar] [CrossRef]
- Toldra-Reig, F.; Serra, J.M. Development of potentiometric sensors for C2H4 detection. Sensors 2018, 18, 2992. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, A.; Kumar, A.; Sim, U.; Im, H.-N.; Song, S.-J. Synergistic enhancement in the sensing performance of a mixed-potential NH3 sensor using SnO2@ CuFe2O4 sensing electrode. Sens. Actuators B Chem. 2020, 308, 127748. [Google Scholar] [CrossRef]
- Fergus, J.W. Solid electrolyte based sensors for the measurement of CO and hydrocarbon gases. Sens. Actuators B Chem. 2007, 122, 683–693. [Google Scholar] [CrossRef]
- Di Bartolomeo, E.; Grilli, M.L.; Traversa, E.; Dutta, A.; Ishihara, T.; Nishiguchi, H.; Takita, Y. Sensing mechanism of potentiometric gas sensors based on stabilized zirconia with oxide electrodes. J. Electrochem. Soc. 2004, 151, H133–H127. [Google Scholar] [CrossRef]
- Chevallier, L.; di Bartolomeo, E.; Grilli, M.L.; Traversa, E. High temperature detection of CO/HCs gases by non-Nernstian planar sensors using Nb2O5 electrode. Sens. Actuators B Chem. 2008, 130, 514–519. [Google Scholar] [CrossRef]
- McCulloch, M.T.; Langford, N.; Duxbury, G. Real-time trace-level detection of carbon dioxide and ethylene in car exhaust gases. Appl. Opt. 2005, 44, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, E.; Rasmussen, R.A.; Grosjean, D. Ambient levels of gas phase pollutants in Porto Alegre, Brazil. Atmos. Environ. 1998, 32, 3371–3379. [Google Scholar] [CrossRef]
- Poulopoulos, S.; Samaras, D.; Philippopoulos, C. Regulated and unregulated emissions from an internal combustion engine operating on ethanol-containing fuels. Atmos. Environ. 2001, 35, 4399–4406. [Google Scholar] [CrossRef]
- Storey, J.M.; Lewis, S.A., Sr.; West, B.H.; Shean, P.H.; Scott Sluder, C.; Wagner, R.M.; Domingo, N.; Thomas, J.; Kass, M. Hydrocarbon Species in the Exhaust of Diesel Engines Equipped with Advanced Emissions Control Devices; Final Report CRC Project No. AVFL-10b-2; Fuels, Engines, and Emissions Research Center, Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2005. [Google Scholar]
- Toldra-Reig, F.; Serra, J.M. Surface functionalization with Ni of Fe0.7Cr1.3O3/8YSZ electrode in a potentiometric sensor to selectively detect C2H4. ACS Appl. Nano Mater. 2018, 1, 6666–6673. [Google Scholar] [CrossRef]
- Tewari, S.; Bhattacharjee, A. Structural, electrical and optical studies on spray-deposited aluminium-doped ZnO thin films. Pramana 2011, 76, 153–163. [Google Scholar] [CrossRef]
- Gajdoš, M.; Eichler, A.; Hafner, J. CO adsorption on close-packed transition and noble metal surfaces: Trends from ab initio calculations. J. Phys. Condens. Matter 2004, 16, 1141–1164. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, J.; Bochentyn, B.; Molin, S.; Gazda, M.; Jasinski, P.; Kusz, B. Solid oxide fuel cells with Ni-infiltrated perovskite anode. Solid State Ion. 2012, 221, 11–14. [Google Scholar] [CrossRef]
- Makgwane, P.R.; Ray, S.S. Nanosized ruthenium particles decorated carbon nanofibers as active catalysts for the oxidation of p-cymene by molecular oxygen. J. Mol. Catal. A Chem. 2013, 373, 1–11. [Google Scholar] [CrossRef]
- Monteiro, N.; Noronha, F.; da Costa, L.; Linardi, M.; Fonseca, F. A direct ethanol anode for solid oxide fuel cell based on a chromite-manganite with catalytic ruthenium nanoparticles. Int. J. Hydrogen Energy 2012, 37, 9816–9829. [Google Scholar] [CrossRef]
- Niranjan, R.; Sainkar, S.; Vijayamohanan, K.; Mulla, I. Ruthenium: Tin oxide thin film as a highly selective hydrocarbon sensor. Sens. Actuators B Chem. 2002, 82, 82–88. [Google Scholar] [CrossRef]
- Pignat, K.; Vallotto, J.; Pinna, F.; Strukul, G. Cationic complexes of palladium (II) and platinum (II) as Lewis acid catalysts for the Diels−Alder reaction. Organometallics 2000, 19, 5160–5167. [Google Scholar] [CrossRef]
- Dilonardo, E.; Penza, M.; Alvisi, M.; Cassano, G.; di Franco, C.; Palmisano, F.; Torsi, L.; Cioffi, N. Sensitive detection of hydrocarbon gases using electrochemically Pd-modified ZnO chemiresistors. Beilstein J. Nanotechnol. 2017, 8, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, H.; Lan, Z.-Q.; Guo, J.; Tan, M.-Q. Carbon-monoxide adsorption and dissociation on Nb (110) surface. Appl. Surf. Sci. 2015, 328, 641–648. [Google Scholar] [CrossRef]
- Chevallier, L.; di Bartolomeo, E.; Grilli, M.L.; Mainas, M.; White, B.; Wachsman, E.D.; Traversa, E. Non-Nernstian Planar Sensors Based on YSZ with a Nb2O5 Electrode. Sens. Actuators B Chem. 2008, 129, 591–598. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Subramaniyam, K. Electrical characterization of a mixed potential propylene sensor. Sens. Actuators B Chem. 2013, 188, 367–371. [Google Scholar] [CrossRef]
- Liu, F.; Wang, B.; Yang, X.; Guan, Y.; Sun, R.; Wang, Q.; Liang, X.; Sun, P.; Lu, G. High-temperature stabilized zirconia-based sensors utilizing MNb2O6 (M: Co, Ni and Zn) sensing electrodes for detection of NO2. Sens. Actuators B Chem. 2016, 232, 523–530. [Google Scholar] [CrossRef]
- Romanytsia, I.; Viricelle, J.-P.; Vernoux, P.; Pijolat, C. Application of advanced morphology Au–X (X = YSZ, ZrO2) composites as sensing electrode for solid state mixed-potential exhaust NOx sensor. Sens. Actuators B Chem. 2015, 207, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Fergus, J.W. Perovskite oxides for semiconductor-based gas sensors. Sens. Actuators B Chem. 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Anggraini, S.A.; Fujio, Y.; Sato, T.; Breedon, M.; Miura, N. Stabilized zirconia-based sensor utilizing SnO2-based sensing electrode with an integrated Cr2O3 catalyst layer for sensitive and selective detection of hydrogen. Int. J. Hydrogen Energy 2013, 38, 305–312. [Google Scholar] [CrossRef]
- Zosel, J. Response behavior of perovskites and Au/oxide composites as HC-electrodes in different combustibles. Solid State Ion. 2004, 175, 531–533. [Google Scholar] [CrossRef]
- Park, C.O.; Fergus, J.; Miura, N.; Park, J.; Choi, A. Solid-state electrochemical gas sensors. Ionics 2009, 15, 261–284. [Google Scholar] [CrossRef]
- Miura, N.; Raisen, T.; Lu, G.; Yamazoe, N. Highly selective CO sensor using stabilized zirconia and a couple of oxide electrodes. Sens. Actuators B Chem. 1998, 47, 84–91. [Google Scholar] [CrossRef]
- Garzon, F. Solid-state mixed potential gas sensors: Theory, experiments and challenges. Solid State Ion. 2000, 136–137, 633–638. [Google Scholar] [CrossRef]
- Miura, N.; Elumalai, P.; Plashnitsa, V.V.; Ueda, T.; Wama, R.; Utiyama, M. Solid-state electrochemical gas sensing. In Solid State Gas Sensing; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 1–27. [Google Scholar]
- Wama, R.; Plashnitsa, V.V.; Elumalai, P.; Kawaguchi, T.; Fujio, Y.; Utiyama, M.; Miura, N. Improvement in propene sensing characteristics by the use of additives to In2O3 sensing electrode of mixed-potential-type zirconia sensor. J. Electrochem. Soc. 2009, 156, J102–J107. [Google Scholar] [CrossRef]
- Sato, T.; Breedon, M.; Miura, N. Reduction in ethanol interference of zirconia-based sensor for selective detection of volatile organic compounds. J. Electrochem. Soc. 2013, 160, B146–B151. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of water vapor on Pd-loaded SnO2 nanoparticles gas sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Murray, E. Dense LaSrMnO3 composite electrodes for NOx sensing. Sens. Actuators B Chem. 2018, 256, 351–358. [Google Scholar] [CrossRef]
- Lee, D.; Ahn, S.-J.; Kim, J.; Moon, J. Influence of water vapor on performance of co-planar single chamber solid oxide fuel cells. J. Power Sources 2010, 195, 6504–6509. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4844. [Google Scholar] [CrossRef]
- Baek, S.-W.; Jeong, J.; Schlegl, H.; Azad, A.; Park, D.S.; Baek, U.B.; Kim, J.H. Metal-supported SOFC with an aerosol deposited in-situ LSM and 8YSZ composite cathode. Ceram. Int. 2016, 42, 2402–2409. [Google Scholar] [CrossRef] [Green Version]
- Lay, E.; Gauthier, G.; Dessemond, L. Preliminary studies of the Ba-doped La/Sr chromo-manganite series as new SOFC anode materials. ECS Trans. 2011, 35, 1345–1356. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toldra-Reig, F.; Serra, J.M. Potentiometric C2H4-Selective Detection on Solid-State Sensors Activated with Bifunctional Catalytic Nanoparticles. Chemosensors 2021, 9, 274. https://doi.org/10.3390/chemosensors9100274
Toldra-Reig F, Serra JM. Potentiometric C2H4-Selective Detection on Solid-State Sensors Activated with Bifunctional Catalytic Nanoparticles. Chemosensors. 2021; 9(10):274. https://doi.org/10.3390/chemosensors9100274
Chicago/Turabian StyleToldra-Reig, Fidel, and Jose Manuel Serra. 2021. "Potentiometric C2H4-Selective Detection on Solid-State Sensors Activated with Bifunctional Catalytic Nanoparticles" Chemosensors 9, no. 10: 274. https://doi.org/10.3390/chemosensors9100274