Efficient Microwave-Assisted Extraction of Nitrites from Cured Meat and Their Voltammetric Detection at Chemically Modified Electrodes Based on Hexamethyl-p-Terphenyl Poly(methylatedbenzimidazolium) Incorporating Nitrogen-Doped Graphite Nanoplatelets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. Preparation of HMT-PMBI, and HMT-PMBI/NGNP Composite Solutions
2.4. Preparation of HMT-PMBI, and HMT-PMBI/NGNP Coated Electrodes
2.5. Extraction of Nitrites Using Microwave-Assisted Heating
3. Results and Discussions
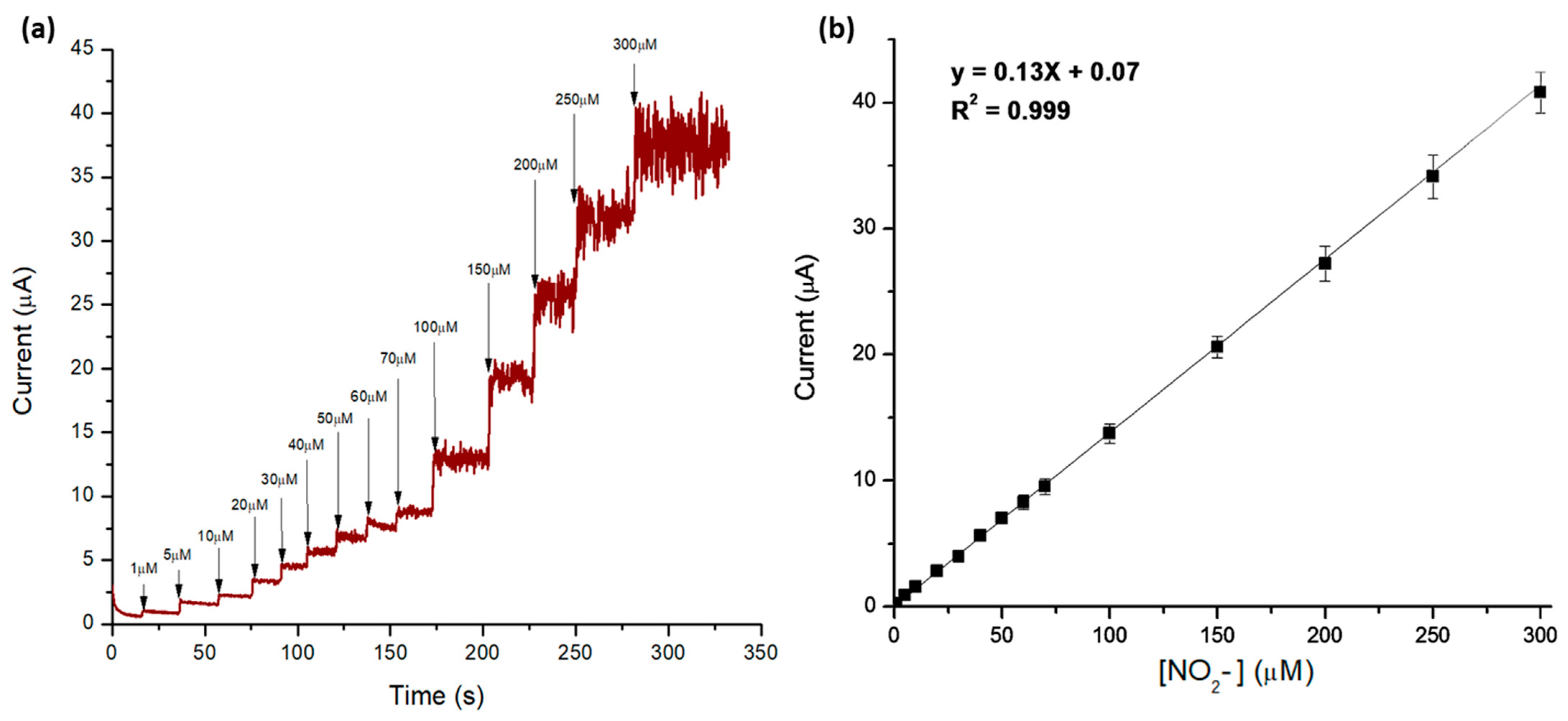
3.1. Electrochemical Detection of Nitrites
3.2. Assessment of Extraction of Nitrites from Meat Products Using Microwave-Assisted Heat Treatment
3.3. Electrochemical Detection of Nitrites in Meat Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Esimbekova, E.N.; Asanova, A.A.; Deeva, A.A.; Kratasyuk, V.A. Inhibition effect of food preservatives on endoproteinases. Food Chem. 2017, 235, 294–297. [Google Scholar] [CrossRef]
- Loganathan, C.; Narayanamoorthi, E.; John, S.A. Leaching of AuNPs from the surface of GO: Sensitive turn on fluorescence detection of toxic preservative. Food Chem. 2020, 309, 125751. [Google Scholar] [CrossRef]
- Della Betta, F.; Pereira, L.M.; Siqueira, M.A.; Valese, A.C.; Daguer, H.; Fett, R.; Vitali, L.; Costa, A.C.O. A sub-minute CZE method to determine nitrate and nitrite in meat products: An alternative for routine analysis. Meat Sci. 2016, 119, 62–68. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Stringer, S.; Fernández, M. A study on the toxigenesis by Clostridium botulinum in nitrate and nitrite-reduced dry fermented sausages. Int. J. Food Microbiol. 2016, 218, 66–70. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products-Invited review. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. In Red Meat and Processed Meat; World Health Organization: Lyon, France, 2018; Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono114.pdf (accessed on 16 November 2021).
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Statement on nitrites in meat products. EFSA J. 2010, 8, 1538. [Google Scholar] [CrossRef]
- Guevara, I.; Iwanejko, J.; Dembińska-Kieć, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Gołąbek, I.; Bartuś, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef]
- Senra-Ferreiro, S.; Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Griess micro-assay for the determination of nitrite by combining fibre optics-based cuvetteless UV–Vis micro-spectrophotometry with liquid-phase microextraction. Anal. Chim. Acta 2010, 668, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.Y.; Fu, L.M.; Ju, W.J.; Wu, P.Y. Microfluidic colorimetric system for nitrite detection in foods. Chem. Eng. J. 2020, 398, 125573. [Google Scholar] [CrossRef]
- Singh, P.; Singh, M.K.; Beg, Y.R.; Nishad, G.R. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta 2019, 191, 364–381. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.B. Kinetics and mechanisms of the Griess reaction. Anal. Chem. 1979, 51, 1493–1502. [Google Scholar] [CrossRef]
- Pourreza, N.; Fat’hi, M.R.; Hatami, A. Indirect cloud point extraction and spectrophotometric determination of nitrite in water and meat products. Microchem. J. 2012, 104, 22–25. [Google Scholar] [CrossRef]
- Butt, S.B.; Riaz, M.; Iqbal, M.Z. Simultaneous determination of nitrite and nitrate by normal phase ion-pair liquid chromatography. Talanta 2001, 55, 789–797. [Google Scholar] [CrossRef]
- Cinquina, A.L.; Calı, A.; Longo, F.; De Santis, L.; Severoni, A.; Abballe, F. Determination of biogenic amines in fish tissues by ion-exchange chromatography with conductivity detection. J. Chromatogr. A 2004, 1032, 73–77. [Google Scholar] [CrossRef]
- Siu, D.C.; Henshall, A. Ion chromatographic determination of nitrate and nitrite in meat products. J. Chromatogr. A 1998, 804, 157–160. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Stalikas, C.D.; Tzouwara-Karayanni, S.M.; Karayannis, M.I. Simultaneous spectrophotometric determination of nitrite and nitrate by flow-injection analysis. Talanta 1996, 43, 1009–1018. [Google Scholar] [CrossRef]
- Kazemzadeh, A.; Ensafi, A.A. Sequential flow injection spectrophotometric determination of nitrite and nitrate in various samples. Anal. Chim. Acta 2001, 442, 319–326. [Google Scholar] [CrossRef]
- Andrade, R.; Viana, C.O.; Guadagnin, S.G.; Reyes, F.G.; Rath, S. A flow-injection spectrophotometric method for nitrate and nitrite determination through nitric oxide generation. Food Chem. 2003, 80, 597–602. [Google Scholar] [CrossRef]
- Cardoso, T.M.G.; Garcia, P.T.; Coltro, W.K.T. Colorimetric determination of nitrite in clinical, food and environmental samples using microfluidic devices stamped in paper platforms. Anal. Methods 2015, 7, 7311–7317. [Google Scholar] [CrossRef]
- Nam, J.; Jung, I.B.; Kim, B.; Lee, S.M.; Kim, S.E.; Lee, K.N.; Shin, D.S. A colorimetric hydrogel biosensor for rapid detection of nitrite ions. Sens. Actuators B Chem. 2018, 270, 112–118. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, S.; Dong, Y.; Chen, X.; Xu, Y.; Ma, Y.; Chen, X. A sensitive colorimetric method for the determination of nitrite in water supplies, meat and dairy products using ionic liquid-modified methyl red as a colour reagent. Food Chem. 2014, 151, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Song, Y.-Z.; Fang, F.; Wu, Z.-Y. Sensitive paper-based analytical device for fast colorimetric detection of nitrite with smartphone. Anal. Bioanal. Chem. 2018, 410, 2665–2669. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895. [Google Scholar] [CrossRef]
- Luo, X.; Pan, J.; Pan, K.; Yu, Y.; Zhong, A.; Wei, S.; Li, J.; Shi, J.; Li, X. An electrochemical sensor for hydrazine and nitrite based on graphene–cobalt hexacyanoferrate nanocomposite: Toward environment and food detection. J. Electrochem. Soc. 2015, 745, 80–87. [Google Scholar] [CrossRef]
- Muthumariappan, A.; Govindasamy, M.; Chen, S.M.; Sakthivel, K.; Mani, V. Screen-printed electrode modified with a composite prepared from graphene oxide nanosheets and Mn3O4 microcubes for ultrasensitive determination of nitrite. Microchim. Acta 2017, 184, 3625–3634. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Settu, R.; Chen, S.M.; Chen, T.W.; Sharmila, G. A new electrochemical sensor for highly sensitive and selective detection of nitrite in food samples based on sonochemical synthesized Calcium Ferrite (CaFe2O4) clusters modified screen printed carbon electrode. J. Colloid Interface Sci. 2018, 524, 417–426. [Google Scholar] [CrossRef]
- Jian, J.-M.; Fu, L.; Ji, J.; Lin, L.; Guo, X.; Ren, T.-L. Electrochemically reduced graphene oxide/gold nanoparticles composite modified screen-printed carbon electrode for effective electrocatalytic analysis of nitrite in foods. Sens. Actuators B Chem. 2018, 262, 125–136. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, Z.; Liu, C.; Huang, H.; Ye, W. Green synthesis of Pd/Fe3O4 composite based on polyDOPA functionalized reduced graphene oxide for electrochemical detection of nitrite in cured food. Electrochim. Acta 2017, 256, 146–154. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.J.; Lima, P.R.; Tanaka, A.A.; Tanaka, S.M.; Kubota, L.T. Determination of nitrite in food samples by anodic voltammetry using a modified electrode. Food Chem. 2009, 113, 1206–1211. [Google Scholar] [CrossRef]
- Guanghan, L.; Hong, J.; Dandan, S. Determination of trace nitrite by anodic stripping voltammetry. Food Chem. 1997, 59, 583–587. [Google Scholar] [CrossRef]
- ISO. Determination of Nitrite Content, ISO 2918:1975 Standard. In International Standards Meat and Meat Product; International Organization for Standarization: Genèva, Switzerland, 1975. [Google Scholar]
- AOAC International. Nitrites in Cured Meat; Colorimetric method AOAC 973.31-1996(1997); AOAC International: Rockville, MD, USA, 1997. [Google Scholar]
- Mohamed, A.A.; Mubarak, A.T.; Fawy, K.F.; El-Shahat, M.F. Modification of AOAC Method 973.31 for Determination of Nitrite in Cured Meats. J. AOAC Int. 2019, 91, 820–827. [Google Scholar] [CrossRef] [Green Version]
- Campillo, N.; Viñas, P.; Martínez-Castillo, N.; Hernández-Córdoba, M. Determination of volatile nitrosamines in meat products by microwave-assisted extraction and dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Raynie, D.E. Modern Extraction Techniques. Anal. Chem. 2006, 78, 3997–4004. [Google Scholar] [CrossRef] [PubMed]
- Luque-García, J.L.; Luque de Castro, M.D. Where is microwave-based analytical equipment for solid sample pre-treatment going? TrAC Trends Anal. Chem. 2003, 22, 90–98. [Google Scholar] [CrossRef]
- Ramezani, H.; Hosseini, H.; Kamankesh, M.; Ghasemzadeh-Mohammadi, V.; Mohammadi, A. Rapid determination of nitrosamines in sausage and salami using microwave-assisted extraction and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Eur. Food Res. Technol. 2015, 240, 441–450. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Xiao, X.; Song, W.; Wang, J.; Li, G. Microwave-assisted extraction performed in low temperature and in vacuo for the extraction of labile compounds in food samples. Anal. Chim. Acta 2012, 712, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Aldave, S.; Tarat, A.; McGettrick, J.D.; Bertoncello, P. Voltammetric Detection of Caffeine in Beverages at Nafion/Graphite Nanoplatelets Layer-by-Layer Films. Nanomaterials 2019, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.G.; Holdcroft, S. Hydroxide-Stable Ionenes. ACS Macro Lett. 2014, 3, 444–447. [Google Scholar] [CrossRef]
- Wright, A.G.; Fan, J.; Britton, B.; Weissbach, T.; Lee, H.F.; Kitching, E.A.; Peckham, T.J.; Holdcroft, S. Hexamethyl-p-terphenyl poly(benzimidazolium): A universal hydroxide-conducting polymer for energy conversion devices. Energy Environ. Sci. 2016, 9, 2130–2142. [Google Scholar] [CrossRef]
- Rees, M.; Wright, A.G.; Holdcroft, S.; Bertoncello, P. Voltammetry at Hexamethyl-P-Terphenyl Poly(Benzimidazolium) (HMT-PMBI)-Coated Glassy Carbon Electrodes: Charge Transport Properties and Detection of Uric and Ascorbic Acid. Sensors 2020, 20, 443. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.R.; Hernandez-Aldave, S.; Kaspar, R.B.; Letterio, M.P.; Yan, Y.; Bertoncello, P. Tris(2,4,6-trimethoxyphenyl)polysulfone-methylene quaternary phosphonium chloride (TPQPCl) ionomer chemically modified electrodes: An electroanalytical study towards sensing applications. Electrochim. Acta 2019, 311, 160–169. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education: Harlow, UK, 2018. [Google Scholar]
- European Medicines Agency. Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 16 November 2021).


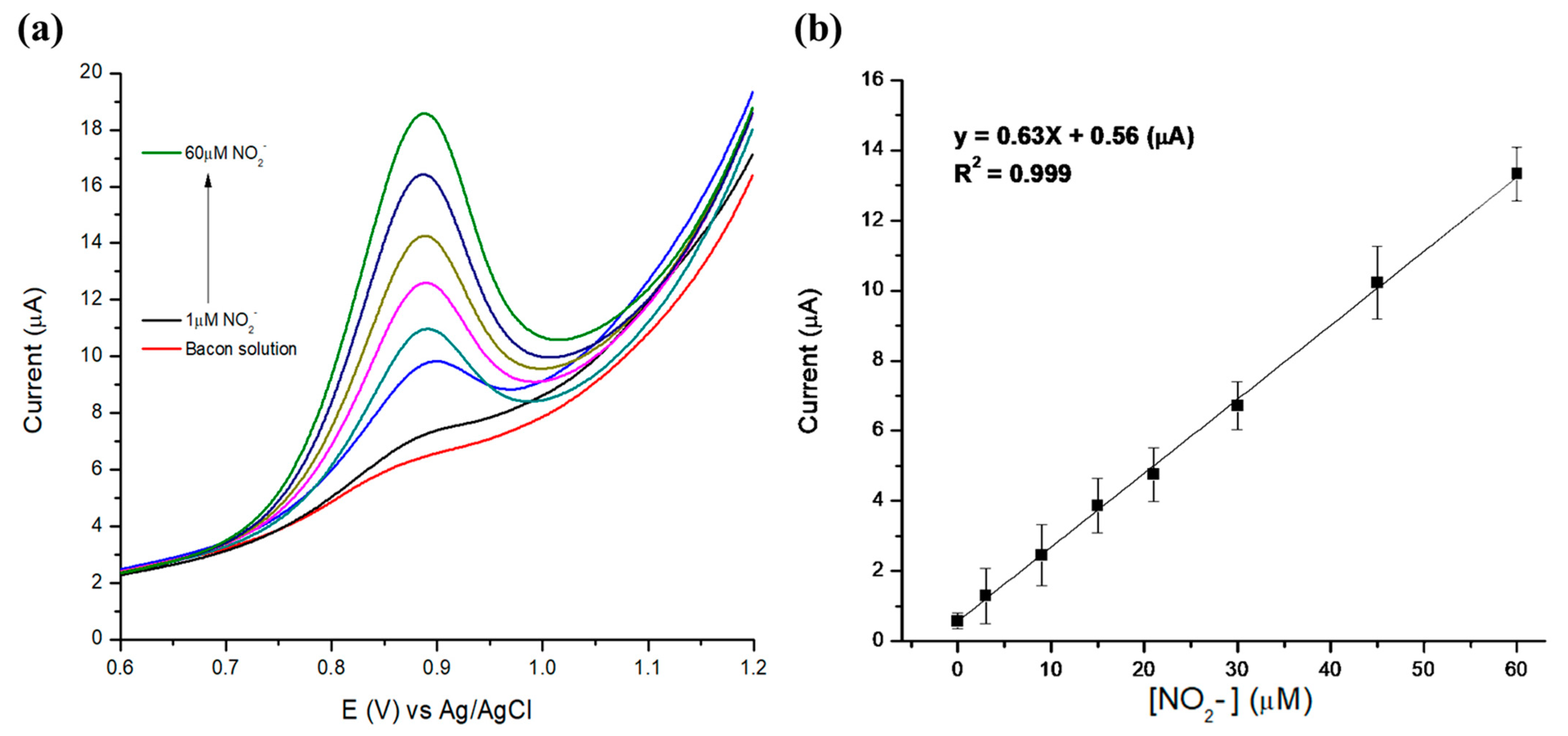
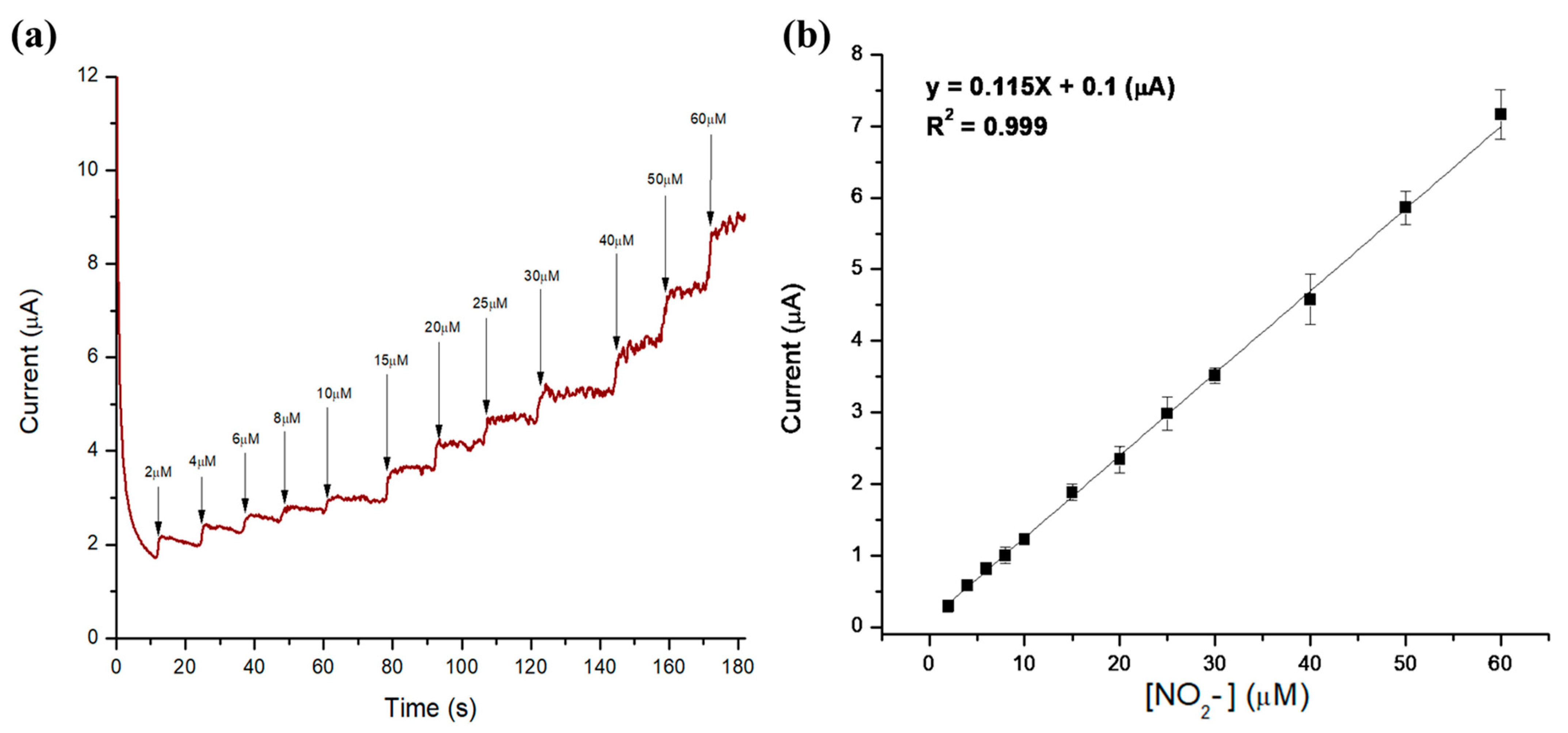
| Method | (Nitrite) (µM) | (NaNO2) (µg/g Bacon) |
|---|---|---|
| ISO (colorimetric) | 0.804 ± 0.02 | 5.56 ± 0.15 |
| AOAC (colorimetric) | 0.814 ± 0.03 | 5.62 ± 0.26 |
| Microwave (colorimetric) | 0.777 ± 0.06 | 5.36 ± 0.48 |
| Microwave (CV curve) | 0.83 ± 0.16 | 5.72 ± 0.92 |
| Microwave (CV standard addition) | 0.89 ± 0.08 | 6.10 ± 0.46 |
| Microwave (CA standard addition) | 0.87 ± 0.11 | 6.00 ± 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Aldave, S.; Tarat, A.; Bertoncello, P. Efficient Microwave-Assisted Extraction of Nitrites from Cured Meat and Their Voltammetric Detection at Chemically Modified Electrodes Based on Hexamethyl-p-Terphenyl Poly(methylatedbenzimidazolium) Incorporating Nitrogen-Doped Graphite Nanoplatelets. Chemosensors 2021, 9, 325. https://doi.org/10.3390/chemosensors9110325
Hernandez-Aldave S, Tarat A, Bertoncello P. Efficient Microwave-Assisted Extraction of Nitrites from Cured Meat and Their Voltammetric Detection at Chemically Modified Electrodes Based on Hexamethyl-p-Terphenyl Poly(methylatedbenzimidazolium) Incorporating Nitrogen-Doped Graphite Nanoplatelets. Chemosensors. 2021; 9(11):325. https://doi.org/10.3390/chemosensors9110325
Chicago/Turabian StyleHernandez-Aldave, Sandra, Afshin Tarat, and Paolo Bertoncello. 2021. "Efficient Microwave-Assisted Extraction of Nitrites from Cured Meat and Their Voltammetric Detection at Chemically Modified Electrodes Based on Hexamethyl-p-Terphenyl Poly(methylatedbenzimidazolium) Incorporating Nitrogen-Doped Graphite Nanoplatelets" Chemosensors 9, no. 11: 325. https://doi.org/10.3390/chemosensors9110325