A Strategy for Magnetic and Electric Stimulation to Enhance Proliferation and Differentiation of NPCs Seeded over PLA Electrospun Membranes
Abstract
:1. Introduction
2. Materials and Methods
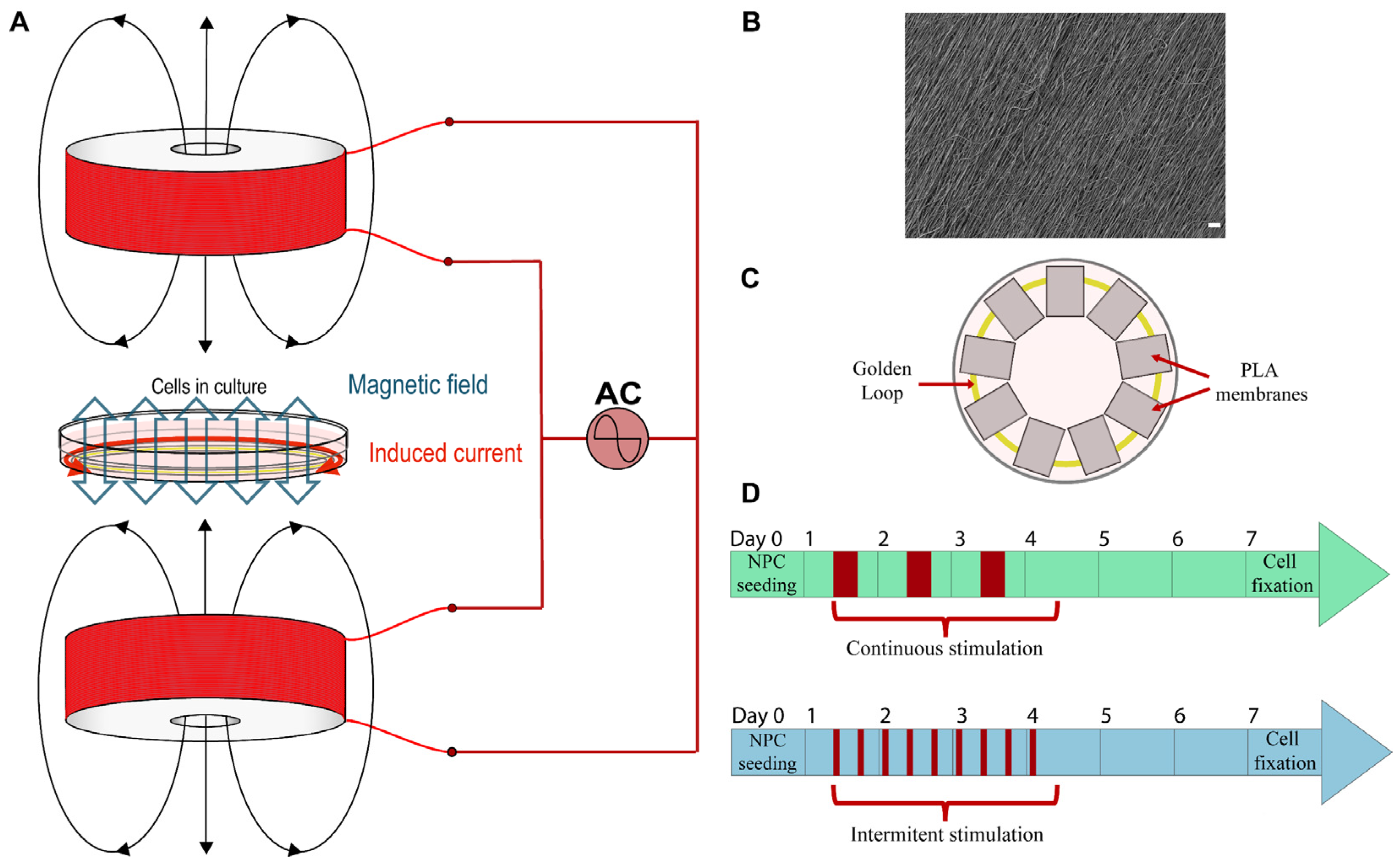
2.1. Bioreactor Description
2.2. PLA Membranes
2.3. Material Sterilization and Preconditioning
2.4. Cell Culture and Electric and Magnetic Stimulation
2.5. Immunocytochemistry
2.6. Statistical Analysis
3. Results and Discussion
3.1. Bioreactor and System Features
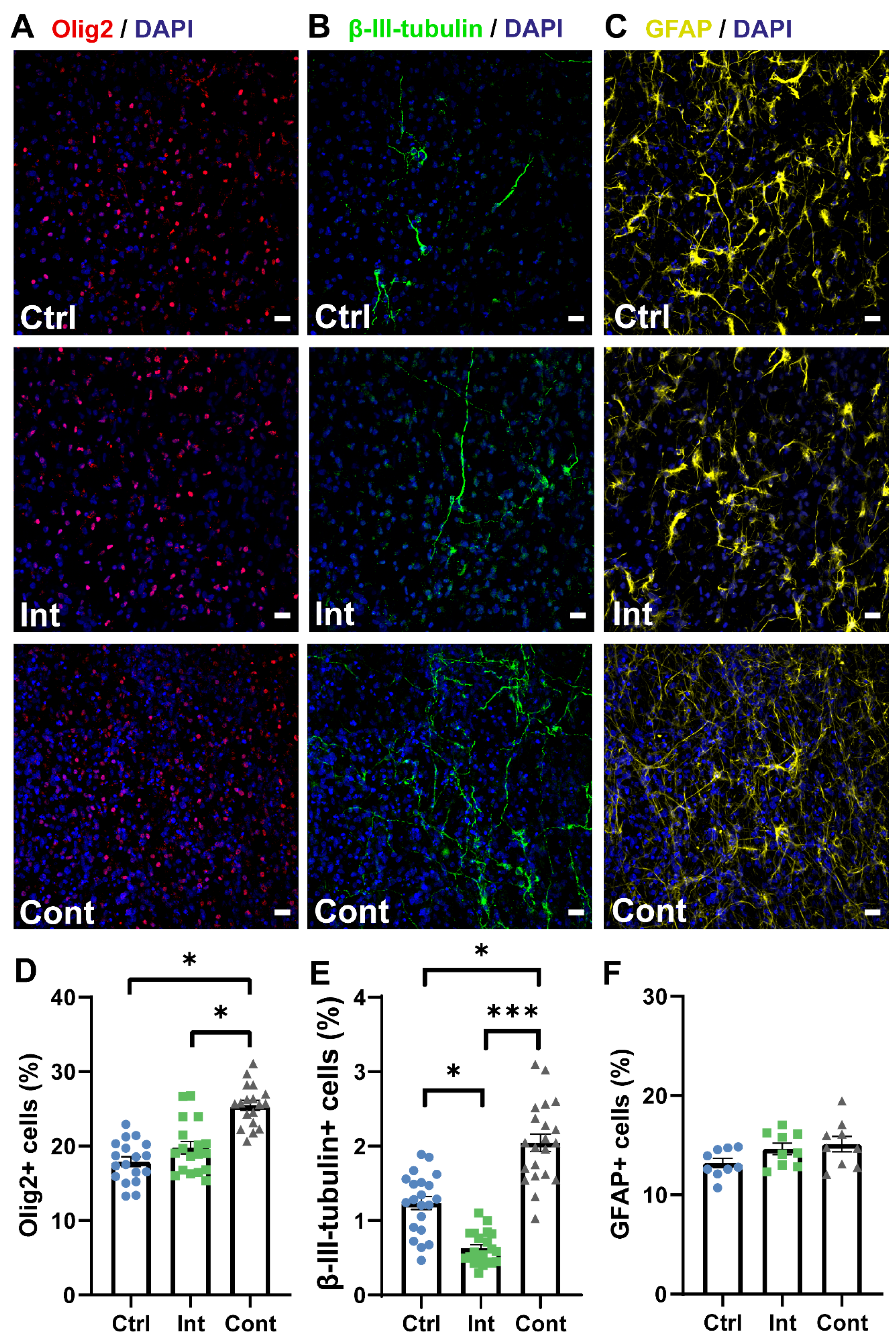
3.2. Continuous Stimulation Promotes NPC Proliferation and Differentiation into Neurons and Oligodendrocytes Progenitors
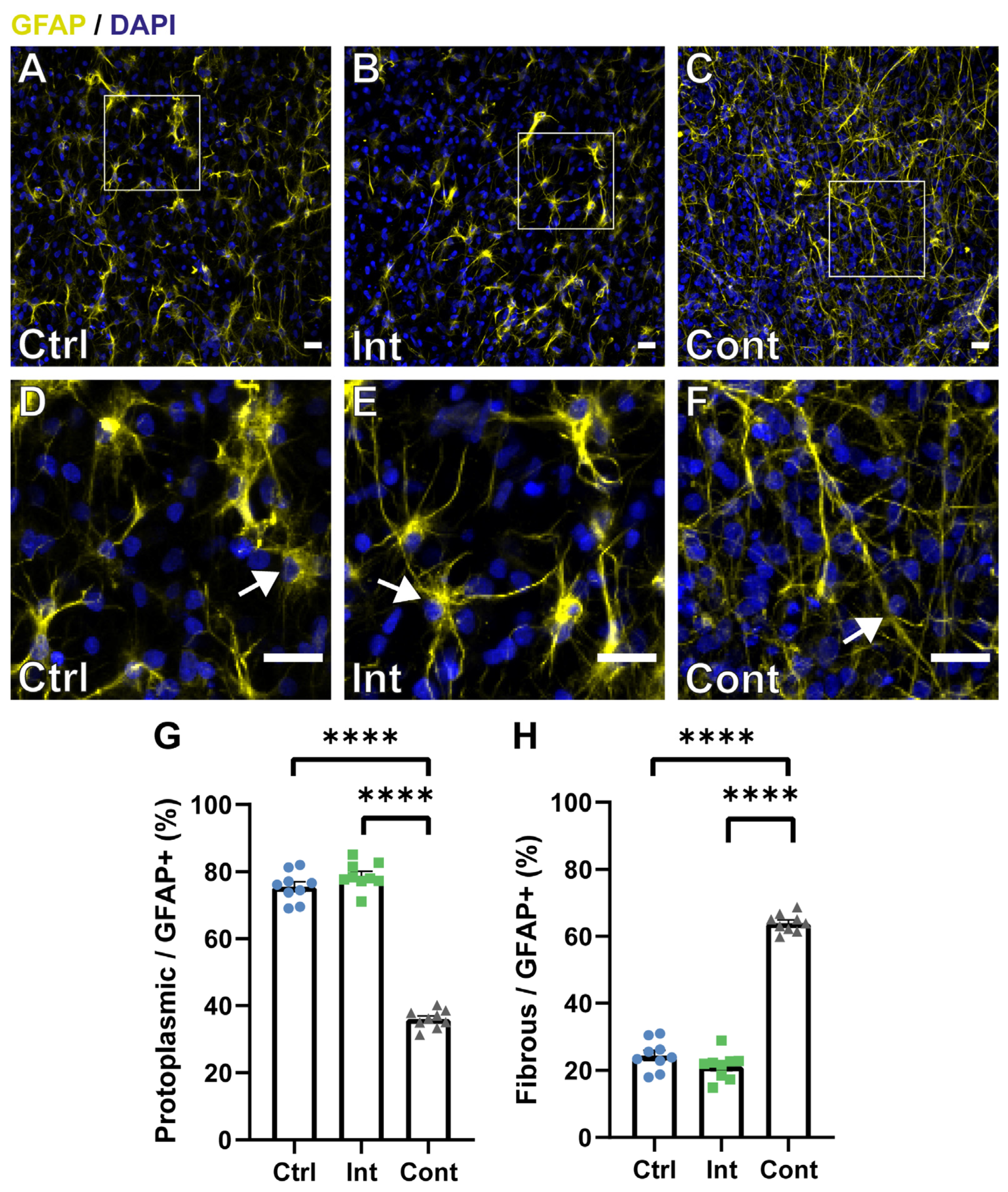
3.3. Continuous Magnetic and Electric Stimulation Affects Astrocyte Maturation into Different Morphological Subtypes
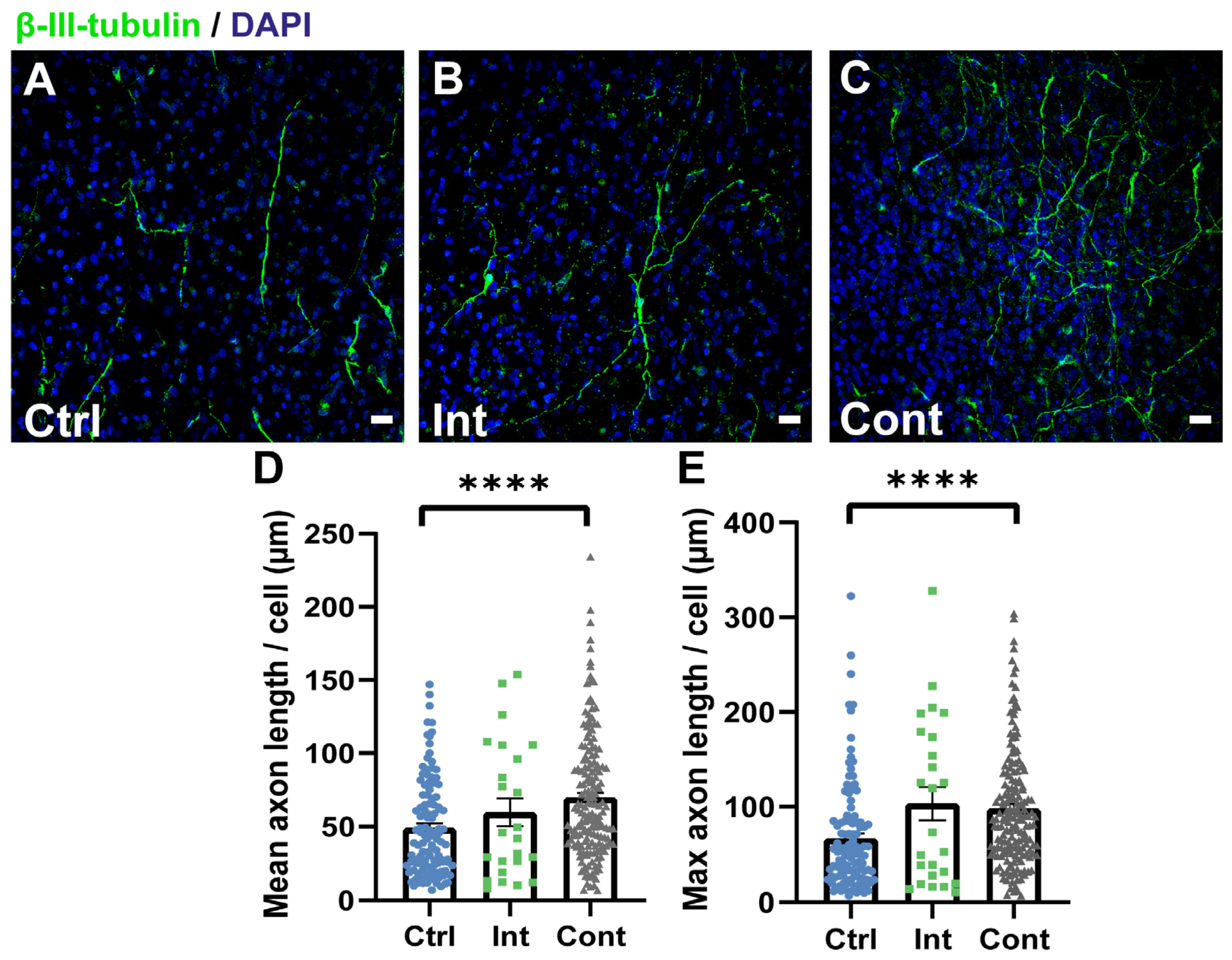
3.4. Continuous Magnetic and Electric Stimulation Promotes Neurite Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalfouh, C.; Guillou, C.; Hardouin, J.; Delarue, Q.; Li, X.; Duclos, C.; Schapman, D.; Marie, J.P.; Cosette, P.; Guérout, N. The Regenerative Effect of Trans-Spinal Magnetic Stimulation After Spinal Cord Injury: Mechanisms and Pathways Underlying the Effect. Neurotherapeutics 2020, 17, 2069–2088. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell Transplantation Therapy for Spinal Cord Injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Csobonyeiova, M.; Polak, S.; Zamborsky, R.; Danisovic, L. Recent Progress in the Regeneration of Spinal Cord Injuries by Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2019, 20, 3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bigio, M.R. The Ependyma: A Protective Barrier between Brain and Cerebrospinal Fluid. Glia 1995, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Redenski, I.; Levenberg, S. Spinal Cord Repair: From Cells and Tissue Engineering to Extracellular Vesicles. Cells 2021, 10, 1872. [Google Scholar] [CrossRef]
- Giraldo, E.; Palmero-Canton, D.; Martinez-Rojas, B.; del Sanchez-Martin, M.M.; Moreno-Manzano, V. Optogenetic Modulation of Neural Progenitor Cells Improves Neuroregenerative Potential. Int. J. Mol. Sci. 2021, 22, 365. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical Stimulation Promotes Stem Cell Neural Differentiation in Tissue Engineering. Stem Cells Int. 2021, 2021, 6697574. [Google Scholar] [CrossRef]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting Neural Progenitor Cells to Restore Connectivity after Spinal Cord Injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef]
- Sabelström, H.; Stenudd, M.; Frisén, J. Neural Stem Cells in the Adult Spinal Cord. Exp. Neurol. 2014, 260, 44–49. [Google Scholar] [CrossRef]
- Mutepfa, A.R.; Hardy, J.G.; Adams, C.F. Electroactive Scaffolds to Improve Neural Stem Cell Therapy for Spinal Cord Injury. Front. Med. Technol. 2022, 4, 693438. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and Novel Polymeric Biomaterials for Neural Tissue Engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, B.; Sun, X.; Liu, R.; Jiang, F.; Yu, H.; Xu, N.; An, Y. Restoring Electrical Connection Using a Conductive Biomaterial Provides a New Therapeutic Strategy for Rats with Spinal Cord Injury. Neurosci. Lett. 2019, 692, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A Novel Magnetic Hydrogel with Aligned Magnetic Colloidal Assemblies Showing Controllable Enhancement of Magnetothermal Effect in the Presence of Alternating Magnetic Field. Adv. Mater. 2015, 27, 2507–2514. [Google Scholar] [CrossRef]
- Bianchi, F.; George, J.H.; Malboubi, M.; Jerusalem, A.; Thompson, M.S.; Ye, H. Engineering a Uniaxial Substrate-Stretching Device for Simultaneous Electrophysiological Measurements and Imaging of Strained Peripheral Neurons. Med. Eng. Phys. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Teh, D.B.L.; Prasad, A.; Jiang, W.; Zhang, N.; Wu, Y.; Yang, H.; Han, S.; Yi, Z.; Yeo, Y.; Ishizuka, T.; et al. Driving Neurogenesis in Neural Stem Cells with High Sensitivity Optogenetics. Neuromol. Med. 2020, 22, 139–149. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 1901714. [Google Scholar] [CrossRef]
- Valente, T.A.M.; Silva, D.M.; Gomes, P.S.; Fernandes, M.H.; Santos, J.D.; Sencadas, V. Effect of Sterilization Methods on Electrospun Poly(Lactic Acid) (PLA) Fiber Alignment for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 3241–3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric Scaffolds for Regenerative Medicine. Polym. Rev. 2011, 51, 23–52. [Google Scholar] [CrossRef]
- Ai, J.; Kiasat-Dolatabadi, A.; Ebrahimi-Barough, S.; Ai, A.; Lotfibakhshaiesh, N.; Norouzi-Javidan, A.; Saberi, H.; Arjmand, B.; Aghayan, H.R. Polymeric Scaffolds in Neural Tissue Engineering: A Review. Arch. Neurosci. 2013, 1, 15–20. [Google Scholar] [CrossRef]
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res. 2018, 67, 335–348. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwcm, M.R.; Willerth, S.M.; Li, X.; Moran, D.W.; Sakiyama-Elbert, S.E.; Xia, Y. Conductive Core-Sheath Nanofibers and Their Potential Application in Neural Tissue Engineering. Adv. Funct. Mater. 2009, 19, 2312–2318. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Arinzeh, T.L. Electrospun Nanofibrous Materials for Neural Tissue Engineering. Polymers 2011, 3, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(Lactic Acid) Nanofibrous Scaffolds for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The Influence of Fiber Diameter of Electrospun Substrates on Neural Stem Cell Differentiation and Proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef]
- Xu, Q.; Jin, L.; Li, C.; Kuddannayai, S.; Zhang, Y. The Effect of Electrical Stimulation on Cortical Cells in 3D Nanofibrous Scaffolds. RSC Adv. 2018, 8, 11027–11035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corey, J.M.; Lin, D.Y.; Mycek, K.B.; Chen, Q.; Samuel, S.; Feldman, E.L.; Martin, D.C. Aligned Electrospun Nanofibers Specify the Direction of Dorsal Root Ganglia Neurite Growth. J. Biomed. Mater. Res. Part A 2007, 83, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Willerth, S.M.; Li, X.; Macewan, M.R.; Rader, A.; Sakiyama-Elbert, S.E.; Xia, Y. The Differentiation of Embryonic Stem Cells Seeded on Electrospun Nanofibers into Neural Lineages. Biomaterials 2009, 30, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(l-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Sudwilai, T.; Ng, J.J.; Boonkrai, C.; Israsena, N.; Chuangchote, S.; Supaphol, P. Polypyrrole-Coated Electrospun Poly(Lactic Acid) Fibrous Scaffold: Effects of Coating on Electrical Conductivity and Neural Cell Growth. J. Biomater. Sci. Polym. Ed. 2014, 25, 1240–1252. [Google Scholar] [CrossRef]
- Kanmaz, D.; Aylin Karahan Toprakci, H.; Olmez, H.; Toprakci, O. Electrospun Polylactic Acid Based Nanofibers for Biomedical Applications. Mater. Sci. Res. India 2018, 15, 224–240. [Google Scholar] [CrossRef]
- Du, J.; Zhen, G.; Chen, H.; Zhang, S.; Qing, L.; Yang, X.; Lee, G.; Mao, H.Q.; Jia, X. Optimal Electrical Stimulation Boosts Stem Cell Therapy in Nerve Regeneration. Biomaterials 2018, 181, 347–359. [Google Scholar] [CrossRef]
- Chang, K.A.; Kim, J.W.; Kim, J.A.; Lee, S.E.; Kim, S.; Suh, W.H.; Kim, H.S.; Kwon, S.; Kim, S.J.; Suh, Y.H. Biphasic Electrical Currents Stimulation Promotes Both Proliferation and Differentiation of Fetal Neural Stem Cells. PLoS ONE 2011, 6, e18738. [Google Scholar]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.V.; Morgado, J.; Ferreira, F.C. Neural Stem Cell Differentiation by Electrical Stimulation Using a Cross-Linked PEDOT Substrate: Expanding the Use of Biocompatible Conjugated Conductive Polymers for Neural Tissue Engineering. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Y.; Pei, Z.; Wang, Y.-L.; Li, Z.; Khan, A.; Meng, X.-T. Ascl1 Regulates Electric Field-Induced Neuronal Differentiation Through PI3K/Akt Pathway. Neuroscience 2019, 404, 141–152. [Google Scholar] [CrossRef]
- Imaninezhad, M.; Pemberton, K.; Xu, F.; Kalinowski, K.; Bera, R.; Zustiak, S.P. Directed and Enhanced Neurite Outgrowth Following Exogenous Electrical Stimulation on Carbon Nanotube-Hydrogel Composites. J. Neural Eng. 2018, 15, 056034. [Google Scholar] [CrossRef] [PubMed]
- Ariza, C.A.; Fleury, A.T.; Tormos, C.J.; Petruk, V.; Chawla, S.; Oh, J.; Sakaguchi, D.S.; Mallapragada, S.K. The Influence of Electric Fields on Hippocampal Neural Progenitor Cells. Stem Cell Rev. Rep. 2010, 6, 585–600. [Google Scholar] [CrossRef]
- Gupta, P.; Agrawal, A.; Murali, K.; Varshney, R.; Beniwal, S.; Manhas, S.; Roy, P.; Lahiri, D. Differential Neural Cell Adhesion and Neurite Outgrowth on Carbon Nanotube and Graphene Reinforced Polymeric Scaffolds. Mater. Sci. Eng. C 2019, 97, 539–551. [Google Scholar] [CrossRef]
- Zhu, R.; Sun, Z.; Li, C.; Ramakrishna, S.; Chiu, K.; He, L. Electrical Stimulation Affects Neural Stem Cell Fate and Function in Vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef]
- Iwasa, S.N.; Shi, H.H.; Hong, S.H.; Chen, T.; Marquez-Chin, M.; Iorio-Morin, C.; Kalia, S.K.; Popovic, M.R.; Naguib, H.E.; Morshead, C.M. Novel Electrode Designs for Neurostimulation in Regenerative Medicine: Activation of Stem Cells. Bioelectricity 2020, 2, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Rowald, A.; Komi, S.; Demesmaeker, R.; Baaklini, E.; Hernandez-Charpak, S.D.; Paoles, E.; Montanaro, H.; Cassara, A.; Becce, F.; Lloyd, B.; et al. Activity-Dependent Spinal Cord Neuromodulation Rapidly Restores Trunk and Leg Motor Functions after Complete Paralysis. Nat. Med. 2022, 28, 260–271. [Google Scholar] [CrossRef]
- Liu, L.; Chen, B.; Liu, K.; Gao, J.; Ye, Y.; Wang, Z.; Qin, N.; Wilson, D.A.; Tu, Y.; Peng, F. Wireless Manipulation of Magnetic/Piezoelectric Micromotors for Precise Neural Stem-Like Cell Stimulation. Adv. Funct. Mater. 2020, 30, 1910108. [Google Scholar] [CrossRef]
- Piech, D.K.; Johnson, B.C.; Shen, K.; Ghanbari, M.M.; Li, K.Y.; Neely, R.M.; Kay, J.E.; Carmena, J.M.; Maharbiz, M.M.; Muller, R. A Wireless Millimetre-Scale Implantable Neural Stimulator with Ultrasonically Powered Bidirectional Communication. Nat. Biomed. Eng. 2020, 4, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; MacEwan, M.R.; Kang, S.K.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.Y.; Shin, J.; et al. Wireless Bioresorbable Electronic System Enables Sustained Nonpharmacological Neuroregenerative Therapy. Nat. Med. 2018, 24, 1830–1836. [Google Scholar] [CrossRef]
- Han, F.; Ma, X.; Zhai, Y.; Cui, L.; Yang, L.; Zhu, Z.; Hao, Y.; Cheng, G. Strategy for Designing a Cell Scaffold to Enable Wireless Electrical Stimulation for Enhanced Neuronal Differentiation of Stem Cells. Adv. Healthc. Mater. 2021, 10, 2100027. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.L.; Young, K.M. How Does Transcranial Magnetic Stimulation Influence Glial Cells in the Central Nervous System? Front. Neural Circuits 2016, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Wang, S.; Guo, Y.; Wang, J.; Lou, M.; Wu, J.; Ding, M.; Tian, M.; Zhang, H. Protective Effects of Repetitive Transcranial Magnetic Stimulation in a Rat Model of Transient Cerebral Ischaemia: A MicroPET Study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 954–961. [Google Scholar] [CrossRef]
- Liu, H.; Li, G.; Ma, C.; Chen, Y.; Wang, J.; Yang, Y. Repetitive Magnetic Stimulation Promotes the Proliferation of Neural Progenitor Cells via Modulating the Expression of MiR-106b. Int. J. Mol. Med. 2018, 42, 3631–3639. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, R.; Ripoli, C.; Mezzogori, D.; Azzena, G.B.; Grassi, C. Extremely Low-Frecuency Electromagnetic Fields Promote in Vitro Neurogenesis via Upregulation of Cav1-Channel Activity. J. Cell. Physiol. 2008, 215, 129–139. [Google Scholar] [CrossRef]
- Cuccurazzu, B.; Leone, L.; Podda, M.V.; Piacentini, R.; Riccardi, E.; Ripoli, C.; Azzena, G.B.; Grassi, C. Exposure to Extremely Low-Frequency (50Hz) Electromagnetic Fields Enhances Adult Hippocampal Neurogenesis in C57BL/6 Mice. Exp. Neurol. 2010, 226, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ge, H.; Zhao, H.; Zou, Y.; Chen, Y.; Feng, H. Electromagnetic Fields for the Regulation of Neural Stem Cells. Stem Cells Int. 2017, 2017, 9898439. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Teh, D.B.L.; Blasiak, A.; Chai, C.; Wu, Y.; Gharibani, P.M.; Yang, I.H.; Phan, T.T.; Lim, K.L.; Yang, H.; et al. Static Magnetic Field Stimulation Enhances Oligodendrocyte Differentiation and Secretion of Neurotrophic Factors. Sci. Rep. 2017, 7, 6743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funnell, J.L.; Ziemba, A.M.; Nowak, J.F.; Awada, H.; Prokopiou, N.; Samuel, J.; Guari, Y.; Nottelet, B.; Gilbert, R.J. Assessing the Combination of Magnetic Field Stimulation, Iron Oxide Nanoparticles, and Aligned Electrospun Fibers for Promoting Neurite Outgrowth from Dorsal Root Ganglia in Vitro. Acta Biomater. 2021, 131, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Cho, H.; Mishig-Ochir, T.; Seo, Y.K.; Park, J.K. Pulsed Electromagnetic Fields Promote Survival and Neuronal Differentiation of Human BM-MSCs. Life Sci. 2016, 151, 130–138. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, C.; Deng, P.; Zhu, G.; Lin, M.; Zhang, L.; Xu, S.; He, M.; Lu, Y.; Duan, W.; et al. Extremely Low-Frequency Electromagnetic Fields Promote in Vitro Neuronal Differentiation and Neurite Outgrowth of Embryonic Neural Stem Cells via up - Regulating TRPC1. PLoS ONE 2016, 11, e0150923. [Google Scholar] [CrossRef] [PubMed]
- Gisbert Roca, F.; Más Estellés, J.; Monleón Pradas, M.; Martínez-Ramos, C. Axonal Extension from Dorsal Root Ganglia on Fibrillar and Highly Aligned Poly(Lactic Acid)-Polypyrrole Substrates Obtained by Two Different Techniques: Electrospun Nanofibres and Extruded Microfibres. Int. J. Biol. Macromol. 2020, 163, 1959–1969. [Google Scholar] [CrossRef]
- Liao, C.F.; Hsu, S.T.; Chen, C.C.; Yao, C.H.; Lin, J.H.; Chen, Y.H.; Chen, Y.S. Effects of Electrical Stimulation on Peripheral Nerve Regeneration in a Silicone Rubber Conduit in Taxol-Treated Rats. Materials 2020, 13, 1063. [Google Scholar]
- Tang, S.; Cuellar, C.A.; Song, P.; Islam, R.; Huang, C.; Wen, H.; Knudsen, B.E.; Gong, P.; Lok, U.W.; Chen, S.; et al. Changes in Spinal Cord Hemodynamics Reflect Modulation of Spinal Network with Different Parameters of Epidural Stimulation. Neuroimage 2020, 221, 117183. [Google Scholar] [CrossRef]
- Kumar, P.J.; Adams, R.D.; Harkins, A.B.; Engeberg, E.D.; Willits, R.K. Stimulation Frequency Alters the Dorsal Root Ganglion Neurite Growth and Directionality In Vitro. IEEE Trans. Biomed. Eng. 2016, 63, 1257–1268. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Kopecký, D.; Vrňata, M.; Prokeš, J.; Varga, M.; Watzlová, E. Polypyrrole Salts and Bases: Superior Conductivity of Nanotubes and Their Stability towards the Loss of Conductivity by Deprotonation. RSC Adv. 2016, 6, 88382–88391. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, N.; Straømme, M.; Fellström, B.; Pradhan, S.; Nyholm, L.; Mihranyan, A. In Vitro and in Vivo Toxicity of Rinsed and Aged Nanocellulose–Polypyrrole Composites. J. Biomed. Mater. Res. Part A 2012, 100A, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Graphene Scaffolds in Progressive Nanotechnology/Stem Cell-Based Tissue Engineering of the Nervous System. J. Mater. Chem. B 2016, 4, 3169. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhao, M.; Forrester, J.V.; McCaig, C.D. Electrical Cues Regulate the Orientation and Frequency of Cell Division and the Rate of Wound Healing in Vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 13577–13582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobelt, L.J.; Wilkinson, A.E.; McCormick, A.M.; Willits, R.K.; Leipzig, N.D. Short Duration Electrical Stimulation to Enhance Neurite Outgrowth and Maturation of Adult Neural Stem Progenitor Cells. Ann. Biomed. Eng. 2014, 42, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.F.; Lee, Y.S.; Tang, T.K.; Cheng, J.Y. Pulsed DC Electric Field-Induced Differentiation of Cortical Neural Precursor Cells. PLoS ONE 2016, 11, e0158133. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.-Y.; Pei, Z.; Li, Z.; Wang, Y.-L.; Khan, A.; Meng, X.-T. Electric Field Stimulation Induced Neuronal Differentiation of Filum Terminale Derived Neural Progenitor Cells. Neurosci. Lett. 2017, 651, 109–115. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, J.; Park, J.H.; Kim, J.H.; Ko, K.N.; Kim, C.W. Extremely Low-Frequency Electromagnetic Fields Induce Neural Differentiation in Bone Marrow Derived Mesenchymal Stem Cells. Exp. Biol. Med. 2013, 238, 923–931. [Google Scholar] [CrossRef]
- Haghighat, N.; Abdolmaleki, P.; Behmanesh, M.; Satari, M. Stable Morphological–Physiological and Neural Protein Expression Changes in Rat Bone Marrow Mesenchymal Stem Cells Treated with Electromagnetic Field and Nitric Oxide. Bioelectromagnetics 2017, 38, 592–601. [Google Scholar] [CrossRef]
- Liu, H.; Han, X.-H.; Chen, H.; Zheng, C.-X.; Yang, Y.; Huang, X.-L. Repetitive Magnetic Stimulation Promotes Neural Stem Cells Proliferation by Upregulating MiR-106b in Vitro. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2015, 35, 766–772. [Google Scholar] [CrossRef]
- Abbasnia, K.; Ghanbari, A.; Abedian, M.; Ghanbari, A.; Sharififar, S.; Azari, H. The Effects of Repetitive Transcranial Magnetic Stimulation on Proliferation and Differentiation of Neural Stem Cells. Anat. Cell Biol. 2015, 48, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Deng, P.; Zhu, G.; Liu, C.; Zhang, L.; Zhou, Z.; Luo, X.; Li, M.; Zhong, M.; Yu, Z.; et al. Extremely Low-Frequency Electromagnetic Fields Affect Transcript Levels of Neuronal Differentiation-Related Genes in Embryonic Neural Stem Cells. PLoS ONE 2014, 9, e90041. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, T.; Czyz, J.; Rolletschek, A.; Blyszczuk, P.; Fuchs, J.; Jovtchev, G.; Schulderer, J.; Kuster, N.; Wobus, A.M. Electromagnetic Fields Affect Transcript Levels of Apoptosis-related Genes in Embryonic Stem Cell-derived Neural Progenitor Cells. FASEB J. 2005, 19, 1686–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabata, H. Diverse Subtypes of Astrocytes and Their Development during Corticogenesis. Front. Neurosci. 2015, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Wang, L.; Weng, W.; Wang, S.; Ma, Y.; Mao, Q.; Gao, G.; Chen, R.; Feng, J. Steered Migration and Changed Morphology of Human Astrocytes by an Applied Electric Field. Exp. Cell Res. 2019, 374, 282–289. [Google Scholar] [CrossRef]
- Fu, C.; Pan, S.; Ma, Y.; Kong, W.; Qi, Z.; Yang, X. Effect of Electrical Stimulation Combined with Graphene-Oxide-Based Membranes on Neural Stem Cell Proliferation and Differentiation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1867–1876. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, Y.; Chen, J.; Zhou, H.; Tan, S. Electrical Stimulation Elicits Neural Stem Cells Activation: New Perspectives in CNS Repair. Front. Hum. Neurosci. 2015, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wu, C.; Chen, S.; Qiao, Z.; Borovskikh, P.; Shchegolkov, A.; Chen, L.; Wei, D.; Sun, J.; Fan, H. Combining Electrospinning and Electrospraying to Prepare a Biomimetic Neural Scaffold with Synergistic Cues of Topography and Electrotransduction. ACS Appl. Bio Mater. 2020, 3, 5148–5159. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Liu, M.; Song, W.; Wu, Q.; Fan, Y. Potential Protective Effect of Biphasic Electrical Stimulation against Growth Factor-Deprived Apoptosis on Olfactory Bulb Neural Progenitor Cells through the Brain-Derived Neurotrophic Factor-Phosphatidylinositol 3′-Kinase/Akt Pathway. Exp. Biol. Med. 2013, 238, 951–959. [Google Scholar] [CrossRef]
- Yuan, T.-F.; Dong, Y.; Zhang, L.; Qi, J.; Yao, C.; Wang, Y.; Chai, R.; Liu, Y.; So, K.-F. Neuromodulation-Based Stem Cell Therapy in Brain Repair: Recent Advances and Future Perspectives. Neurosci. Bull. 2021, 37, 735–745. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Lang, N.; Antal, A.; Tergau, F.; Paulus, W.; Priori, A. Safety Criteria for Transcranial Direct Current Stimulation (TDCS) in Humans. Clin. Neurophysiol. 2003, 114, 2220–2222. [Google Scholar] [CrossRef]
- Rossini, P.M.; Rossi, S. Transcranial Magnetic Stimulation: Diagnostic, Therapeutic, and Research Potential. Neurology 2007, 68, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, J.M.; Ramakers, G.M.J.; Di Lazzaro, V. Physiology of Repetitive Transcranial Magnetic Stimulation of the Human Brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.P.; Fouad, C.; Lanzinger, W.; Willits, R.K. Effect of Intraoperative Electrical Stimulation on Recovery after Rat Sciatic Nerve Isograft Repair. Neurotrauma Rep. 2020, 1, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, L.C.; Fu, H.T.; Deng, J.H.; Xu, G.X.; Li, T.; Ji, X.R.; Tang, P.F. Epidural Electrical Stimulation Effectively Restores Locomotion Function in Rats with Complete Spinal Cord Injury. Neural Regen. Res. 2021, 16, 579. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuenca-Ortolá, I.; Martínez-Rojas, B.; Moreno-Manzano, V.; García Castelló, M.; Monleón Pradas, M.; Martínez-Ramos, C.; Más Estellés, J. A Strategy for Magnetic and Electric Stimulation to Enhance Proliferation and Differentiation of NPCs Seeded over PLA Electrospun Membranes. Biomedicines 2022, 10, 2736. https://doi.org/10.3390/biomedicines10112736
Cuenca-Ortolá I, Martínez-Rojas B, Moreno-Manzano V, García Castelló M, Monleón Pradas M, Martínez-Ramos C, Más Estellés J. A Strategy for Magnetic and Electric Stimulation to Enhance Proliferation and Differentiation of NPCs Seeded over PLA Electrospun Membranes. Biomedicines. 2022; 10(11):2736. https://doi.org/10.3390/biomedicines10112736
Chicago/Turabian StyleCuenca-Ortolá, Irene, Beatriz Martínez-Rojas, Victoria Moreno-Manzano, Marcos García Castelló, Manuel Monleón Pradas, Cristina Martínez-Ramos, and Jorge Más Estellés. 2022. "A Strategy for Magnetic and Electric Stimulation to Enhance Proliferation and Differentiation of NPCs Seeded over PLA Electrospun Membranes" Biomedicines 10, no. 11: 2736. https://doi.org/10.3390/biomedicines10112736




