A New Labdane-Type Diterpene, 6-O-Acetyl-(12R)-epiblumdane, from Stevia rebaudiana Leaves with Insulin Secretion Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
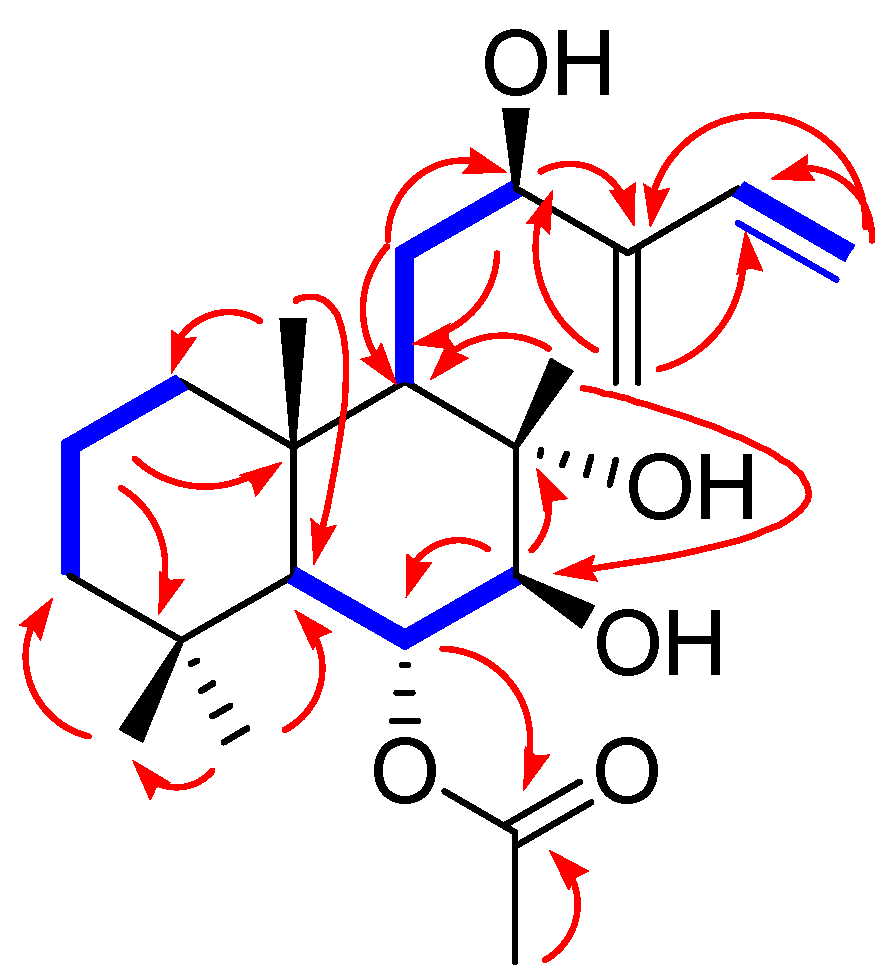
6-O-Acetyl-(12R)-epiblumdane (1)
2.4. Computational Analysis
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Glucose-Stimulated Insulin Secretion Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Compounds 1–10
3.2. Structural Elucidation of the Isolated Compounds
3.3. Effect of Compounds on Glucose-Stimulated Insulin Secretion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lemus-Mondaca, R.; Vega-Galvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.; Hohnova, B.; Hyotylainen, T. Characterisation of Stevia rebaudiana by comprehensive two-dimensional liquid chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1150, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chatsudthipong, V.; Muanprasat, C. Stevioside and related compounds: Therapeutic benefits beyond sweetness. Pharmacol. Ther. 2009, 121, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Ryu, H.W.; So, Y.; Cho, J.K.; Woo, H.S.; Jin, C.H.; Seo, K.I.; Park, J.C.; Jeong, I.Y. Anti-inflammatory effect of austroinulin and 6-O-acetyl-austroinulin from Stevia rebaudiana in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 62, 638–644. [Google Scholar] [CrossRef]
- Lee, S.R.; Kang, H.; Yoo, M.J.; Yu, J.S.; Lee, S.; Yi, S.A.; Beemelmanns, C.; Lee, J.; Kim, K.H. Anti-adipogenic pregnane steroid from a Hydractinia-associated fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An important source of natural bioactive compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]
- Yu, J.S.; Park, M.; Pang, C.; Rashan, L.; Jung, W.H.; Kim, K.H. Antifungal Phenols from Woodfordia uniflora Collected in Oman. J. Nat. Prod. 2020, 83, 2261–2268. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.-C.; Ko, Y.-J.; Kim, S.-H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharm. Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a Styrylpyrone-Fused Steroid with a Hexacyclic 6/5/6/6/6/5 Skeleton from a Mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.-C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a sterol possessing a 6/5/6/5-fused ring system with a contracted tetrahydrofuran B-ring, from the fruiting bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Li, C.; Kwon, M.; Oh, T.; Lee, T.H.; Kim, D.H.; Ahn, J.S.; Ko, S.K.; Kim, C.S.; Cao, S.; et al. Herqueilenone A, a unique rearranged benzoquinone-chromanone from the Hawaiian volcanic soil-associated fungal strain Penicillium herquei FT729. Bioorg. Chem. 2020, 105, 104397. [Google Scholar] [CrossRef] [PubMed]
- Rischer, M.; Lee, S.R.; Eom, H.J.; Park, H.B.; Vollmers, J.; Kaster, A.K.; Shin, Y.H.; Oh, D.C.; Kim, K.H.; Beemelmanns, C. Spirocyclic cladosporicin A and cladosporiumins I and J from a Hydractinia-associated Cladosporium sphaerospermum SW67. Org. Chem. Front. 2019, 6, 1084–1093. [Google Scholar] [CrossRef]
- Kevin, E.; Jonathan, M. The optimal DFT approach in DP4 NMR structure analysis–pushing the limits of relative configuration elucidation. Org. Biomol. Chem. 2019, 17, 5886–5890. [Google Scholar]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Willoughby, P.H.; Jansma, M.J.; Hoye, T.R. A guide to small-molecule structure assignment through computation of (H-1 and C-13) NMR chemical shifts. Nat. Protoc. 2014, 9, 643–660. [Google Scholar] [CrossRef]
- Shen, C.-C.; Wei, W.-C.; Lin, L.-C. Diterpenoids and Bisnorditerpenoids from Blumea aromatica. J. Nat. Prod. 2019, 82, 3181–3185. [Google Scholar] [CrossRef]
- Oshima, Y.; Saito, J.-i.; Hikino, H. Sterebins A, B, C and D, bisnorditerpenoids of Stevia rebaudiana leaves. Tetrahedron 1986, 42, 6443–6446. [Google Scholar] [CrossRef]
- Oshima, Y.; Saito, J.-I.; Hikino, H. Sterebins E, F, G and H, diterpenoids of Stevia rebaudiana leaves. Phytochemistry 1988, 27, 624–626. [Google Scholar] [CrossRef]
- Park, K.-E.; Kim, Y.-A.; Jung, H.-A.; Lee, H.-J.; Ahn, J.-W.; Lee, B.-J.; Seo, Y.-W. Three norisoprenoids from the brown alga Sargassum thunbergii. J. Korean Chem. Soc. 2004, 48, 394–398. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Khlebnikov, V. Carotenoids and Degraded Carotenoids, VIII–Synthesis of (+)-Dihydroactinidiolide, (+)- and (−)-Actinidiolide, (+)- and (−)-Loliolide as well as (+)- and (−)-Epiloliolide. Liebigs Ann. Chem. 1993, 1993, 77–82. [Google Scholar] [CrossRef]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol Long-Chain Fatty Acid Esters with Antimalarial Activity from Holarrhena f loribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.B.; Sowemimo, A.A.; Venables, L.; Koorbanally, N.; Awolola, G.V.; Sofidiya, M.O.; Odukoya, O.A.; Koekemoer, T.; van de Venter, M. Biological evaluation of phytoconstituents from Markhamia tomentosa ethanolic leaf extract. South Afr. J. Bot. 2018, 115, 31–36. [Google Scholar] [CrossRef]
- Ahmad, U.; Ahmad, R.S. Anti diabetic property of aqueous extract of Stevia rebaudiana Bertoni leaves in Streptozotocin-induced diabetes in albino rats. BMC Complement. Med. Ther. 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhu, N.-L.; Kong, J.; Peng, P.; Li, L.-F.; Wei, X.-L.; Jiang, Y.-Y.; Zhang, Y.-L.; Bian, B.-L.; She, G.-M. A newly discovered phenylethanoid glycoside from Stevia rebaudiana Bertoni affects insulin secretion in rat INS-1 islet β cells. Molecules 2019, 24, 4178. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Giacca, A.; Carpentier, A.; Lewis, G. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 2006, 49, 1371–1379. [Google Scholar] [CrossRef] [Green Version]






| Position | Compound 1 | |
|---|---|---|
| δH (J in Hz) | δC, Multiplicity | |
| 1 | 1.20 m/1.44 m | 39.5 t |
| 2 | 1.45 m/1.58 m | 17.6 t |
| 3 | 1.24 m b/1.37 m b | 43.3 t |
| 4 | 33.4 s | |
| 5 | 1.47 m b | 56.5 d |
| 6 | 5.14 dd (11.0, 10.0) | 73.1 d |
| 7 | 3.58 d (10.0) | 83.5 d |
| 8 | 77.8 d | |
| 9 | 1.81 d (4.0) | 52.9 d |
| 10 | 39.1 s | |
| 11 | 1.56 m b, 2.13 m b | 25.9 t |
| 12 | 4.50 dd (11.0, 2.0) | 83.4 d |
| 13 | 145.1 s | |
| 14 | 6.41 dd (17.5, 11.0) | 136.7 d |
| 15 | 5.15 d (11.0)/5.46 d (17.5) | 114.8 t |
| 16 | 5.30 s/5.33 s | 116.5 t |
| 17 | 1.28 s | 19.8 q |
| 18 | 0.88 s | 21.8 q |
| 19 | 1.02 s | 35.7 q |
| 20 | 0.95 s | 16.6 q |
| 1’ | 172.1 s | |
| 2’ | 2.15 s | 21.6 q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Lee, D.; Kang, K.S.; Kim, K.H. A New Labdane-Type Diterpene, 6-O-Acetyl-(12R)-epiblumdane, from Stevia rebaudiana Leaves with Insulin Secretion Effect. Biomedicines 2022, 10, 839. https://doi.org/10.3390/biomedicines10040839
Kang H, Lee D, Kang KS, Kim KH. A New Labdane-Type Diterpene, 6-O-Acetyl-(12R)-epiblumdane, from Stevia rebaudiana Leaves with Insulin Secretion Effect. Biomedicines. 2022; 10(4):839. https://doi.org/10.3390/biomedicines10040839
Chicago/Turabian StyleKang, Heesun, Dahae Lee, Ki Sung Kang, and Ki Hyun Kim. 2022. "A New Labdane-Type Diterpene, 6-O-Acetyl-(12R)-epiblumdane, from Stevia rebaudiana Leaves with Insulin Secretion Effect" Biomedicines 10, no. 4: 839. https://doi.org/10.3390/biomedicines10040839







