The Role of Hsp27 in Chemotherapy Resistance
Abstract
:1. Introduction
2. The Role of Hsp27 in Cancer
2.1. Modulation of the “Salvador-Warts-Hippo” (SWH) Pathway
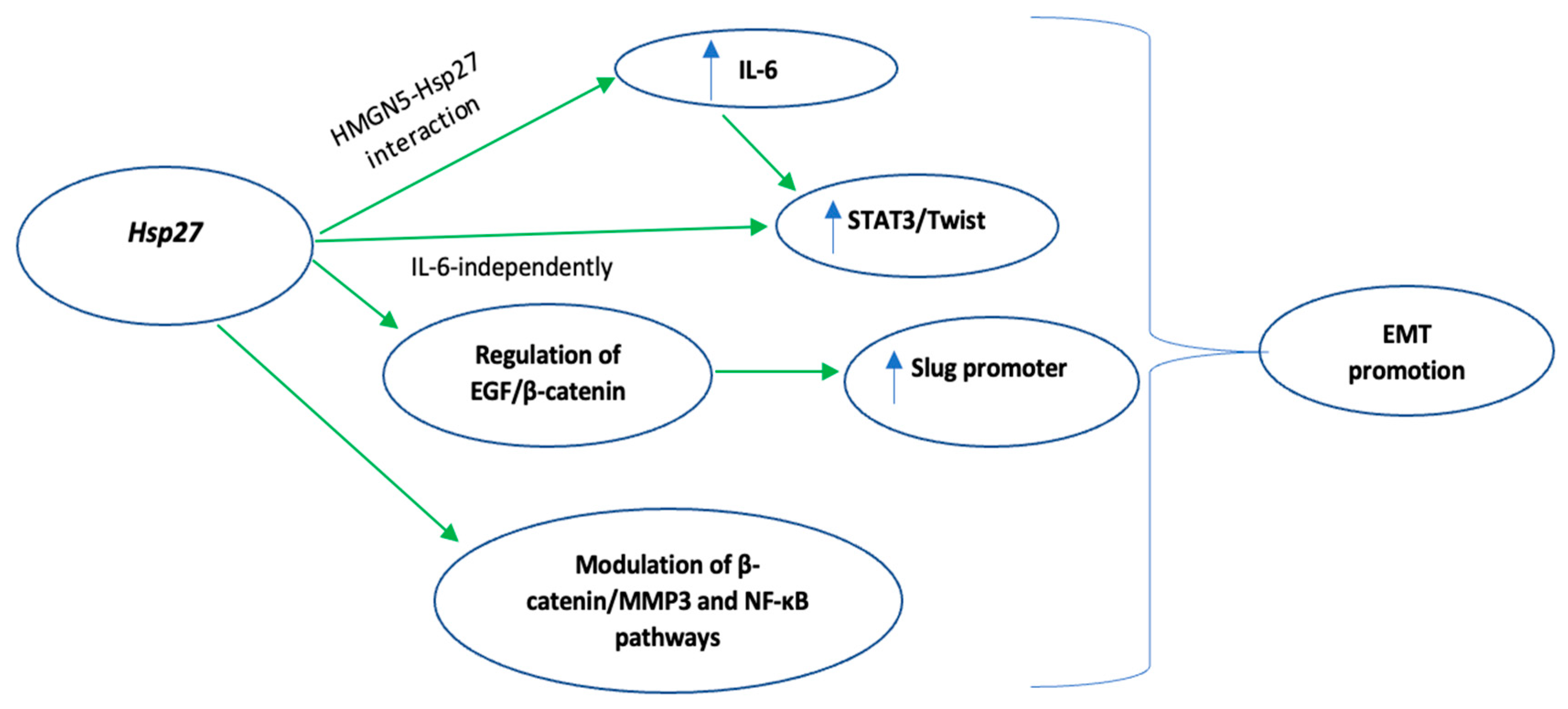
2.2. Promotion of Epithelial-Mesenchymal Transition (EMT)
2.3. Adaptation of CSCs to Stresses of the Tumor Microenvironment
2.4. Induction of Angiogenesis
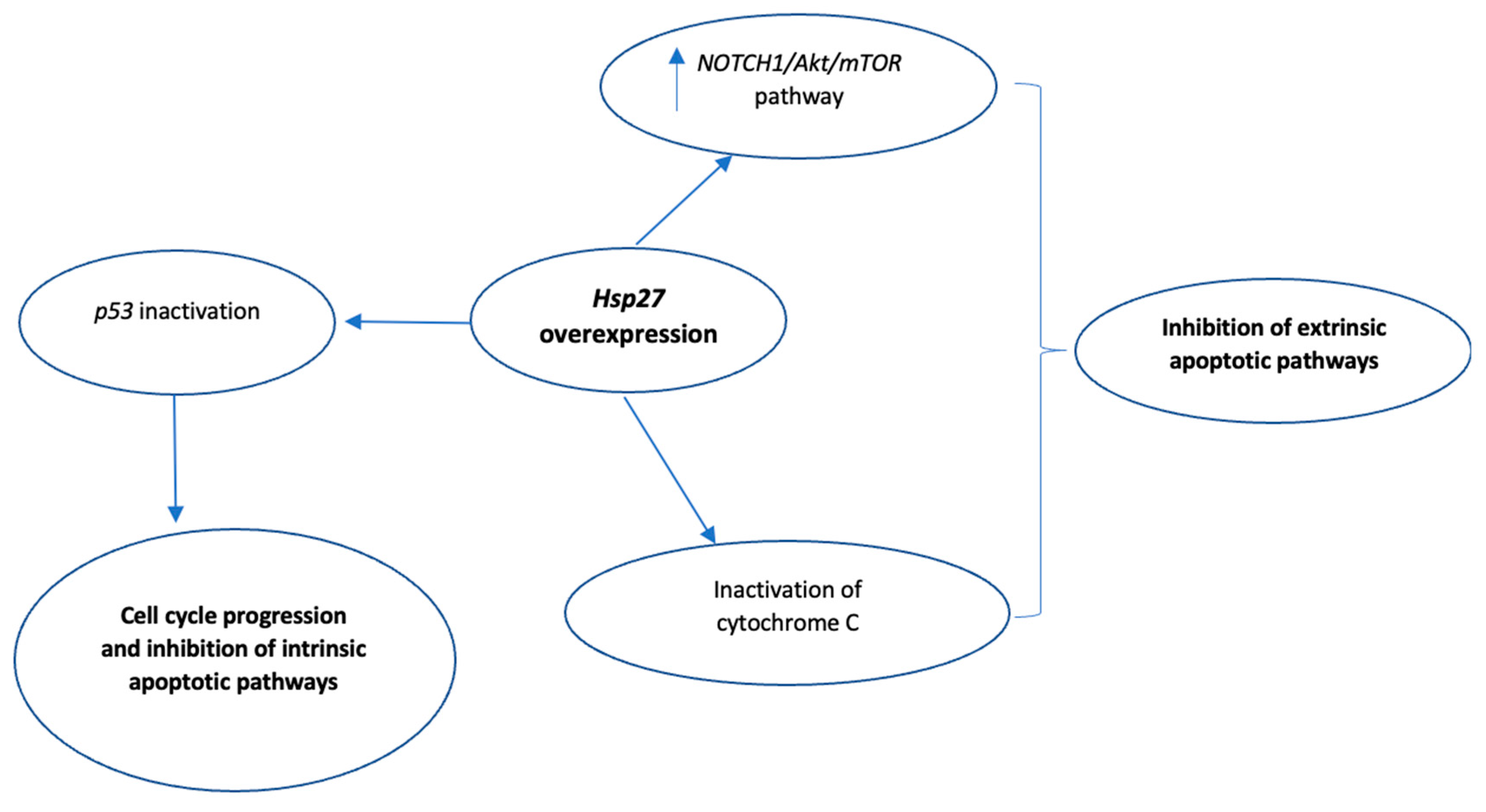
2.5. Inhibition of Apoptosis
3. The Role of Hsp27 in Chemotherapy Resistance
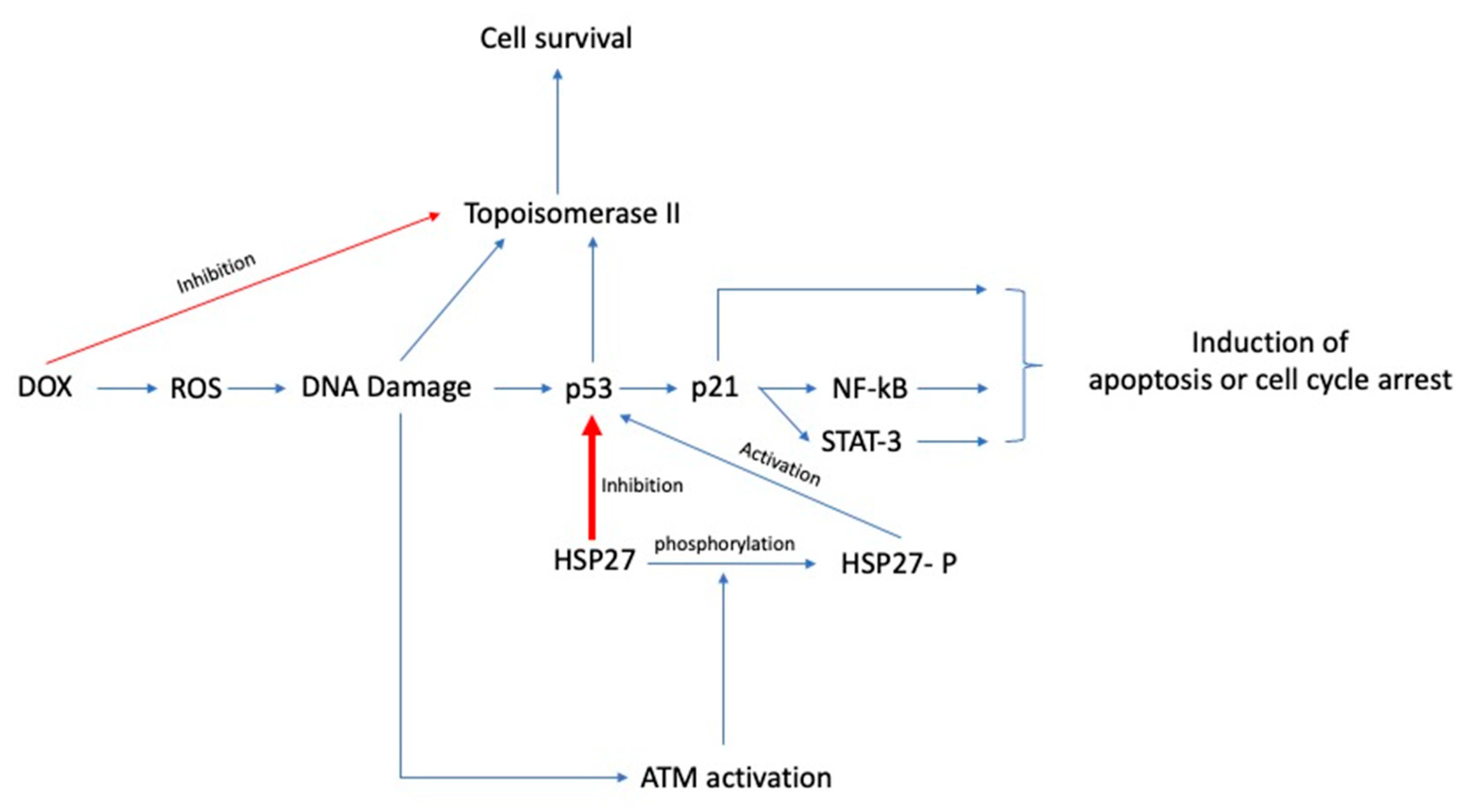
3.1. Doxorubicin
3.2. Herceptin/Trastuzumab
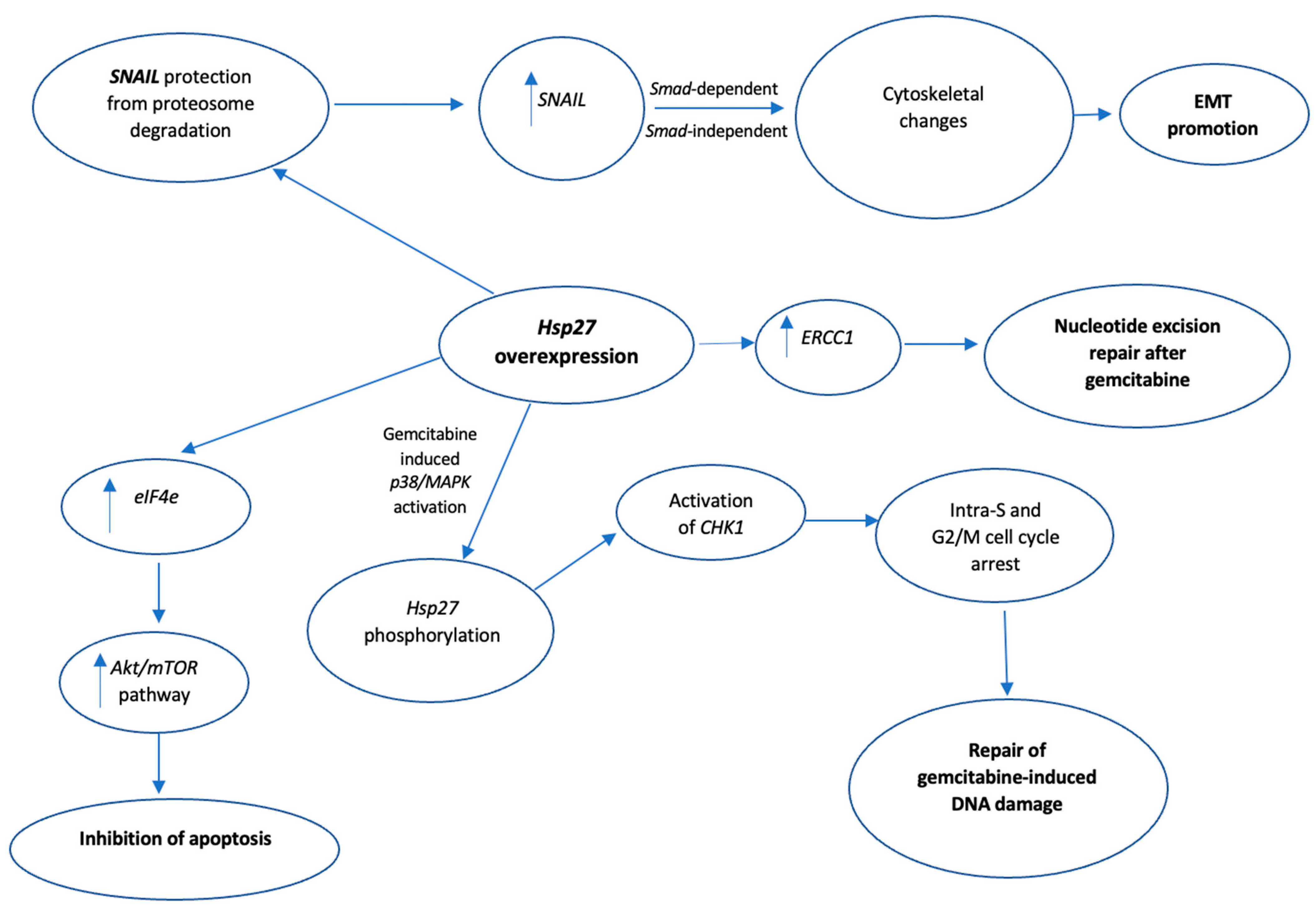
3.3. Gemcitabine
3.4. 5-FU
3.5. Temozolomide
3.6. Paclitaxel
4. Other Hsp27 Inhibitors
5. Clinical Trials with Hsp27 Inhibitors
6. Hsp27 as a Potential Biomarker
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Choi, S.K.; Kam, H.; Kim, K.Y.; Park, S.I.; Lee, Y.S. Targeting Heat Shock Protein 27 in Cancer: A Druggable Target for Cancer Treatment? Cancers 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat Shock Protein 27 (HSP27): Biomarker of Disease and Therapeutic Target. Fibrogenesis Tissue Repair 2012, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lianos, G.D.; Alexiou, G.A.; Mangano, A.; Mangano, A.; Rausei, S.; Boni, L.; Dionigi, G.; Roukos, D.H. The Role of Heat Shock Proteins in Cancer. Cancer Lett. 2015, 360, 114–118. [Google Scholar] [CrossRef]
- Houlden, H.; Laura, M.; Wavrant-De Vrieze, F.; Blake, J.; Wood, N.; Reilly, M.M. Mutations in the HSP27 (HSPB1) Gene Cause Dominant, Recessive, and Sporadic Distal HMN/CMT Type 2. Neurology 2008, 71, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Kokolakis, G.; Tatari, M.; Zacharopoulou, A.; Mintzas, A.C. The Hsp27 Gene of the Mediterranean Fruit Fly, Ceratitis Capitata: Structural Characterization, Regulation and Developmental Expression. Insect Mol. Biol. 2008, 17, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Rogalla, T.; Ehrnsperger, M.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.-P.; Buchner, J.; et al. Regulation of Hsp27 Oligomerization, Chaperone Function, and Protective Activity against Oxidative Stress/Tumor Necrosis Factor α by Phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrigo, A.-P.; Gibert, B. HspB1 Dynamic Phospho-Oligomeric Structure Dependent Interactome as Cancer Therapeutic Target. Curr. Mol. Med. 2012, 12, 1151–1163. [Google Scholar] [CrossRef]
- Mehlen, P.; Hickey, E.; Weber, L.A.; Arrigo, A.P. Large Unphosphorylated Aggregates as the Active Form of Hsp27 Which Controls Intracellular Reactive Oxygen Species and Glutathione Levels and Generates a Protection against TNFalpha in NIH-3T3-Ras Cells. Biochem. Biophys. Res. Commun. 1997, 241, 187–192. [Google Scholar] [CrossRef]
- Baldwin, A.J.; Hilton, G.R.; Lioe, H.; Bagnéris, C.; Benesch, J.L.P.; Kay, L.E. Quaternary Dynamics of AB-Crystallin as a Direct Consequence of Localised Tertiary Fluctuations in the C-Terminus. J. Mol. Biol. 2011, 413, 310–320. [Google Scholar] [CrossRef]
- Delbecq, S.P.; Klevit, R.E. One Size Does Not Fit All: The Oligomeric States of AB Crystallin. FEBS Lett. 2013, 587, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- McDonald, E.T.; Bortolus, M.; Koteiche, H.A.; Mchaourab, H.S. Sequence, Structure, and Dynamic Determinants of Hsp27 (HspB1) Equilibrium Dissociation Are Encoded by the N-Terminal Domain. Biochemistry 2012, 51, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- Lelj-Garolla, B.; Mauk, A.G. Roles of the N- and C-Terminal Sequences in Hsp27 Self-Association and Chaperone Activity. Protein Sci. 2011, 21, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thériault, J.R.; Lambert, H.; Chávez-Zobel, A.T.; Charest, G.; Lavigne, P.; Landry, J. Essential Role of the NH2-Terminal WD/EPF Motif in the Phosphorylation-Activated Protective Function of Mammalian Hsp27. J. Biol. Chem. 2004, 279, 23463–23471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsogiannou, M.; Andrieu, C.; Rocchi, P. Heat Shock Protein 27 Phosphorylation State Is Associated with Cancer Progression. Front. Genet. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lentze, N.; Narberhaus, F. Detection of Oligomerisation and Substrate Recognition Sites of Small Heat Shock Proteins by Peptide Arrays. Biochem. Biophys. Res. Commun. 2004, 325, 401–407. [Google Scholar] [CrossRef]
- Hayes, D.; Napoli, V.; Mazurkie, A.; Stafford, W.F.; Graceffa, P. Phosphorylation Dependence of Hsp27 Multimeric Size and Molecular Chaperone Function. J. Biol. Chem. 2009, 284, 18801–18807. [Google Scholar] [CrossRef] [Green Version]
- Charette, S.J.; Landry, J. The Interaction of HSP27 with Daxx Identifies a Potential Regulatory Role of HSP27 in Fas-Induced Apoptosis. Ann. N. Y. Acad. Sci. 2006, 926, 126–131. [Google Scholar] [CrossRef]
- Lambert, H.; Charette, S.J.; Bernier, A.F.; Guimond, A.; Landry, J. HSP27 Multimerization Mediated by Phosphorylation-Sensitive Intermolecular Interactions at the Amino Terminus. J. Biol. Chem. 1999, 274, 9378–9385. [Google Scholar] [CrossRef] [Green Version]
- Gusev, N.B.; Bogatcheva, N.V.; Marston, S.B. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochem. (Mosc.) 2002, 67, 511–519. [Google Scholar] [CrossRef]
- Kostenko, S.; Moens, U. Heat Shock Protein 27 Phosphorylation: Kinases, Phosphatases, Functions and Pathology. Cell. Mol. Life Sci. 2009, 66, 3289–3307. [Google Scholar] [CrossRef]
- Matsushima-Nishiwaki, R.; Takai, S.; Adachi, S.; Minamitani, C.; Yasuda, E.; Noda, T.; Kato, K.; Toyoda, H.; Kaneoka, Y.; Yamaguchi, A.; et al. Phosphorylated Heat Shock Protein 27 Represses Growth of Hepatocellular Carcinoma via Inhibition of Extracellular Signal-Regulated Kinase. J. Biol. Chem. 2008, 283, 18852–18860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatakrishnan, C.D.; Dunsmore, K.; Wong, H.; Roy, S.; Sen, C.K.; Wani, A.; Zweier, J.L.; Ilangovan, G. HSP27 Regulates P53 Transcriptional Activity in Doxorubicin-Treated Fibroblasts and Cardiac H9c2 Cells: P21 Upregulation and G2/M Phase Cell Cycle Arrest. American Journal of Physiology. Heart Circ. Physiol. 2008, 294, H1736–H1744. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Simon, S.; Gibert, B.; Virot, S.; Manero, F.; Arrigo, A.-P. Dynamic Processes That Reflect Anti-Apoptotic Strategies Set up by HspB1 (Hsp27). Exp. Cell Res. 2010, 316, 1535–1552. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Huang, C.-J.; Lee, Y.-J.; Tien, L.-T.; Ku, W.-C.; Chien, R.; Lee, F.-K.; Chien, C.-C. Knocking down of Heat-Shock Protein 27 Directs Differentiation of Functional Glutamatergic Neurons from Placenta-Derived Multipotent Cells. Sci. Rep. 2016, 6, 30314. [Google Scholar] [CrossRef]
- Heinrich, J.C.; Donakonda, S.; Haupt, V.J.; Lennig, P.; Zhang, Y.; Schroeder, M. New HSP27 Inhibitors Efficiently Suppress Drug Resistance Development in Cancer Cells. Oncotarget 2016, 7, 68156. [Google Scholar] [CrossRef] [Green Version]
- Jovcevski, B.; Kelly, M.A.; Rote, A.P.; Berg, T.; Gastall, H.Y.; Benesch, J.L.; Aquilina, J.A.; Ecroyd, H. Phosphomimics Destabilize Hsp27 Oligomeric Assemblies and Enhance Chaperone Activity. Chem. Biol. 2015, 22, 186–195. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Vartholomatos, G.; Stefanaki, K.; Patereli, A.; Dova, L.; Karamoutsios, A.; Lallas, G.; Sfakianos, G.; Moschovi, M.; Prodromou, N. Expression of heat shock proteins in medulloblastoma. J. Neurosurg. Pediatr. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, X.; Jin, G.; Jin, H.; Wang, N.; Hu, F.; Luo, Q.; Shu, H.; Zhao, F.; Yao, M.; et al. A Targetable Molecular Chaperone Hsp27 Confers Aggressiveness in Hepatocellular Carcinoma. Theranostics 2016, 6, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Alexiou, G.A.; Karamoutsios, A.; Lallas, G.; Ragos, V.; Goussia, A.; Kyritsis, A.P.; Voulgaris, S.; Vartholomatos, G. Expression of heat shock proteins in brain tumors. Turk. Neurosurg. 2014, 24, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, W.; Fan, J.; Lai, Y.; Che, G. Clinicopathological and Prognostic Significance of Heat Shock Protein 27 (HSP27) Expression in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. SpringerPlus 2016, 5, 1165. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.A.; Crafa, P.; Desenzani, S.; Graiani, G.; Lagrasta, C.; Sianesi, M.; Soliani, P.; Borghetti, A.F. The Expression of HSP27 Is Associated with Poor Clinical Outcome in Intrahepatic Cholangiocarcinoma. BMC Cancer 2007, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zhi, J.; Peng, X.; Zhong, X.; Xu, A. Clinical Significance of HSP27 Expression in Colorectal Cancer. Mol. Med. Rep. 2010, 3, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Lo Muzio, L.; Campisi, G.; Farina, A.; Rubini, C.; Ferrari, F.; Falaschini, S.; Leonardi, R.; Carinci, F.; Stalbano, S.; De Rosa, G. Prognostic Value of HSP27 in Head and Neck Squamous Cell Carcinoma: A Retrospective Analysis of 57 Tumours. Anticancer Res. 2006, 26, 1343–1349. [Google Scholar] [PubMed]
- Liang, C.; Xu, Y.; Ge, H.; Li, G.; Wu, J. The Clinicopathological and Prognostic Value of HSP27 in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. OncoTargets Ther. 2018, 11, 1293–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahid, S.; Thaper, D.; Gibson, K.F.; Bishop, J.L.; Zoubeidi, A. Molecular Chaperone Hsp27 Regulates the Hippo Tumor Suppressor Pathway in Cancer. Sci. Rep. 2016, 6, 31842. [Google Scholar] [CrossRef] [PubMed]
- Maugeri-Saccà, M.; De Maria, R. The Hippo Pathway in Normal Development and Cancer. Pharmacol. Ther. 2018, 186, 60–72. [Google Scholar] [CrossRef]
- Harvey, K.F.; Zhang, X.; Thomas, D.M. The Hippo Pathway and Human Cancer. Nat. Rev. Cancer 2013, 13, 246–257. [Google Scholar] [CrossRef]
- Zhao, M.; Mishra, L.; Deng, C.-X. The Role of TGF-β/SMAD4 Signaling in Cancer. Int. J. Biol. Sci. 2018, 14, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Hannigan, G.; Troussard, A.A.; Dedhar, S. Integrin-Linked Kinase: A Cancer Therapeutic Target Unique among Its ILK. Nat. Rev. Cancer 2005, 5, 51–63. [Google Scholar] [CrossRef]
- Shiota, M.; Bishop, J.L.; Nip, K.M.; Zardan, A.; Takeuchi, A.; Cordonnier, T.; Beraldi, E.; Bazov, J.; Fazli, L.; Chi, K.; et al. Hsp27 Regulates Epithelial Mesenchymal Transition, Metastasis, and Circulating Tumor Cells in Prostate Cancer. Cancer Res. 2013, 73, 3109–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordonnier, T.; Bishop, J.L.; Shiota, M.; Nip, K.M.; Thaper, D.; Vahid, S.; Heroux, D.; Gleave, M.; Zoubeidi, A. Hsp27 Regulates EGF/β-Catenin Mediated Epithelial to Mesenchymal Transition in Prostate Cancer. Int. J. Cancer 2014, 136, E496–E507. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Yang, J.; Tian, B.; Sun, H.; Kalita, M.; Ju, H.; Paulucci-Holthauzen, A.; Zhao, Y.; Brasier, A.R.; Sadygov, R.G. Mixed-Effects Model of Epithelial-Mesenchymal Transition Reveals Rewiring of Signaling Networks. Cell. Signal. 2015, 27, 1413–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, D.M.; Medici, D. Signaling Mechanisms of the Epithelial-Mesenchymal Transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yun, F.; Shi, L.; Li, Z.-H.; Luo, N.-R.; Jia, Y.-F. Roles of Signaling Pathways in the Epithelial-Mesenchymal Transition in Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 6201–6206. [Google Scholar] [CrossRef] [Green Version]
- Roche, J. The Epithelial-To-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in Cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Yao, K.; He, L.; Gan, Y.; Liu, J.; Tang, J.; Long, Z.; Tan, J. HMGN5 Promotes IL-6-Induced Epithelial-Mesenchymal Transition of Bladder Cancer by Interacting with Hsp27. Aging 2020, 12, 7282–7298. [Google Scholar] [CrossRef]
- Zheng, M.; Jiang, Y.; Chen, W.; Li, K.; Liu, X.; Gao, S.; Feng, H.; Wang, S.; Jiang, J.; Ma, X.; et al. Snail and Slug Collaborate on EMT and Tumor Metastasis through MiR-101-Mediated EZH2 Axis in Oral Tongue Squamous Cell Carcinoma. Oncotarget 2015, 6, 6794–6810. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, H.; Okano, T.; Minegishi, Y.; Matsuda, K.; Sudoh, J.; Kitamura, K.; Noro, R.; Soeno, C.; Yoshimura, A.; Seike, M.; et al. HSP27 Modulates Epithelial to Mesenchymal Transition of Lung Cancer Cells in a Smad-Independent Manner. Oncol. Lett. 2010, 1, 1011–1016. [Google Scholar] [CrossRef] [Green Version]
- Wettstein, G.; Bellaye, P.-S.; Kolb, M.; Hammann, A.; Crestani, B.; Soler, P.; Marchal-Somme, J.; Hazoume, A.; Gauldie, J.; Gunther, A.; et al. Inhibition of HSP27 Blocks Fibrosis Development and EMT Features by Promoting Snail Degradation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ren, X.; Wu, J.; Gao, X.; Cen, X.; Wang, S.; Sheng, S.; Chen, Q.; Tang, Y.-J.; Liang, X.-H.; et al. HSP27 Associates with Epithelial-Mesenchymal Transition, Stemness and Radioresistance of Salivary Adenoid Cystic Carcinoma. J. Cell. Mol. Med. 2018, 22, 2283–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Qian, J.; Li, X.; Chen, W.; Xu, A.; Zhao, K.; Hua, Y.; Huang, Z.; Zhang, J.; Liang, C.; et al. Long Noncoding RNA BX357664 Regulates Cell Proliferation and Epithelial-To-Mesenchymal Transition via Inhibition of TGF-β1/P38/HSP27 Signaling in Renal Cell Carcinoma. Oncotarget 2016, 7, 81410–81422. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liang, W.; Luo, L. HSP27 Promotes Epithelial-Mesenchymal Transition through Activation of the β-Catenin/MMP3 Pathway in Pancreatic Ductal Adenocarcinoma Cells. Transl. Cancer Res. 2019, 8, 1268–1278. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Qian, Y.; Dai, X.; Yang, L.; Chen, J.; Guo, S.; Hisamitsu, T. Research on the Efficacy of Celastrus Orbiculatus in Suppressing TGF-β1-Induced Epithelial-Mesenchymal Transition by Inhibiting HSP27 and TNF-α-Induced NF-κ B/Snail Signaling Pathway in Human Gastric Adenocarcinoma. BMC Complement. Altern. Med. 2014, 14, 433. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chou, K.; Hsu, J.; Lin, J.; Hsu, T.; Yen, D.H.; Hung, S.; Hsu, H. High Metabolic Rate and Stem Cell Characteristics of Esophageal Cancer Stem-like Cells Depend on the Hsp27–AKT–HK2 Pathway. Int. J. Cancer 2019, 145, 2144–2156. [Google Scholar] [CrossRef]
- Tan, B.T.; Park, C.Y.; Ailles, L.E.; Weissman, I.L. The Cancer Stem Cell Hypothesis: A Work in Progress. Lab. Investig. 2006, 86, 1203–1207. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, J.D.; Barr, M.; Fennell, D.; Richard, D.; Reynolds, J.; O’Leary, J.; O’Byrne, K. The Cancer Stem-Cell Hypothesis: Its Emerging Role in Lung Cancer Biology and Its Relevance for Future Therapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2012, 7, 1880–1890. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-P.; Lee, Y.-T.; Wang, J.-Y.; Miller, S.A.; Chiou, S.-H.; Hung, M.-C.; Hung, S.-C. Survival of Cancer Stem Cells under Hypoxia and Serum Depletion via Decrease in PP2A Activity and Activation of P38-MAPKAPK2-Hsp27. PLoS ONE 2012, 7, e49605. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-P.; Lee, Y.-T.; Yang, S.-H.; Miller, S.A.; Chiou, S.-H.; Hung, M.-C.; Hung, S.-C. Colon Cancer Stem Cells Resist Antiangiogenesis Therapy-Induced Apoptosis. Cancer Lett. 2013, 328, 226–234. [Google Scholar] [CrossRef]
- Thuringer, D.; Jego, G.; Wettstein, G.; Terrier, O.; Cronier, L.; Yousfi, N.; Hébrard, S.; Bouchot, A.; Hazoumé, A.; Joly, A.-L.; et al. Extracellular HSP27 Mediates Angiogenesis through Toll-like Receptor 3. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 4169–4183. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef] [PubMed]
- Straume, O.; Shimamura, T.; Lampa, M.J.G.; Carretero, J.; Oyan, A.M.; Jia, D.; Borgman, C.L.; Soucheray, M.; Downing, S.R.; Short, S.M.; et al. Suppression of Heat Shock Protein 27 Induces Long-Term Dormancy in Human Breast Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 8699–8704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat Shock Proteins 27 and 70: Anti-Apoptotic Proteins with Tumorigenic Properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrigo, A.-P. The Cellular “Networking” of Mammalian Hsp27 and Its Functions in the Control of Protein Folding, Redox State and Apoptosis. Adv. Exp. Med. Biol. 2007, 594, 14–26. [Google Scholar] [CrossRef]
- Havasi, A.; Li, Z.; Wang, Z.; Martin, J.L.; Botla, V.; Ruchalski, K.; Schwartz, J.H.; Borkan, S.C. Hsp27 Inhibits Bax Activation and Apoptosis via a Phosphatidylinositol 3-Kinase-Dependent Mechanism. J. Biol. Chem. 2008, 283, 12305–12313. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, D.; Li, G.; Hideshima, T.; Podar, K.; Mitsiades, C.; Mitsiades, N.; Catley, L.; Tai, Y.T.; Hayashi, T.; Shringarpure, R.; et al. Hsp27 Inhibits Release of Mitochondrial Protein Smac in Multiple Myeloma Cells and Confers Dexamethasone Resistance. Blood 2003, 102, 3379–3386. [Google Scholar] [CrossRef]
- Schmitt, E.; Gehrmann, M.; Brunet, M.; Multhoff, G.; Garrido, C. Intracellular and Extracellular Functions of Heat Shock Proteins: Repercussions in Cancer Therapy. J. Leukoc. Biol. 2007, 81, 15–27. [Google Scholar] [CrossRef]
- Voss, O.H.; Batra, S.; Kolattukudy, S.J.; Gonzalez-Mejia, M.E.; Smith, J.B.; Doseff, A.I. Binding of Caspase-3 Prodomain to Heat Shock Protein 27 Regulates Monocyte Apoptosis by Inhibiting Caspase-3 Proteolytic Activation. J. Biol. Chem. 2007, 282, 25088–25099. [Google Scholar] [CrossRef] [Green Version]
- Acunzo, J.; Katsogiannou, M.; Rocchi, P. Small Heat Shock Proteins HSP27 (HspB1), AB-Crystallin (HspB5) and HSP22 (HspB8) as Regulators of Cell Death. Int. J. Biochem. Cell Biol. 2012, 44, 1622–1631. [Google Scholar] [CrossRef]
- WANG, X.; CHEN, M.; ZHOU, J.; ZHANG, X. HSP27, 70 and 90, Anti-Apoptotic Proteins, in Clinical Cancer Therapy. Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Kabakov, A.; Yakimova, A.; Matchuk, O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.-B.; Huang, G.-X.; Lu, J.-J.; Ma, J.; Yuan, Q.-J.; Cao, Y.; Zhu, L. Up-Regulation of Heat Shock Protein 27 Inhibits Apoptosis in Lumbosacral Nerve Root Avulsion-Induced Neurons. Sci. Rep. 2019, 9, 11468. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Fuqua, S.A.; Lock-Lim, S.; Toft, D.O.; Welch, W.J.; McGuire, W.L. Response of Human Breast Cancer Cells to Heat Shock and Chemotherapeutic Drugs. Cancer Res. 1992, 52, 3648–3654. [Google Scholar]
- Oesterreich, S.; Weng, C.N.; Qiu, M.; Hilsenbeck, S.G.; Osborne, C.K.; Fuqua, S.A. The Small Heat Shock Protein Hsp27 Is Correlated with Growth and Drug Resistance in Human Breast Cancer Cell Lines. Cancer Res. 1993, 53, 4443–4448. [Google Scholar]
- Huot, J.; Roy, G.; Lambert, H.; Chrétien, P.; Landry, J. Increased Survival after Treatments with Anticancer Agents of Chinese Hamster Cells Expressing the Human Mr 27,000 Heat Shock Protein. Cancer Res. 1991, 51, 5245–5252. [Google Scholar]
- Garrido, C.; Mehlen, P.; Fromentin, A.; Hammann, A.; Assem, M.; Arrigo, A.-P.; Chauffert, B. Inconstant Association between 27-KDa Heat-Shock Protein (Hsp27) Content and Doxorubicin Resistance in Human Colon Cancer Cells. The Doxorubicin-Protecting Effect of Hsp27. Eur. J. Biochem. 1996, 237, 653–659. [Google Scholar] [CrossRef]
- Hansen, R.K.; Parra, I.; Lemieux, P.; Oesterreich, S.; Hilsenbeck, S.G.; Fuqua, S.A.W. Hsp27 Overexpression Inhibits Doxorubicin–Induced Apoptosis in Human Breast Cancer Cells. Breast Cancer Res. Treat. 1999, 56, 185–194. [Google Scholar] [CrossRef]
- Kwon, Y.; Shin, B.S.; Chung, I.K. The P53 Tumor Suppressor Stimulates the Catalytic Activity of Human Topoisomerase IIalpha by Enhancing the Rate of ATP Hydrolysis. J. Biol. Chem. 2000, 275, 18503–18510. [Google Scholar] [CrossRef] [Green Version]
- Stros, M.; Bacikova, A.; Polanska, E.; Stokrova, J.; Strauss, F. HMGB1 Interacts with Human Topoisomerase II and Stimulates Its Catalytic Activity. Nucleic Acids Res. 2007, 35, 5001–5013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.; Wang, M.; Jiang, L.; Liu, H.; Sun, J. Paclitaxel-Doxorubicin Sequence Is More Effective in Breast Cancer Cells with Heat Shock Protein 27 Overexpression. Chin. Med. J. 2008, 121, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Fujiwara, K.; Tanaka, H.; Maehata, K.; Kohno, I. Paclitaxel Inhibits Expression of Heat Shock Protein 27 in Ovarian and Uterine Cancer Cells. Int. J. Gynecol. Cancer 2004, 14, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.T.; Ganguly, S.S.; Bennett, H.; Friend, J.W.; Tepe, J.; Plattner, R. Imatinib Reverses Doxorubicin Resistance by Affecting Activation of STAT3-Dependent NF-ΚB and HSP27/P38/AKT Pathways and by Inhibiting ABCB1. PLoS ONE 2013, 8, e55509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan-Sunol, C.; Gabai, V.L.; Sherman, M.Y. Hsp27 Modulates P53 Signaling and Suppresses Cellular Senescence. Cancer Res. 2007, 67, 11779–11788. [Google Scholar] [CrossRef] [Green Version]
- Ramani, S.; Park, S. HSP27 Role in Cardioprotection by Modulating Chemotherapeutic Doxorubicin-Induced Cell Death. J. Mol. Med. 2021, 99, 771–784. [Google Scholar] [CrossRef]
- Zhang, D.; Putti, T.C. Over-Expression of ERp29 Attenuates Doxorubicin-Induced Cell Apoptosis through Up-Regulation of Hsp27 in Breast Cancer Cells. Exp. Cell Res. 2010, 316, 3522–3531. [Google Scholar] [CrossRef]
- Cnop, M.; Toivonen, S.; Igoillo-Esteve, M.; Salpea, P. Endoplasmic Reticulum Stress and EIF2α Phosphorylation: The Achilles Heel of Pancreatic β Cells. Mol. Metab. 2017, 6, 1024–1039. [Google Scholar] [CrossRef]
- Andrieu, C.; Taieb, D.; Baylot, V.; Ettinger, S.; Soubeyran, P.; De-Thonel, A.; Nelson, C.; Garrido, C.; So, A.; Fazli, L.; et al. Heat Shock Protein 27 Confers Resistance to Androgen Ablation and Chemotherapy in Prostate Cancer Cells through EIF4E. Oncogene 2010, 29, 1883–1896. [Google Scholar] [CrossRef]
- Kanagasabai, R.; Krishnamurthy, K.; Druhan, L.J.; Ilangovan, G. Forced Expression of Heat Shock Protein 27 (Hsp27) Reverses P-Glycoprotein (ABCB1)-Mediated Drug Efflux AndMDR1Gene Expression in Adriamycin-Resistant Human Breast Cancer Cells. J. Biol. Chem. 2011, 286, 33289–33300. [Google Scholar] [CrossRef] [Green Version]
- Mahvi, D.M.; Carper, S.W.; Yu, C.O.; McCausland, T.A.; Kristian Storm, F. Toremifene, a Novel Antiestrogen, Can Overcome Hsp27-Induced Drug Resistance in Human Breast Cancer Cells. Endocrine 1996, 4, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kang, K.W.; Kim, K.-H.; Kwon, B.; Kim, S.-K.; Lee, H.-Y.; Kong, S.-Y.; Lee, E.S.; Jang, S.-G.; Yoo, B.C. Upregulated HSP27 in Human Breast Cancer Cells Reduces Herceptin Susceptibility by Increasing Her2 Protein Stability. BMC Cancer 2008, 8, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.-Y.; Choi, S.-K.; Seo, S.H.; Jo, H.; Shin, J.-H.; Na, Y.; Lee, Y.-S.; Kwon, Y. Specific Roles of HSP27 S15 Phosphorylation Augmenting the Nuclear Function of HER2 to Promote Trastuzumab Resistance. Cancers 2020, 12, 1540. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Jalili-Nik, M.; Avan, A.; Ferns, G.A.; Khazaei, M.; Hassanian, S.M. The Role of HSP27 in the Development of Drug Resistance of Gastrointestinal Malignancies: Current Status and Perspectives. J. Cell. Physiol. 2018, 234, 8241–8248. [Google Scholar] [CrossRef]
- Mori-Iwamoto, S.; Kuramitsu, Y.; Ryozawa, S.; Mikuria, K.; Fujimoto, M.; Maehara, S.-I.; Maehara, Y.; Okita, K.; Nakamura, K.; Sakaida, I. Proteomics Finding Heat Shock Protein 27 as a Biomarker for Resistance of Pancreatic Cancer Cells to Gemcitabine. Int. J. Oncol. 2007, 31, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhang, X.; Huang, S.; Chen, M.; Shen, S.; Ding, X.; Lv, Y.; Zou, X. The Effects of HSP27 on Gemcitabine-Resistant Pancreatic Cancer Cell Line through Snail. Pancreas 2015, 44, 1121–1129. [Google Scholar] [CrossRef]
- Ye, W.; Li, Z.; Tang, T.; Du, J.; Zhou, X.; Wu, H.; Li, X.; Qin, G. ERp29 Downregulation Enhances Lung Adenocarcinoma Cell Chemosensitivity to Gemcitabine by Upregulating HSP27 Phosphorylation. Exp. Ther. Med. 2019, 17, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Suenaga, S.; Kuramitsu, Y.; Kaino, S.; Maehara, S.-I.; Maehara, Y.; Sakaida, I.; Nakamura, K. Active Hexose-Correlated Compound Down-Regulates HSP27 of Pancreatic Cancer Cells, and Helps the Cytotoxic Effect of Gemcitabine. Anticancer Res. 2014, 34, 141–146. [Google Scholar]
- Kuramitsu, Y.; Wang, Y.; Taba, K.; Suenaga, S.; Ryozawa, S.; Kaino, S.; Sakaida, I.; Nakamura, K. Heat-Shock Protein 27 Plays the Key Role in Gemcitabine-Resistance of Pancreatic Cancer Cells. Anticancer Res. 2012, 32, 2295–2299. [Google Scholar]
- Taba, K.; Kuramitsu, Y.; Ryozawa, S.; Yoshida, K.; Tanaka, T.; Maehara, S.-I.; Maehara, Y.; Sakaida, I.; Nakamura, K. Heat-Shock Protein 27 Is Phosphorylated in Gemcitabine-Resistant Pancreatic Cancer Cells. Anticancer Res. 2010, 30, 2539–2543. [Google Scholar]
- Tokunaga, M.; Baron, B.; Kitagawa, T.; Tokuda, K.; Kuramitsu, Y. Active Hexose-Correlated Compound Down-Regulates Heat Shock Factor 1, a Transcription Factor for HSP27, in Gemcitabine-Resistant Human Pancreatic Cancer Cells. Anticancer Res. 2015, 35, 6063–6067. [Google Scholar] [PubMed]
- Kawano, M.; Kaino, S.; Amano, S.; Shinoda, S.; Suenaga, S.; Sen-Yo, M.; Sakaida, I. Heat Shock Protein 27 Expression in EUS-FNA Samples Can Predict Gemcitabine Sensitivity in Pancreatic Cancer. In Vivo 2018, 32, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Choi, H.J.; Kang, S.; Kim, S.Y.; Hwang, Y.; Je, S.; Han, Z.; Kim, J.-H.; Song, J.J. Ratio of Phosphorylated HSP27 to Nonphosphorylated HSP27 Biphasically Acts as a Determinant of Cellular Fate in Gemcitabine-Resistant Pancreatic Cancer Cells. Cell. Signal. 2015, 27, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Kawaguchi, J.; Itani, M.; Yoshioka, T.; Matsushima-Nishiwaki, R.; Hirose, Y.; Kozawa, O.; et al. Phosphorylation Status of Heat Shock Protein 27 Plays a Key Role in Gemcitabine-Induced Apoptosis of Pancreatic Cancer Cells. Cancer Lett. 2011, 313, 218–225. [Google Scholar] [CrossRef]
- de Laat, W.L.; Jaspers, N.G.; Hoeijmakers, J.H. Molecular Mechanism of Nucleotide Excision Repair. Genes Dev. 1999, 13, 768–785. [Google Scholar] [CrossRef] [Green Version]
- Neizer-Ashun, F.; Bhattacharya, R. Reality CHEK: Understanding the Biology and Clinical Potential of CHK1. Cancer Lett. 2021, 497, 202–211. [Google Scholar] [CrossRef]
- Baylot, V.; Andrieu, C.; Katsogiannou, M.; Taieb, D.; Garcia, S.; Giusiano, S.; Acunzo, J.; Iovanna, J.; Gleave, M.; Garrido, C.; et al. OGX-427 Inhibits Tumor Progression and Enhances Gemcitabine Chemotherapy in Pancreatic Cancer. Cell Death Dis. 2011, 2, e221. [Google Scholar] [CrossRef] [Green Version]
- Mori-Iwamoto, S.; Taba, K.; Kuramitsu, Y.; Ryozawa, S.; Tanaka, T.; Maehara, S.; Maehara, Y.; Okita, K.; Nakamura, K.; Sakaida, I. Interferon-γ Down-Regulates Heat Shock Protein 27 of Pancreatic Cancer Cells and Helps in the Cytotoxic Effect of Gemcitabine. Pancreas 2009, 38, 224–226. [Google Scholar] [CrossRef]
- Taba, K.; Kuramitsu, Y.; Ryozawa, S.; Yoshida, K.; Tanaka, T.; Mori-Iwamoto, S.; Maehara, S.; Maehara, Y.; Sakaida, I.; Nakamura, K. KNK437 Downregulates Heat Shock Protein 27 of Pancreatic Cancer Cells and Enhances the Cytotoxic Effect of Gemcitabine. Chemotherapy 2011, 57, 12–16. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Wu, S.; Li, S.; Le, L.; Su, X.; Qiu, P.; Hu, H.; Yan, G. Triptolide Cooperates with Cisplatin to Induce Apoptosis in Gemcitabine-Resistant Pancreatic Cancer. Pancreas 2012, 41, 1029–1038. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Long, Y.; Liu, B.; Wang, X. Role of HSP27 in the Multidrug Sensitivity and Resistance of Colon Cancer Cells. Oncol. Lett. 2020, 19, 2021–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.-X.; Dai, M.-S.; Lu, H. 5-Fluorouracil Activation of P53 Involves an MDM2-Ribosomal Protein Interaction. J. Biol. Chem. 2007, 282, 8052–8059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Bao, Y.; Yang, G.-K.; Wan, J.; Du, L.-J.; Ma, Z.-H. MiR-214 Sensitizes Human Colon Cancer Cells to 5-FU by Targeting Hsp27. Cell. Mol. Biol. Lett. 2019, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ju, H.; Zhang, L.; Lu, H.; Jie, K. MicroRNA-577 Suppresses Tumor Growth and Enhances Chemosensitivity in Colorectal Cancer. J. Biochem. Mol. Toxicol. 2017, 31, e21888. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Upadhyay, A.K.; Bhat, M.K. Inhibition of Hsp27 and Hsp40 Potentiates 5-Fluorouracil and Carboplatin Mediated Cell Killing in Hepatoma Cells. Cancer Biol. Ther. 2009, 8, 2106–2113. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; Gander, M.; Leyvraz, S.; Newlands, E. Current and Future Developments in the Use of Temozolomide for the Treatment of Brain Tumours. Lancet Oncol. 2001, 2, 552–560. [Google Scholar] [CrossRef]
- Sang, D.; Li, R.; Lan, Q. Quercetin Sensitizes Human Glioblastoma Cells to Temozolomide in Vitro via Inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-F.; Nieh, S.; Jao, S.-W.; Liu, C.-L.; Wu, C.-H.; Chang, Y.-C.; Yang, C.-Y.; Lin, Y.-S. Quercetin Suppresses Drug-Resistant Spheres via the P38 MAPK–Hsp27 Apoptotic Pathway in Oral Cancer Cells. PLoS ONE 2012, 7, e49275. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Dong, X.-S.; Gao, H.-Y.; Jiang, Y.-F.; Jin, Y.-L.; Chang, Y.-Y.; Chen, L.-Y.; Wang, J.-H. Suppression of HSP27 Increases the Anti-Tumor Effects of Quercetin in Human Leukemia U937 Cells. Mol. Med. Rep. 2016, 13, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin Sensitizes Glioblastoma to T-AUCB by Dual Inhibition of Hsp27 and COX-2 in Vitro and in Vivo. J. Exp. Clin. Cancer Res. 2016, 35, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Tang, C.; Li, L.; Li, R.; Fan, Y. Quercetin Blocks T-AUCB-Induced Autophagy by Hsp27 and Atg7 Inhibition in Glioblastoma Cells in Vitro. J. Neuro-Oncol. 2016, 129, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Apoptosis Induction in Human Glioblastoma Multiforme T98G Cells upon Temozolomide and Quercetin Treatment. Tumor Biol. 2013, 34, 2367–2378. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Silencing of Hsp27 and Hsp72 in Glioma Cells as a Tool for Programmed Cell Death Induction upon Temozolomide and Quercetin Treatment. Toxicol. Appl. Pharmacol. 2013, 273, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Sumorek-Wiadro, J.; Langner, E.; Wertel, I.; Maciejczyk, A.; Pawlikowska-Pawlęga, B.; Pawelec, J.; Wasiak, M.; Hułas-Stasiak, M.; Bądziul, D.; et al. Involvement of PI3K Pathway in Glioma Cell Resistance to Temozolomide Treatment. Int. J. Mol. Sci. 2021, 22, 5155. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Sun, C.; Zhou, T.; Zhou, B.; Guo, E.; Shan, W.; Xia, M.; Li, K.; Weng, D.; Meng, L.; et al. HSP27 Knockdown Increases Cytoplasmic P21 and Cisplatin Sensitivity in Ovarian Carcinoma Cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 23, 119–128. [Google Scholar] [CrossRef]
- Song, T.; Zhang, Z.; Liu, L.; Yang, T.; Jiang, J.; Li, P. Small Interfering RNA-Mediated Silencing of Heat Shock Protein 27 (HSP27) Increases Chemosensitivity to Paclitaxel by Increasing Production of Reactive Oxygen Species in Human Ovarian Cancer Cells (HO8910). J. Int. Med. Res. 2009, 37, 1375–1388. [Google Scholar] [CrossRef]
- Kamada, M.; So, A.; Muramaki, M.; Rocchi, P.; Beraldi, E.; Gleave, M. Hsp27 Knockdown Using Nucleotide-Based Therapies Inhibit Tumor Growth and Enhance Chemotherapy in Human Bladder Cancer Cells. Mol. Cancer Ther. 2007, 6, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Stope, M.B.; Weiss, M.; Preuss, M.; Streitbörger, A.; Ritter, C.A.; Zimmermann, U.; Walther, R.; Burchardt, M. Immediate and Transient Phosphorylation of the Heat Shock Protein 27 Initiates Chemoresistance in Prostate Cancer Cells. Oncol. Rep. 2014, 32, 2380–2386. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, J.-C.; Tuukkanen, A.; Schroeder, M.; Fahrig, T.; Fahrig, R. RP101 (Brivudine) Binds to Heat Shock Protein HSP27 (HSPB1) and Enhances Survival in Animals and Pancreatic Cancer Patients. J. Cancer Res. Clin. Oncol. 2011, 137, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Nappi, L.; Aguda, A.H.; Nakouzi, N.A.; Lelj-Garolla, B.; Beraldi, E.; Lallous, N.; Thi, M.; Moore, S.; Fazli, L.; Battsogt, D.; et al. Ivermectin Inhibits HSP27 and Potentiates Efficacy of Oncogene Targeting in Tumor Models. J. Clin. Investig. 2020, 130, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Yu, E.Y.; Jacobs, C.; Bazov, J.; Kollmannsberger, C.; Higano, C.S.; Mukherjee, S.D.; Gleave, M.E.; Stewart, P.S.; Hotte, S.J. A Phase I Dose-Escalation Study of Apatorsen (OGX-427), an Antisense Inhibitor Targeting Heat Shock Protein 27 (Hsp27), in Patients with Castration-Resistant Prostate Cancer and Other Advanced Cancers. Ann. Oncol. 2016, 27, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Hahn, N.M.; Regan, M.M.; Werner, L.; Alva, A.; George, S.; Picus, J.; Alter, R.; Balar, A.; Hoffman-Censits, J.; et al. Apatorsen plus Docetaxel versus Docetaxel Alone in Platinum-Resistant Metastatic Urothelial Carcinoma (Borealis-2). Br. J. Cancer 2018, 118, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Shipley, D.L.; Waterhouse, D.M.; Jones, S.F.; Ward, P.J.; Shih, K.C.; Hemphill, B.; McCleod, M.; Whorf, R.C.; Page, R.D.; et al. A Randomized, Double-Blinded, Phase II Trial of Carboplatin and Pemetrexed with or without Apatorsen (OGX-427) in Patients with Previously Untreated Stage IV Non-Squamous-Non-Small-Cell Lung Cancer: The SPRUCE Trial. Oncologist 2019, 24, e1409–e1416. [Google Scholar] [CrossRef] [Green Version]
- Ko, A.H.; Murphy, P.B.; Peyton, J.D.; Shipley, D.L.; Al-Hazzouri, A.; Rodriguez, F.A.; Womack, M.S.; Xiong, H.Q.; Waterhouse, D.M.; Tempero, M.A.; et al. A Randomized, Double-Blinded, Phase II Trial of Gemcitabine and Nab-Paclitaxel plus Apatorsen or Placebo in Patients with Metastatic Pancreatic Cancer: The RAINIER Trial. Oncologist 2017, 22, 1427-e129. [Google Scholar] [CrossRef] [Green Version]
- Sheng, B.; Qi, C.; Liu, B.; Lin, Y.; Fu, T.; Zeng, Q. Increased HSP27 Correlates with Malignant Biological Behavior of Non-Small Cell Lung Cancer and Predicts Patient’s Survival. Sci. Rep. 2017, 7, 13807. [Google Scholar] [CrossRef]
- Tweedle, E.M.; Khattak, I.; Ang, C.W.; Nedjadi, T.; Jenkins, R.; Park, B.K.; Kalirai, H.; Dodson, A.; Azadeh, B.; Terlizzo, M.; et al. Low Molecular Weight Heat Shock Protein HSP27 Is a Prognostic Indicator in Rectal Cancer but Not Colon Cancer. Gut 2010, 59, 1501–1510. [Google Scholar] [CrossRef]
- Zoubeidi, A.; Gleave, M. Small Heat Shock Proteins in Cancer Therapy and Prognosis. Int. J. Biochem. Cell Biol. 2012, 44, 1646–1656. [Google Scholar] [CrossRef]
- Zanini, C.; Pulerà, F.; Carta, F.; Giribaldi, G.; Mandili, G.; Maule, M.M.; Forni, M.; Turrini, F. Proteomic Identification of Heat Shock Protein 27 as a Differentiation and Prognostic Marker in Neuroblastoma but Not in Ewing’s Sarcoma. Virchows Arch. Int. J. Pathol. 2008, 452, 157–167. [Google Scholar] [CrossRef]



| Sensitizer | Chemotherapy Agent | Cancer Type | Reference |
|---|---|---|---|
| Paclitaxel | Doxorubicin | Breast cancer | [82] |
| Imatinib | Doxorubicin | Colorectal | [84] |
| OGX-427 | Gemcitabine | Pancreatic | [107] |
| Erlotinib | Lung | [12] | |
| AHCCC | Gemcitabine | Pancreatic | [101] |
| KNK437 | Gemcitabine | Pancreatic | [109] |
| Triptolide | Gemcitabine | Pancreatic | [110] |
| Quercetin | 5-FU | Hepatoma | [115] |
| TMZ | GBM | [118] | |
| t-AUCB | GBM | [121] | |
| Toremifene | Doxorubicin | Breast cancer | [91] |
| BVDU | Bortezomib | Multiple myeloma | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampros, M.; Vlachos, N.; Voulgaris, S.; Alexiou, G.A. The Role of Hsp27 in Chemotherapy Resistance. Biomedicines 2022, 10, 897. https://doi.org/10.3390/biomedicines10040897
Lampros M, Vlachos N, Voulgaris S, Alexiou GA. The Role of Hsp27 in Chemotherapy Resistance. Biomedicines. 2022; 10(4):897. https://doi.org/10.3390/biomedicines10040897
Chicago/Turabian StyleLampros, Marios, Nikolaos Vlachos, Spyridon Voulgaris, and George A. Alexiou. 2022. "The Role of Hsp27 in Chemotherapy Resistance" Biomedicines 10, no. 4: 897. https://doi.org/10.3390/biomedicines10040897





