Abnormal Prefrontal Functional Connectivity Is Associated with Inflexible Information Processing in Patients with Autism Spectrum Disorder (ASD): An fNIRS Study
Abstract
:1. Introduction
2. Materials and Methods
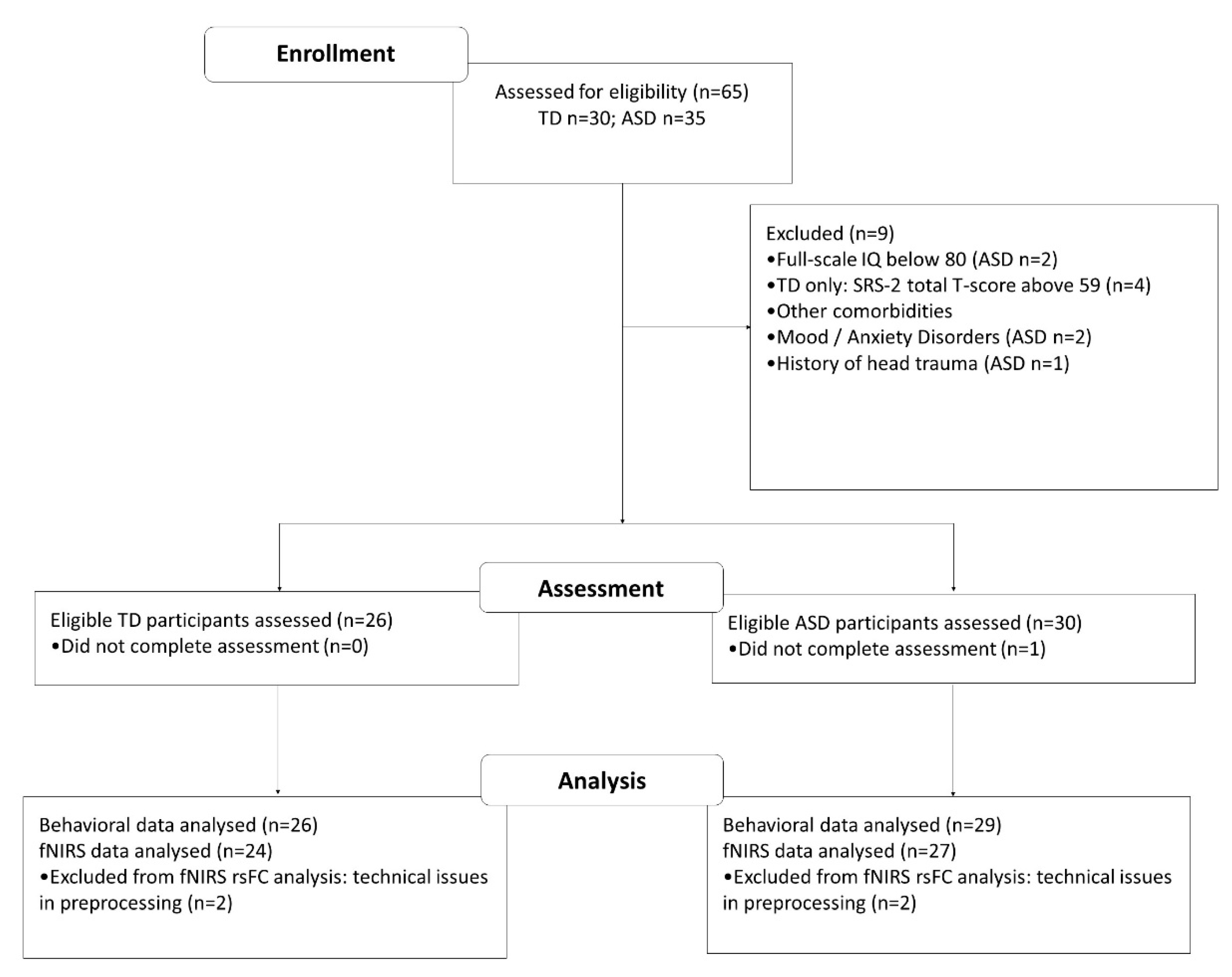
2.1. Participants
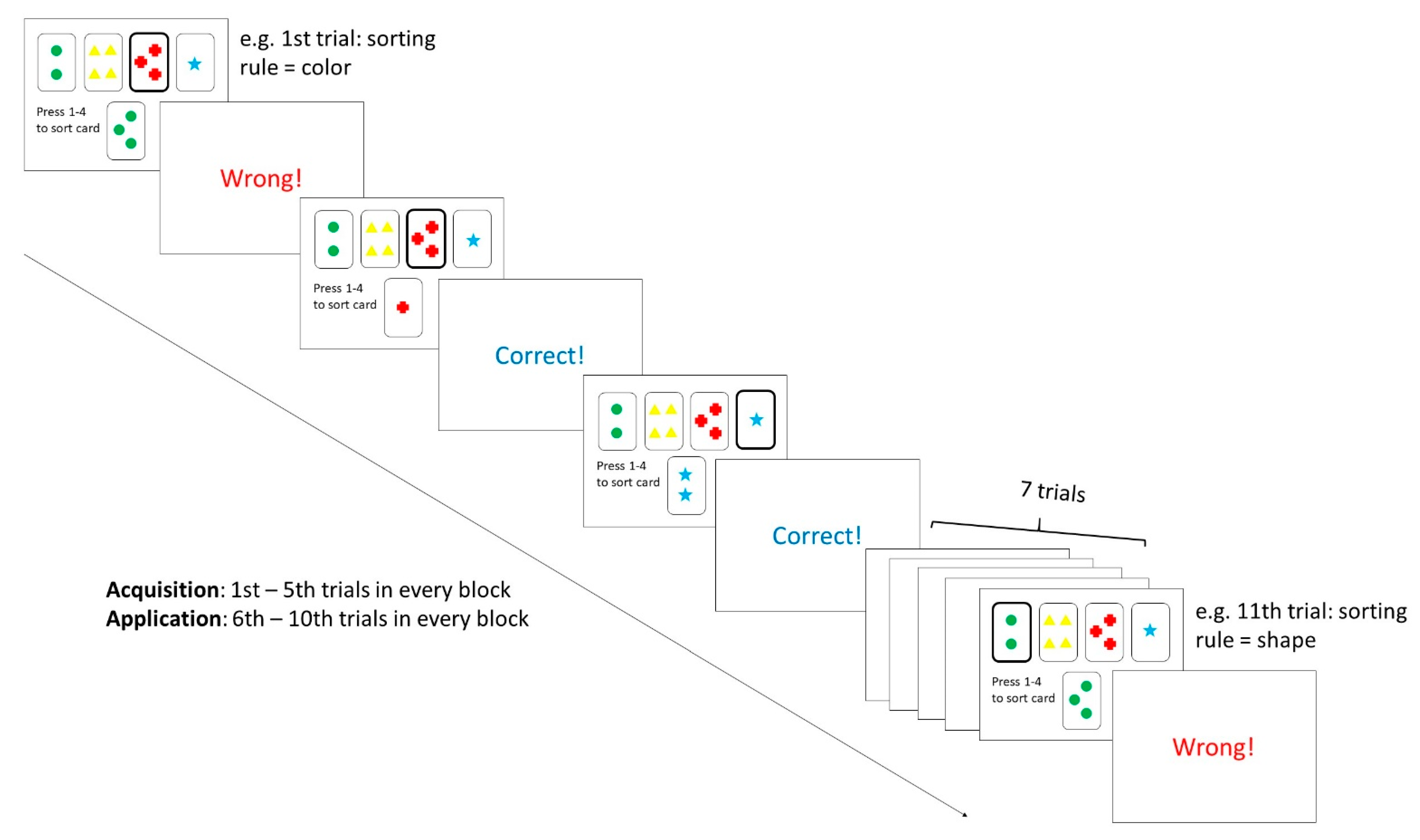
2.2. Procedure and Materials
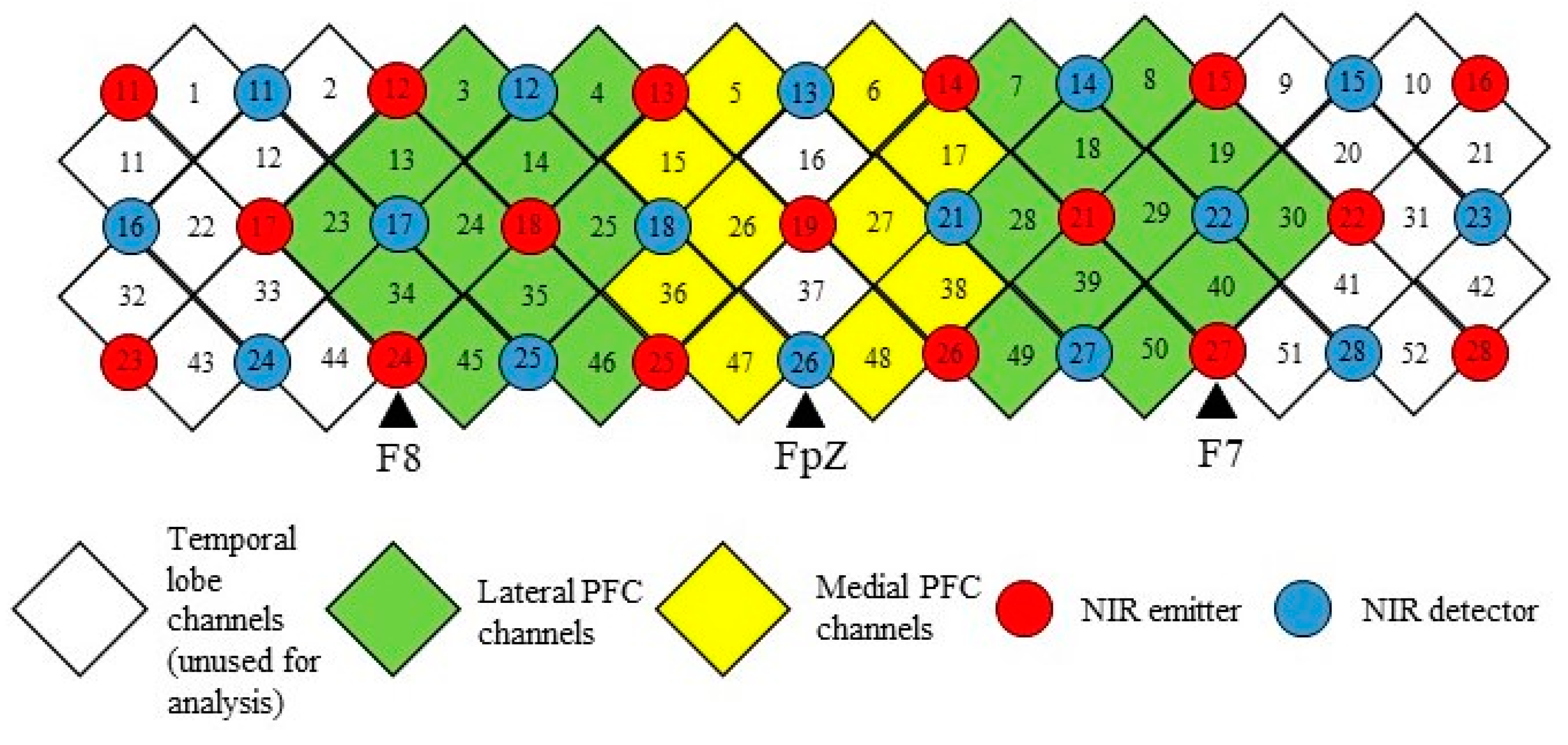
2.3. fNIRS Measurement
2.4. Data Analysis
3. Results
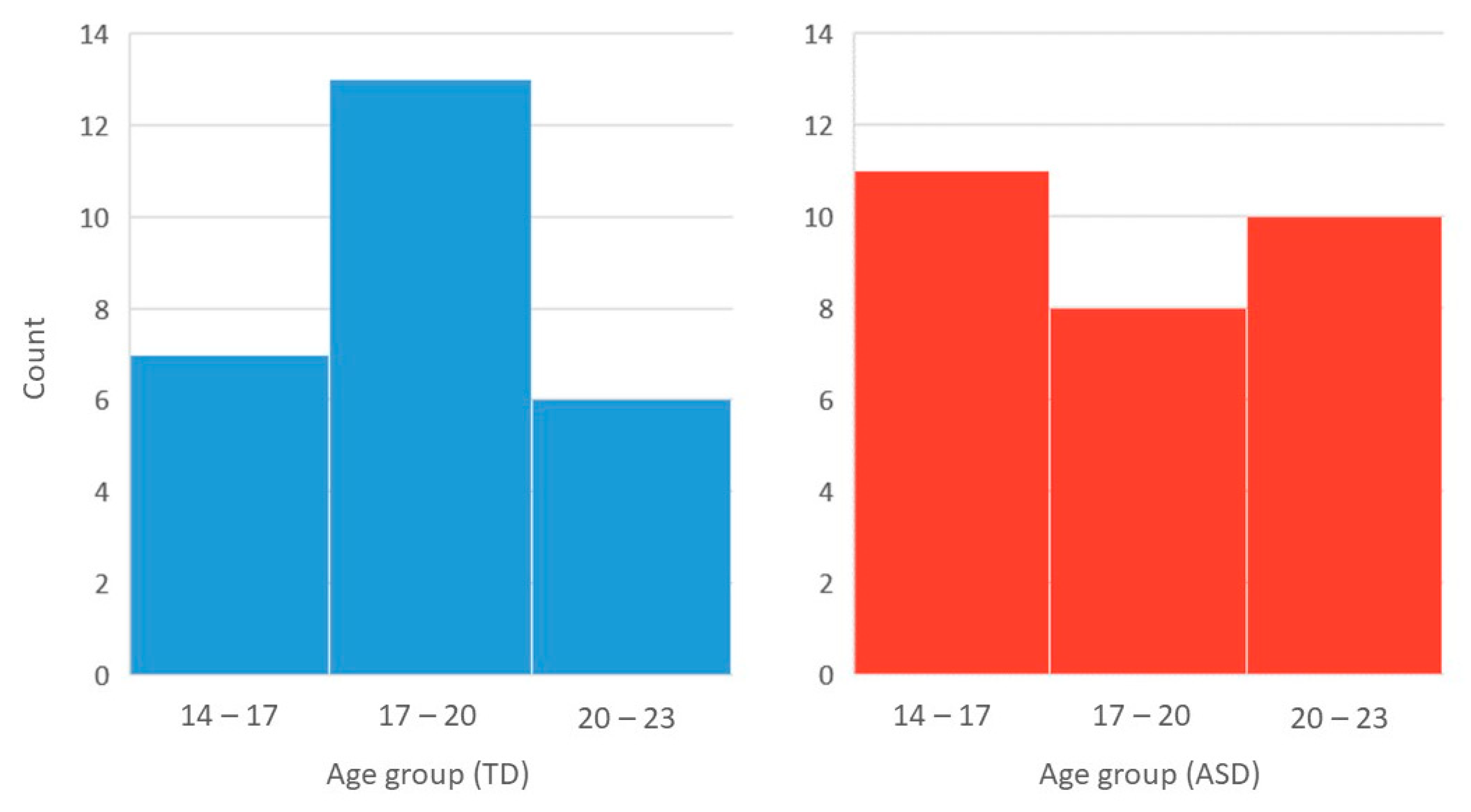
3.1. Demographic Details
3.2. WCST Behavioral Performance
3.3. fNIRS Prefrontal Activation during the WCST
3.4. fNIRS Prefrontal Functional Connectivity during the WCST
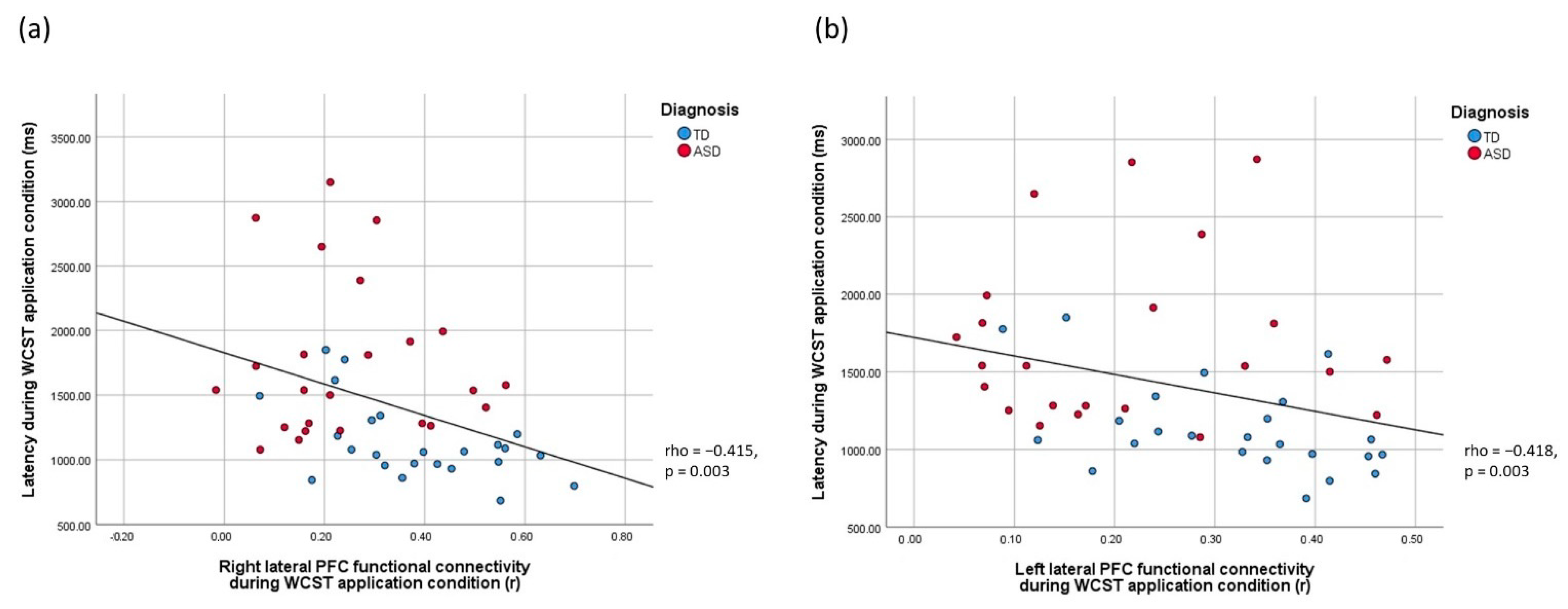
3.5. Brain-Behavior Relationships
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maenner, M.J.; Shaw, K.A.; Baio, J. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1. [Google Scholar] [CrossRef] [PubMed]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013; p. 947. [Google Scholar]
- Russell, G.; Golding, J.; Norwich, B.; Emond, A.; Ford, T.; Steer, C. Social and behavioural outcomes in children diagnosed with autism spectrum disorders: A longitudinal cohort study. J. Child Psychol. Psychiatry 2012, 53, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.-Y.; Chan, A.S.-Y. Neural Basis of Learning Issues in Children with Autism: A Bridge to Remediation Planning. In Routledge International Handbook of Schools and Schooling in Asia; Routledge: London, UK, 2018; pp. 542–554. [Google Scholar]
- Duncan, J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn. Sci. 2010, 14, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Memari, A.H.; Ziaee, V.; Shayestehfar, M.; Ghanouni, P.; Mansournia, M.A.; Moshayedi, P. Cognitive flexibility impairments in children with autism spectrum disorders: Links to age, gender and child outcomes. Res. Dev. Disabil. 2013, 34, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Van Eylen, L.; Boets, B.; Steyaert, J.; Evers, K.; Wagemans, J.; Noens, I. Cognitive flexibility in autism spectrum disorder: Explaining the inconsistencies? Res. Autism Spectr. Disord. 2011, 5, 1390–1401. [Google Scholar] [CrossRef] [Green Version]
- Vatansever, D.; Menon, D.K.; Stamatakis, E.A. Default mode contributions to automated information processing. Proc. Natl. Acad. Sci. USA 2017, 114, 12821–12826. [Google Scholar] [CrossRef] [Green Version]
- Armbruster-Genç, D.J.N.; Ueltzhöffer, K.; Fiebach, C.J. Brain Signal Variability Differentially Affects Cognitive Flexibility and Cognitive Stability. J. Neurosci. 2016, 36, 3978–3987. [Google Scholar] [CrossRef] [Green Version]
- Kehagia, A.A.; Murray, G.; Robbins, T. Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Curr. Opin. Neurobiol. 2010, 20, 199–204. [Google Scholar] [CrossRef]
- Miles, S.; Howlett, C.A.; Berryman, C.; Nedeljkovic, M.; Moseley, G.L.; Phillipou, A. Considerations for using the Wisconsin Card Sorting Test to assess cognitive flexibility. Behav. Res. Methods 2021, 53, 2083–2091. [Google Scholar] [CrossRef]
- Westwood, H.; Stahl, D.; Mandy, W.; Tchanturia, K. The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: A systematic review and meta-analysis. Psychol. Med. 2016, 46, 1809–1827. [Google Scholar] [CrossRef] [Green Version]
- Uddin, L.Q. Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Coulacoglou, C.; Saklofske, D.H. Chapter 5-Executive Function, Theory of Mind, and Adaptive Behavior. In Psychometrics and Psychological Assessment; Coulacoglou, C., Saklofske, D.H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 91–130. [Google Scholar]
- Landry, O.; Al-Taie, S. A Meta-analysis of the Wisconsin Card Sort Task in Autism. J. Autism Dev. Disord. 2016, 46, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Han, Y.M.Y.; Sze, S.L.; Chan, A.S. Abnormal frontal theta oscillations underlie the cognitive flexibility deficits in children with high-functioning autism spectrum disorders. Neuropsychology 2016, 30, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.C.; Fink, A. Fluid intelligence and neural efficiency: Effects of task complexity and sex. Pers. Individ. Differ. 2003, 35, 811–827. [Google Scholar] [CrossRef]
- Han, Y.M.; Chan, A. Disordered cortical connectivity underlies the executive function deficits in children with autism spectrum disorders. Res. Dev. Disabil. 2017, 61, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Goldstein, G.; Minshew, N.J. Neuropsychologic Functioning in Children with Autism: Further Evidence for Disordered Complex Information-Processing. Child Neuropsychol. 2006, 12, 279–298. [Google Scholar] [CrossRef] [Green Version]
- Buchsbaum, B.R.; Greer, S.; Chang, W.-L.; Berman, K.F. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Hum. Brain Mapp. 2005, 25, 35–45. [Google Scholar] [CrossRef]
- Carrillo-De-La-Peña, M.; Garcia-Larrea, L. Right frontal event related EEG coherence (ERCoh) differentiates good from bad performers of the Wisconsin Card Sorting Test (WCST). Neurophysiol. Clin. Neurophysiol. 2007, 37, 63–75. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.; Qin, W.; Liu, C.; Qi, Z.; Chen, N.; Li, K. Characteristics of resting-state functional connectivity in intractable unilateral temporal lobe epilepsy patients with impaired executive control function. Front. Hum. Neurosci. 2017, 11, 609. [Google Scholar] [CrossRef] [Green Version]
- Teffer, K.; Semendeferi, K. Human prefrontal cortex: Evolution, development, and pathology. Prog. Brain Res. 2012, 195, 191–218. [Google Scholar]
- Gilbert, S.J.; Bird, G.; Brindley, R.; Frith, C.; Burgess, P.W. Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia 2008, 46, 2281–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yerys, B.E.; Antezana, L.; Weinblatt, R.; Jankowski, K.; Strang, J.; Vaidya, C.J.; Schultz, R.T.; Gaillard, W.D.; Kenworthy, L. Neural Correlates of Set-Shifting in Children With Autism. Autism Res. 2015, 8, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Functional and Effective Connectivity: A Review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Villringer, A.; Chance, B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997, 20, 435–442. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chou, P.-H.; Wei, H.-L.; Sun, C.-W.; Lin, W.-H. Functional Connectivity During Phonemic and Semantic Verbal Fluency Test: A Multichannel Near Infrared Spectroscopy Study. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 43–48. [Google Scholar] [CrossRef]
- Yeung, M.K.; Lee, T.L.; Chan, A.S. Frontal lobe dysfunction underlies the differential word retrieval impairment in adolescents with high-functioning autism. Autism Res. 2019, 12, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D.; Kodama, H. Wechsler Intelligence Scale for Children; Psychological Corporation: New York, NY, USA, 2012; Volume 1. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV); Technical Manual: WAIS-IV Technical & Interpretive Manual; The Psychological Corporation: San Antonio, TX, USA, 2008. [Google Scholar]
- Lai, M.-C.; Lombardo, M.; Ruigrok, A.; Chakrabarti, B.; Wheelwright, S.; Auyeung, B.; Allison, C.; Baron-Cohen, S. MRC AIMS Consortium Cognition in Males and Females with Autism: Similarities and Differences. PLoS ONE 2012, 7, e47198. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Wang, J.; Yu, C.; Chen, H. Effect of handedness on brain activity patterns and effective connectivity network during the semantic task of Chinese characters. Sci. Rep. 2015, 5, 18262. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale: SRS-2; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Kongs, S.K.; Thompson, L.L.; Iverson, G.L.; Heaton, R.K. Wisconsin Card Sorting Test-, 64 Card Version: WCST-64; PAR: Lutz, FL, USA, 2000. [Google Scholar]
- Jasper, H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The NIRS Brain AnalyzIR Toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- Pinti, P.; Aichelburg, C.; Gilbert, S.; Hamilton, A.; Hirsch, J.; Burgess, P.; Tachtsidis, I. A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments. Jpn. Psychol. Res. 2018, 60, 347–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedev, A.V. Does the resting state connectivity have hemispheric asymmetry? A near-infrared spectroscopy study. NeuroImage 2014, 85, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Perlman, S.B.; Luna, B.; Hein, T.C.; Huppert, T.J. fNIRS evidence of prefrontal regulation of frustration in early childhood. NeuroImage 2014, 85, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Pinti, P.; Scholkmann, F.; Hamilton, A.; Burgess, P.; Tachtsidis, I. Current Status and Issues Regarding Pre-processing of fNIRS Neuroimaging Data: An Investigation of Diverse Signal Filtering Methods Within a General Linear Model Framework. Front. Hum. Neurosci. 2019, 12, 505. [Google Scholar] [CrossRef] [Green Version]
- Yeung, M.K.; Lin, J. Probing depression, schizophrenia, and other psychiatric disorders using fNIRS and the verbal fluency test: A systematic review and meta-analysis. J. Psychiatr. Res. 2021, 140, 416–435. [Google Scholar] [CrossRef]
- Barker, J.W.; Aarabi, A.; Huppert, T.J. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed. Opt. Express 2013, 4, 1366–1379. [Google Scholar] [CrossRef] [Green Version]
- Santosa, H.; Aarabi, A.; Perlman, S.B.; Huppert, T.J. Characterization and correction of the false-discovery rates in resting state connectivity using functional near-infrared spectroscopy. J. Biomed. Opt. 2017, 22, 055002. [Google Scholar] [CrossRef]
- Young, N.; Hudry, K.; Trembath, D.; Vivanti, G. Children With Autism Show Reduced Information Seeking When Learning New Tasks. Am. J. Intellect. Dev. Disabil. 2016, 121, 65–73. [Google Scholar] [CrossRef]
- Miller, H.L.; Ragozzino, M.; Cook, E.H.; Sweeney, J.A.; Mosconi, M.W. Cognitive Set Shifting Deficits and Their Relationship to Repetitive Behaviors in Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Ehlen, F.; Roepke, S.; Klostermann, F.; Baskow, I.; Geise, P.; Belica, C.; Tiedt, H.O.; Behnia, B. Small Semantic Networks in Individuals with Autism Spectrum Disorder Without Intellectual Impairment: A Verbal Fluency Approach. J. Autism Dev. Disord. 2020, 50, 3967–3987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haigh, S.M.; Walsh, J.A.; Mazefsky, C.A.; Minshew, N.J.; Eack, S.M. Processing Speed is Impaired in Adults with Autism Spectrum Disorder, and Relates to Social Communication Abilities. J. Autism Dev. Disord. 2018, 48, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Eack, S.M.; Bahorik, A.L.; McKnight, S.A.; Hogarty, S.S.; Greenwald, D.P.; Newhill, C.E.; Phillips, M.L.; Keshavan, M.S.; Minshew, N.J. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr. Res. 2013, 148, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Faja, S.; Webb, S.J.; Merkle, K.; Aylward, E.; Dawson, G. Brief Report: Face Configuration Accuracy and Processing Speed Among Adults with High-Functioning Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Silva PH, R.D.; Secchinato, K.F.; Rondinoni, C.; Leoni, R.F. Brain Structural–Functional Connectivity Relationship Underlying the Information Processing Speed. Brain Connect. 2020, 10, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rizzo, A.L.; Sorg, C.; Napiórkowski, N.; Neitzel, J.; Menegaux, A.; Müller, H.J.; Vangkilde, S.; Finke, K. Decreased cingulo-opercular network functional connectivity mediates the impact of aging on visual processing speed. Neurobiol. Aging 2019, 73, 50–60. [Google Scholar] [CrossRef]
- Haupt, M.; Ruiz-Rizzo, A.L.; Sorg, C.; Finke, K. Right-lateralized fronto-parietal network and phasic alertness in healthy aging. Sci. Rep. 2020, 10, 4823. [Google Scholar] [CrossRef] [Green Version]
- Küchenhoff, S.; Sorg, C.; Schneider, S.C.; Kohl, O.; Müller, H.J.; Napiórkowski, N.; Menegaux, A.; Finke, K.; Ruiz-Rizzo, A.L. Visual processing speed is linked to functional connectivity between right frontoparietal and visual networks. Eur. J. Neurosci. 2021, 53, 3362–3377. [Google Scholar] [CrossRef]
- Minshew, N.J.; Williams, D.L.; McFadden, K. Information Processing, Neural Connectivity, and Neuronal Organization, in Autism; Springer: Berlin/Heidelberg, Germany, 2008; pp. 381–405. [Google Scholar]
- Fishman, I.; Keown, C.L.; Lincoln, A.J.; Pineda, J.A.; Müller, R.-A. Atypical Cross Talk Between Mentalizing and Mirror Neuron Networks in Autism Spectrum Disorder. JAMA Psychiatry 2014, 71, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Koshino, H.; Carpenter, P.A.; Minshew, N.J.; Cherkassky, V.L.; Keller, T.A.; Just, M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage 2005, 24, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, K.; Yeung, M.K.; Chan, A.S.; Han, Y.M.Y. Effortful Control and Prefrontal Cortex Functioning in Children with Autism Spectrum Disorder: An fNIRS Study. Brain Sci. 2020, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Koshino, H.; Kana, R.K.; Keller, T.A.; Cherkassky, V.L.; Minshew, N.J.; Just, M.A. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb. Cortex 2008, 18, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.J.; Barraclough, N.E.; Andrews, T.J. Reduced connectivity between mentalizing and mirror systems in autism spectrum condition. Neuropsychologia 2019, 122, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Gläscher, J.; Adolphs, R.; Tranel, D. Model-based lesion mapping of cognitive control using the Wisconsin Card Sorting Test. Nat. Commun. 2019, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.L.; Mazefsky, C.A.; Walker, J.D.; Minshew, N.J.; Goldstein, G. Associations Between Conceptual Reasoning, Problem Solving, and Adaptive Ability in High-functioning Autism. J. Autism Dev. Disord. 2014, 44, 2908–2920. [Google Scholar] [CrossRef]
- D’Cruz, A.-M.; Mosconi, M.W.; Steele, S.; Rubin, L.H.; Luna, B.; Minshew, N.; Sweeney, J.A. Lateralized Response Timing Deficits in Autism. Biol. Psychiatry 2009, 66, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Rinehart, N.J.; Bradshaw, J.L.; Brereton, A.V.; Tonge, B.J. Lateralization in individuals with high-functioning autism and Asperger’s disorder: A frontostriatal model. J. Autism Dev. Disord. 2002, 32, 321–332. [Google Scholar] [CrossRef]
- Müller, V.I.; Langner, R.; Cieslik, E.C.; Rottschy, C.; Eickhoff, S.B. Interindividual differences in cognitive flexibility: Influence of gray matter volume, functional connectivity and trait impulsivity. Anat. Embryol. 2015, 220, 2401–2414. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.M.; Yau, S.S.; Han, Y.M. The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: A systematic review and meta-analyses of human and rodent studies. Neurosci. Biobehav. Rev. 2021, 125, 392–416. [Google Scholar] [CrossRef]
- Plewnia, C.; Schroeder, P.; Kunze, R.; Faehling, F.; Wolkenstein, L. Keep Calm and Carry On: Improved Frustration Tolerance and Processing Speed by Transcranial Direct Current Stimulation (tDCS). PLoS ONE 2015, 10, e0122578. [Google Scholar] [CrossRef] [Green Version]
- Gögler, N.; Willacker, L.; Funk, J.; Strube, W.; Langgartner, S.; Napiórkowski, N.; Hasan, A.; Finke, K. Single-session transcranial direct current stimulation induces enduring enhancement of visual processing speed in patients with major depression. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, E.; Duncan, J.; Kanwisher, N. Broad domain generality in focal regions of frontal and parietal cortex. Proc. Natl. Acad. Sci. USA 2013, 110, 16616–16621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, T.M.; McLaren, D.G.; Moody, T.D.; Feusner, J.D.; Bookheimer, S.Y. Generalized Psychophysiological Interaction (PPI) analysis of memory related connectivity in individuals at genetic risk for alzheimer’s disease. JoVE J. Vis. Exp. 2017, 129, e55394. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Group | ||||
|---|---|---|---|---|---|
| ASD | TD | t | df | p | |
| Mean chronological age in years (S.D.) | 17.75 (2.31) | 18.47 (2.36) | 1.14 | 53 | 0.259 |
| Mean full Scale IQ (S.D.) | 97.93 (15.98) | 101.73 (8.95) | 1.07 | 53 | 0.293 |
| Mean SRS-2 total score (S.D.) | 98.86 (25.15) | 55.60 (24.29) | −6.00 *** | 53 | <0.001 |
| Mean ADI-R domain scores (S.D.) | |||||
| Social interaction | 19.30 (3.60) | N/A | N/A | ||
| Communication | 14.67 (4.29) | ||||
| Restricted, repetitive behavior | 3.30 (1.92) | ||||
| Parameters | Group | ||||
|---|---|---|---|---|---|
| ASD | TD | t | df | p | |
| Accuracy (% of correct responses) | |||||
| Acquisition | 55.17 (12.07) | 60.03 (12.63) | 1.46 | 53 | 0.151 |
| Application | 76.97 (14.47) | 83.77 (15.20) | 1.70 | 53 | 0.095 |
| Reaction time (ms) | |||||
| Acquisition | 1882.33 (581.46) | 1346.39 (394.43) | 3.95 *** | 53 | <0.001 |
| Application | 1647.53 (597.26) | 1123.10 (291.22) | 4.20 *** | 53 | <0.001 |
| Right Hemisphere | Left Hemisphere | |||
|---|---|---|---|---|
| Group | Group | |||
| ASD | TD | ASD | TD | |
| Condition 1: Acquisition | ||||
| Medial | 1.58 (40.83) | 7.86 (38.89) | 9.85 (34.54) | 7.97 (40.32) |
| Lateral | 1.60 (35.96) | 16.35 (35.88) | 7.86 (36.96) | 8.40 (46.22) |
| Condition 2: Application | ||||
| Medial | 2.40 (40.50) | 8.27 (39.67) | 7.96 (37.41) | 6.22 (40.67) |
| Lateral | −0.24 (37.33) | 16.00 (35.66) | 8.21 (38.23) | 6.54 (45.37) |
| Right Hemisphere | Left Hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Group | |||||||||
| ASD | TD | t | df | p | ASD | TD | t | df | p | |
| Condition 1: Acquisition | ||||||||||
| Medial | 0.22 (.15) | 0.31 (.17) | 2.04 | 49 | 0.047 | 0.19 (0.09) | 0.24 (0.10) | 1.95 | 49 | .057 |
| Lateral | 0.21 (.17) | 0.34 (.17) | 2.91 ** | 49 | 0.005 | 0.21 (0.13) | 0.30 (0.12) | 2.69 | 49 | 0.010 |
| Condition 2: Application | ||||||||||
| Medial | 0.22 (.14) | 0.34 (.18) | 2.56 | 49 | 0.014 | 0.20 (0.08) | 0.25 (0.12) | 1.86 | 49 | 0.069 |
| Lateral | 0.25 (.16) | 0.38 (.16) | 2.89 ** | 49 | 0.006 | 0.21 (0.13) | 0.32 (0.11) | 2.89 ** | 49 | 0.006 |
| Parameters | R_lPFC_Acquisition | R_lPFC_Application | L_lPFC_Application |
|---|---|---|---|
| RT_acquisition | −0.348 | −0.362 | −0.344 |
| RT_application | −0.353 | −0.415 ** | −0.418 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.M.Y.; Chan, M.-C.; Lai, O.L.-H.; Krishnamurthy, K.; Han, Y.M.Y. Abnormal Prefrontal Functional Connectivity Is Associated with Inflexible Information Processing in Patients with Autism Spectrum Disorder (ASD): An fNIRS Study. Biomedicines 2022, 10, 1132. https://doi.org/10.3390/biomedicines10051132
Chan MMY, Chan M-C, Lai OL-H, Krishnamurthy K, Han YMY. Abnormal Prefrontal Functional Connectivity Is Associated with Inflexible Information Processing in Patients with Autism Spectrum Disorder (ASD): An fNIRS Study. Biomedicines. 2022; 10(5):1132. https://doi.org/10.3390/biomedicines10051132
Chicago/Turabian StyleChan, Melody M. Y., Ming-Chung Chan, Oscar Long-Hin Lai, Karthikeyan Krishnamurthy, and Yvonne M. Y. Han. 2022. "Abnormal Prefrontal Functional Connectivity Is Associated with Inflexible Information Processing in Patients with Autism Spectrum Disorder (ASD): An fNIRS Study" Biomedicines 10, no. 5: 1132. https://doi.org/10.3390/biomedicines10051132






