Plasma Amino Acid Concentrations in Patients with Alcohol and/or Cocaine Use Disorders and Their Association with Psychiatric Comorbidity and Sex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Recruitment and Participants
2.3. Eligibility Criteria
2.4. Clinical Assessment
2.4.1. Patients with Substance Use Disorders
2.4.2. Control Subjects
2.5. Blood Collection, Plasma Extraction and Rapid Detection Tests for Infections
2.6. Quantification of Amino Acids
2.7. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics in the Sample Groups
3.2. Amino Acids in the Sample Groups
3.3. Amino Acids in Relation to Sex and Diagnosis of Substance Use Disorder
3.3.1. Age and Body Mass Index as Covariates
3.3.2. Sex
3.3.3. Diagnosis of Substance Use Disorder
3.4. Amino Acids and Variables Related to Substance Use Disorder
3.5. Amino Acids and Psychiatric Comorbidity in Patients with Substance Use Disorders
3.5.1. Prevalence of Psychiatric Comorbidity
3.5.2. Amino Acids in Patients with Psychiatric Comorbidity
3.6. Amino Acids in Relation to Sex and Psychiatric Comorbidity
3.6.1. Patients with Alcohol Use Disorder
3.6.2. Patients with Cocaine Use Disorder
3.6.3. Patients with Alcohol and Cocaine Use Disorders
4. Discussion
4.1. Age and Body Mass Index
4.2. Sex
4.3. Substance Use Disorder
4.4. Psychiatric Comorbidity
5. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKay, J.R.; Pettinati, H.M.; Morrison, R.; Feeley, M.; Mulvaney, F.D.; Gallop, R. Relation of depression diagnoses to 2-year outcomes in cocaine-dependent patients in a randomized continuing care study. Psychol. Addict. Behav. 2002, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.F.; Brooks, A.J.; Gordon, S.M.; Green, C.A.; Kropp, F.; McHugh, R.K.; Lincoln, M.; Hien, D.; Miele, G.M. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug Alcohol. Depend. 2007, 86, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boileau-Falardeau, M.; Contreras, G.; Gariepy, G.; Laprise, C. Patterns and motivations of polysubstance use: A rapid review of the qualitative evidence. Health Promot. Chronic Dis. Prev. Can. 2022, 42, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Salom, C.L.; Betts, K.S.; Williams, G.M.; Najman, J.M.; Alati, R. Predictors of comorbid polysubstance use and mental health disorders in young adults-a latent class analysis. Addiction 2016, 111, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008, 1141, 105–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araos, P.; Vergara-Moragues, E.; Pedraz, M.; Pavon, F.J.; Campos Cloute, R.; Calado, M.; Ruiz, J.J.; Garcia-Marchena, N.; Gornemann, I.; Torrens, M.; et al. Psychopathological comorbidity in cocaine users in outpatient treatment. Adicciones 2014, 26, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwell, K.J.; Tolliver, B.K.; Brady, K.T. Biologic Commonalities between Mental Illness and Addiction. Prim. Psychiatry 2009, 16, 33–39. [Google Scholar]
- Hasin, D.S.; O’Brien, C.P.; Auriacombe, M.; Borges, G.; Bucholz, K.; Budney, A.; Compton, W.M.; Crowley, T.; Ling, W.; Petry, N.M.; et al. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am. J. Psychiatry 2013, 170, 834–851. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Koob, G.; Baler, R. Biomarkers in substance use disorders. ACS Chem. Neurosci. 2015, 6, 522–525. [Google Scholar] [CrossRef]
- Garcia-Marchena, N.; Barrera, M.; Mestre-Pinto, J.I.; Araos, P.; Serrano, A.; Perez-Mana, C.; Papaseit, E.; Fonseca, F.; Ruiz, J.J.; Rodriguez de Fonseca, F.; et al. Inflammatory mediators and dual depression: Potential biomarkers in plasma of primary and substance-induced major depression in cocaine and alcohol use disorders. PLoS ONE 2019, 14, e0213791. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Shurtleff, D.; Harris, R.A. Neuroimmune mechanisms of alcohol and drug addiction. Int. Rev. Neurobiol. 2014, 118, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedraz, M.; Araos, P.; Garcia-Marchena, N.; Serrano, A.; Romero-Sanchiz, P.; Suarez, J.; Castilla-Ortega, E.; Mayoral-Cleries, F.; Ruiz, J.J.; Pastor, A.; et al. Sex differences in psychiatric comorbidity and plasma biomarkers for cocaine addiction in abstinent cocaine-addicted subjects in outpatient settings. Front. Psychiatry 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araos, P.; Vidal, R.; O’Shea, E.; Pedraz, M.; Garcia-Marchena, N.; Serrano, A.; Suarez, J.; Castilla-Ortega, E.; Ruiz, J.J.; Campos-Cloute, R.; et al. Serotonin is the main tryptophan metabolite associated with psychiatric comorbidity in abstinent cocaine-addicted patients. Sci. Rep. 2019, 9, 16842. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; Garcia-Marchena, N.; O’Shea, E.; Requena-Ocana, N.; Flores-Lopez, M.; Araos, P.; Serrano, A.; Suarez, J.; Rubio, G.; Rodriguez de Fonseca, F.; et al. Plasma tryptophan and kynurenine pathway metabolites in abstinent patients with alcohol use disorder and high prevalence of psychiatric comorbidity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109958. [Google Scholar] [CrossRef]
- Fonseca, F.; Mestre-Pinto, J.I.; Gomez-Gomez, A.; Martinez-Sanvisens, D.; Rodriguez-Minguela, R.; Papaseit, E.; Perez-Mana, C.; Langohr, K.; Valverde, O.; Pozo, O.J.; et al. The Tryptophan System in Cocaine-Induced Depression. J. Clin. Med. 2020, 9, 4103. [Google Scholar] [CrossRef]
- Tanaka, M.; Toth, F.; Polyak, H.; Szabo, A.; Mandi, Y.; Vecsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef]
- Takehana, S.; Yoshida, H.; Ozawa, S.; Yamazaki, J.; Shimbo, K.; Nakayama, A.; Mizukoshi, T.; Miyano, H. The effects of pre-analysis sample handling on human plasma amino acid concentrations. Clin. Chim. Acta 2016, 455, 68–74. [Google Scholar] [CrossRef]
- Rajendram, R.; Preedy, V.R.; Patel, V.B. Branched Chain Amino Acids in Clinical Nutrition; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Maes, M.; Verkerk, R.; Vandoolaeghe, E.; Lin, A.; Scharpe, S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: Modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr. Scand. 1998, 97, 302–308. [Google Scholar] [CrossRef]
- Martin, D.; Swartzwelder, H.S. Ethanol inhibits release of excitatory amino acids from slices of hippocampal area CA1. Eur. J. Pharmacol. 1992, 219, 469–472. [Google Scholar] [CrossRef]
- Cotman, C.W.; Monaghan, D.T. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu. Rev. Neurosci. 1988, 11, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Akanuma, S.; Sakurai, T.; Tachikawa, M.; Kubo, Y.; Hosoya, K. Transporter-mediated L-glutamate elimination from cerebrospinal fluid: Possible involvement of excitatory amino acid transporters expressed in ependymal cells and choroid plexus epithelial cells. Fluids Barriers CNS 2015, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleva, R.M.; Gass, J.T.; Widholm, J.J.; Olive, M.F. Glutamatergic targets for enhancing extinction learning in drug addiction. Curr. Neuropharmacol. 2010, 8, 394–408. [Google Scholar] [CrossRef] [Green Version]
- Tsai, G.; Coyle, J.T. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu. Rev. Med. 1998, 49, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Faingold, C.L.; N’Gouemo, P.; Riaz, A. Ethanol and neurotransmitter interactions--from molecular to integrative effects. Prog. Neurobiol. 1998, 55, 509–535. [Google Scholar] [CrossRef]
- Weitlauf, C.; Woodward, J.J. Ethanol selectively attenuates NMDAR-mediated synaptic transmission in the prefrontal cortex. Alcohol. Clin. Exp. Res. 2008, 32, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.L.; Forster, G.L.; Unterwald, E.M. Repeated cocaine enhances ventral hippocampal-stimulated dopamine efflux in the nucleus accumbens and alters ventral hippocampal NMDA receptor subunit expression. J. Neurochem. 2014, 130, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Pomierny-Chamiolo, L.; Miszkiel, J.; Frankowska, M.; Mizera, J.; Filip, M. Neuroadaptive changes in metabotropic glutamate mGlu2/3R expression during different phases of cocaine addiction in rats. Pharmacol. Rep. PR 2017, 69, 1073–1081. [Google Scholar] [CrossRef]
- Sun, W.; Rebec, G.V. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 8004–8008. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Salmeron, B.J.; Ross, T.J.; Xi, Z.X.; Stein, E.A.; Yang, Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users—A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 2009, 174, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Tong, Q.; Xu, Q.; Xia, Q.; Yuan, Y.; Zhang, L.; Sun, H.; Shan, H.; Zhang, K. Correlations between plasma levels of amino acids and nonmotor symptoms in Parkinson’s disease. J. Neural Transm. 2015, 122, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Rosenberg, P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994, 330, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Sanacora, G.; Krystal, J.H. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol. Disord. Drug Targets 2007, 6, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sawa, A.; Iyo, M. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry 2007, 62, 1310–1316. [Google Scholar] [CrossRef]
- Altamura, C.A.; Mauri, M.C.; Ferrara, A.; Moro, A.R.; D’Andrea, G.; Zamberlan, F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am. J. Psychiatry 1993, 150, 1731–1733. [Google Scholar] [CrossRef]
- Paul, I.A.; Skolnick, P. Glutamate and depression: Clinical and preclinical studies. Ann. N. Y. Acad. Sci. 2003, 1003, 250–272. [Google Scholar] [CrossRef]
- Pinto, V.L.; de Souza, P.F.; Brunini, T.M.; Oliveira, M.B.; Moss, M.B.; Siqueira, M.A.; Ferraz, M.R.; Mendes-Ribeiro, A.C. Low plasma levels of L-arginine, impaired intraplatelet nitric oxide and platelet hyperaggregability: Implications for cardiovascular disease in depressive patients. J. Affect. Disord. 2012, 140, 187–192. [Google Scholar] [CrossRef]
- Waziri, R.; Mott, J. Drug effects on serine metabolism in psychiatric patients. Psychiatry Res. 1986, 18, 119–126. [Google Scholar] [CrossRef]
- Garip, B.; Kayir, H. Alteration in NMDAR-related amino acids in first episode psychosis. Synapse 2019, 73, e22127. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Marcos, A.; Ambrosio, E.; Mayboroda, O.A.; Marina, M.L.; Crego, A.L. Investigation on the combined effect of cocaine and ethanol administration through a liquid chromatography-mass spectrometry metabolomics approach. J. Pharm. Biomed. Anal. 2017, 140, 313–321. [Google Scholar] [CrossRef]
- McHugh, R.K.; Votaw, V.R.; Sugarman, D.E.; Greenfield, S.F. Sex and gender differences in substance use disorders. Clin Psychol. Rev. 2018, 66, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lopez, M.; Garcia-Marchena, N.; Pavon, F.J.; Lara, E.; Porras-Perales, O.; Araos, P.; Requena-Ocana, N.; Torres-Galvan, S.; Manas-Padilla, M.C.; Rubio, G.; et al. Plasma Concentrations of Lysophosphatidic Acid and Autotaxin in Abstinent Patients with Alcohol Use Disorder and Comorbid Liver Disease. Biomedicines 2021, 9, 1207. [Google Scholar] [CrossRef] [PubMed]
- Torrens, M.; Serrano, D.; Astals, M.; Perez-Dominguez, G.; Martin-Santos, R. Diagnosing comorbid psychiatric disorders in substance abusers: Validity of the Spanish versions of the Psychiatric Research Interview for Substance and Mental Disorders and the Structured Clinical Interview for DSM-IV. Am. J. Psychiatry 2004, 161, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Hasin, D.; Samet, S.; Nunes, E.; Meydan, J.; Matseoane, K.; Waxman, R. Diagnosis of comorbid psychiatric disorders in substance users assessed with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. Am. J. Psychiatry 2006, 163, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Quiroga, J.A.; Diaz-Digon, L.; Comin, M.; Bosch, R.; Palomar, G.; Chalita, J.P.; Roncero, C.; Nogueira, M.; Torrens, M.; Casas, M. Criteria and Concurrent Validity of Adult ADHD Section of the Psychiatry Research Interview for Substance and Mental Disorders. J. Atten. Disord. 2015, 19, 999–1006. [Google Scholar] [CrossRef]
- Hasin, D. DSM-5 SUD diagnoses: Changes, reactions, remaining open questions. Drug Alcohol. Depend. 2015, 148, 226–229. [Google Scholar]
- Wittchen, H.U.; Robins, L.N.; Cottler, L.B.; Sartorius, N.; Burke, J.D.; Regier, D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. Br. J. Psychiatry 1991, 159, 645–653. [Google Scholar] [CrossRef]
- Lorenzo, M.P.; Villasenor, A.; Ramamoorthy, A.; Garcia, A. Optimization and validation of a capillary electrophoresis laser-induced fluorescence method for amino acids determination in human plasma: Application to bipolar disorder study. Electrophoresis 2013, 34, 1701–1709. [Google Scholar] [CrossRef]
- Walter, H.; Schlaff, W.B.; Lesch, O.M.; Vitek, L.; Zima, T.; Hartl, D.; Dvorak, A.; Gutierrez-Lobos, K.; Thau, K.; Witte, P.D. Breath Alcohol Level and Plasma Amino Acids: A Comparison between Older and Younger Chronic Alcohol-Dependent Patients. Alcohol Alcohol. 2008, 43, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Guevara-Cruz, M.; Vargas-Morales, J.M.; Mendez-Garcia, A.L.; Lopez-Barradas, A.M.; Granados-Portillo, O.; Ordaz-Nava, G.; Rocha-Viggiano, A.K.; Gutierrez-Leyte, C.A.; Medina-Cerda, E.; Rosado, J.L.; et al. Amino acid profiles of young adults differ by sex, body mass index and insulin resistance. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 393–401. [Google Scholar] [CrossRef]
- Maltais-Payette, I.; Boulet, M.M.; Prehn, C.; Adamski, J.; Tchernof, A. Circulating glutamate concentration as a biomarker of visceral obesity and associated metabolic alterations. Nutr. Metab. 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N.; et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. 2016, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, M.; Vancampfort, D.; Nguyen, T.T.; Ekblom-Bak, E.; Wallin, P.; Andersson, G.; Lundin, A. Physical Activity, Sedentary Behavior, and Cardiorespiratory Fitness in Hazardous and Non-Hazardous Alcohol Consumers. Am. J. Health Promot. 2021, 35, 669–678. [Google Scholar] [CrossRef]
- Requena-Ocana, N.; Flores-Lopez, M.; Martin, A.S.; Garcia-Marchena, N.; Pedraz, M.; Ruiz, J.J.; Serrano, A.; Suarez, J.; Pavon, F.J.; de Fonseca, F.R.; et al. Influence of gender and education on cocaine users in an outpatient cohort in Spain. Sci. Rep. 2021, 11, 20928. [Google Scholar] [CrossRef] [PubMed]
- Yamada, C.; Kondo, M.; Kishimoto, N.; Shibata, T.; Nagai, Y.; Imanishi, T.; Oroguchi, T.; Ishii, N.; Nishizaki, Y. Association between insulin resistance and plasma amino acid profile in non-diabetic Japanese subjects. J. Diabetes Investig. 2015, 6, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Colpaert, F.; Canton, H. Glutamate and glycine co-activate while polyamines merely modulate the NMDA receptor complex. Prog. Neuropsychopharmacol. Biol. Psychiatry 1991, 15, 183–190. [Google Scholar] [CrossRef]
- Shimosato, K.; Watanabe, S.; Marley, R.J.; Saito, T. Increased polyamine levels and changes in the sensitivity to convulsions during chronic treatment with cocaine in mice. Brain Res. 1995, 684, 243–247. [Google Scholar] [CrossRef]
- Miyakawa, T.; Yagi, T.; Kitazawa, H.; Yasuda, M.; Kawai, N.; Tsuboi, K.; Niki, H. Fyn-kinase as a determinant of ethanol sensitivity: Relation to NMDA-receptor function. Science 1997, 278, 698–701. [Google Scholar] [CrossRef]
- Yaka, R.; Tang, K.C.; Camarini, R.; Janak, P.H.; Ron, D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin. Exp. Res. 2003, 27, 1736–1742. [Google Scholar] [CrossRef]
- Scofield, M.D.; Kalivas, P.W. Astrocytic dysfunction and addiction: Consequences of impaired glutamate homeostasis. Neuroscientist 2014, 20, 610–622. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Vina, J.R. How Glutamate Is Managed by the Blood-Brain Barrier. Biology 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhou, Y.G. Homeostasis of the Intraparenchymal-Blood Glutamate Concentration Gradient: Maintenance, Imbalance, and Regulation. Front. Mol. Neurosci. 2017, 10, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmaal, L.; Veltman, D.J.; Nederveen, A.; van den Brink, W.; Goudriaan, A.E. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: A randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 2143–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Marchena, N.; Ladron de Guevara-Miranda, D.; Pedraz, M.; Araos, P.F.; Rubio, G.; Ruiz, J.J.; Pavon, F.J.; Serrano, A.; Castilla-Ortega, E.; Santin, L.J.; et al. Higher Impulsivity As a Distinctive Trait of Severe Cocaine Addiction among Individuals Treated for Cocaine or Alcohol Use Disorders. Front. Psychiatry 2018, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilberman, M.L.; Tavares, H.; Blume, S.B.; el-Guebaly, N. Substance use disorders: Sex differences and psychiatric comorbidities. Can. J. Psychiatry 2003, 48, 5–13. [Google Scholar] [CrossRef]
- Goldstein, R.B.; Dawson, D.A.; Chou, S.P.; Grant, B.F. Sex differences in prevalence and comorbidity of alcohol and drug use disorders: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Stud. Alcohol Drugs 2012, 73, 938–950. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, S.; Koga, N.; Hattori, K.; Matsuo, J.; Ota, M.; Hori, H.; Sasayama, D.; Teraishi, T.; Ishida, I.; Yoshida, F.; et al. Plasma amino acid profile in major depressive disorder: Analyses in two independent case-control sample sets. J. Psychiatr. Res. 2018, 96, 23–32. [Google Scholar] [CrossRef]
- Mitani, H.; Shirayama, Y.; Yamada, T.; Maeda, K.; Ashby, C.R., Jr.; Kawahara, R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1155–1158. [Google Scholar] [CrossRef]
- Altamura, C.; Maes, M.; Dai, J.; Meltzer, H.Y. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol. 1995, 5, 71–75. [Google Scholar] [CrossRef]



| Variable | Sample Group | p-Value | ||||
|---|---|---|---|---|---|---|
| Control n = 130 | AUD n = 60 | CUD n = 41 | AUD + CUD n = 64 | |||
| Age Mean ± SD | years | 39.4 ± 12.1 | 47.8 ± 5.7 *** | 35.0 ± 8.1 * | 38.4 ± 7.8 | <0.001 (1) |
| BMI Mean ± SD | kg/m2 | 25.1 ± 4.5 | 26.4 ± 4.7 | 24.9 ± 4.9 | 25.9 ± 4.4 | 0.190 (1) |
| Sex n (%) | Women | 65 (50.0) | 24 (40.0) | 11 (26.8) | 8 (12.5) | <0.001 (2) |
| Men | 65 (50.0) | 36 (60.0) | 30 (73.2) | 56 (87.5) | ||
| Marital Status n (%) | Single | 52 (40.0) | 15 (25.0) | 15 (36.6) | 29 (45.3) | 0.001 (3) |
| Cohabiting | 64 (49.2) | 26 (43.3) | 20 (48.8) | 14 (21.9) | ||
| Separated | 13 (10.0) | 18 (30.0) | 6 (14.6) | 21 (32.8) | ||
| Widow | 1 (0.8) | 1 (1.7) | 0 (0.0) | 0 (0.0) | ||
| Education n (%) | Elementary | 4 (3.1) | 14 (23.3) | 6 (14.6) | 14 (21.9) | <0.001 (2) |
| Secondary | 38 (29.2) | 36 (60.0) | 33 (80.5) | 38 (59.4) | ||
| Tertiary | 88 (67.7) | 10 (16.7) | 2 (4.9) | 12 (18.8) | ||
| Employment n (%) | Yes | 103 (79.2) | 30 (50.0) | 17 (41.5) | 21 (32.8) | <0.001 (2) |
| No | 27 (20.8) | 30 (50.0) | 24 (58.5) | 43 (67.2) | ||
| Variable | Sample Group | p-Value (1) | ||||
|---|---|---|---|---|---|---|
| Control n = 130 | AUD n = 60 | CUD n = 41 | AUD + CUD n = 64 | |||
| Iso Median (IQR) | μM | 67.5 (55.2–82.1) | 67.7 (52.5–85.7) | 73.4 (55.7–99.3) | 74.3 (59.6–97.7) | 0.273 |
| Leu Median (IQR) | μM | 128.6 (104.8–152.0) | 128.5 (96.0–149.7) | 138.0 (103.3–179.3) | 142.9 (112.1–182.4) | 0.074 |
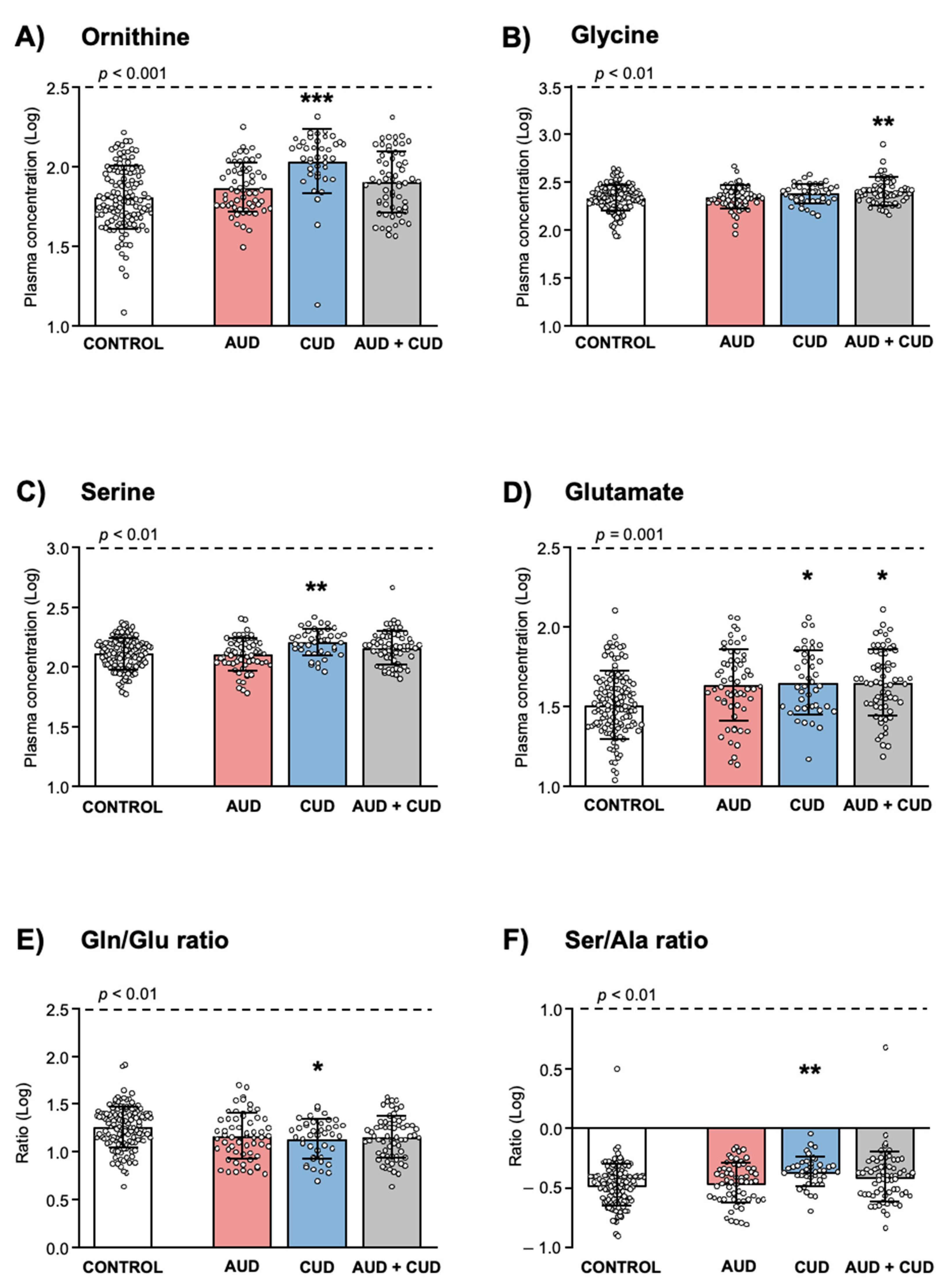
| Orn Median (IQR) | μM | 62.30 (48.2–93.8) | 71.2 (57.1–97.1) | 115.8 (90.5–143.6) *** | 81.1 (55.7–118.8) * | <0.001 |
| Gln Median (IQR) | μM | 590.4 (526.5–674.5) | 633.2 (560.2–730.6) | 599.2 (545.2–730.6) | 645.5 (560.4–710.8) | 0.050 |
| Ala Median (IQR) | μM | 394.0 (332.6–445.4) | 374.0 (292.5–458.5) | 396.9 (296.6–440.5) | 381.0 (318.8–475.6) | 0.672 |
| Thr Median (IQR) | μM | 139.1 (115.2–169.0) | 138.9 (117.5–183.0) | 149.7 (127.4–171.6) | 154.7 (123.4–176.5) | 0.128 |
| Gly Median (IQR) | μM | 220.6 (177.9–271.6) | 216.9 (192.6–268.2) | 251.4 (203.9–288.6) | 243.6 (207.0–288.3) * | 0.018 |
| Ser Median (IQR) | μM | 130.2 (104.5–160.1) | 128.8 (108.8–159.2) | 168.0 (134.2–193.0) *** | 148.9 (116.0–181.7) | <0.001 |
| Tau Median (IQR) | μM | 48.3 (38.4–62.2) | 43.3 (34.6–53.0) | 50.2 (41.1–57.4) | 47.3 (38.2–57.5) | 0.086 |
| Glu Median (IQR) | μM | 31.8 (24.3–44.4) | 42.2 (32.3–62.0) *** | 42.6 (31.5–66.2) ** | 44.9 (32.3–69.0) *** | <0.001 |
| Ser/Gly Median (IQR) | 0.59 (0.50–0.71) | 0.58 (0.49–0.70) | 0.68 * (0.57–0.76) | 0.58 (0.48–0.69) | 0.010 | |
| Gln/Glu Median (IQR) | 18.7 (13.3–25.5) | 14.5 (9.7–22.3) * | 15.3 (8.7–19.4) ** | 14.6 (9.2–19.9) ** | 0.001 | |
| Ser/Ala Median (IQR) | 0.35 (0.26–0.42) | 0.36 (0.26–0.47) | 0.44 (0.36–0.51) *** | 0.41 (0.28–0.49) | <0.001 | |
| Variable | Group | p-Value (1) | ||
|---|---|---|---|---|
| AUD n = 60 | CUD n = 41 | AUD + CUD n = 64 | ||
| Psychiatric Comorbidity n (%) | 36 (60.0) | 21 (51.2) | 46 (71.9) | 0.092 |
| Mood disorders n (%) | 27 (45.0) | 10 (24.4) | 21 (32.8) | 0.091 |
| Anxiety disorders n (%) | 15 (25.0) | 8 (19.5) | 16 (25.0) | 0.514 |
| Psychotic disorders n (%) | 7 (11.7) | 6 (14.6) | 6 (9.4) | 0.712 |
| Eating disorders n (%) | 0 (0.0) | 1 (2.4) | 4 (6.3) | 0.124 |
| ADHD in childhood n (%) | 7 (11.7) | 7 (17.1) | 15 (23.4) | 0.226 |
| Borderline personality n (%) | 6 (10.0) | 8 (19.5) | 20 (31.3) | 0.014 |
| Antisocial personality n (%) | 2 (3.3) | 9 (22.0) | 12 (19.0) | 0.010 |
| Psychiatric medication (2) n (%) | 44 (73.3) | 18 (43.9) | 37 (57.8) | 0.011 |
| Variable | Group | p-Value (1) | ||
|---|---|---|---|---|
| Non-Comorbid Psychiatric Disorders n = 62 | Comorbid Psychiatric Disorders n = 103 | |||
| Iso Median (IQR) | μM | 72.62 (55.18–87.08) | 68.71 (58.24–100.3) | 0.919 |
| Leu Median (IQR) | μM | 135.3 (104.7–161.3) | 133.3 (105.8–178.2) | 0.698 |
| Orn Median (IQR) | μM | 91.26 (73.34–128.7) | 80.05 (55.41–116.7) | 0.025 |
| Gln Median (IQR) | μM | 625.5 (564.4–729.6) | 641.3 (548.6–715.7) | 0.739 |
| Ala Median (IQR) | μM | 362.7 (291.4–433.4) | 389.4 (311.1–477.7) | 0.147 |
| Thr Median (IQR) | μM | 148.9 (124.6–184.4) | 146.6 (119.9–175.6) | 0.642 |
| Gly Median (IQR) | μM | 240.4 (207.1–272.3) | 236.5 (194.0–288.6) | >0.999 |
| Ser Median (IQR) | μM | 149.8 (119.5–177.9) | 141.1 (108.1–175.9) | 0.371 |
| Tau Median (IQR) | μM | 45.99 (34.68–54.48) | 46.98 (38.42–57.29) | 0.230 |
| Glu Median (IQR) | μM | 42.23 (31.94–57.66) | 44.83 (32.07–69.16) | 0.860 |
| Ser/Gly Median (IQR) | 0.62 (0.51–0.75) | 0.60 (0.51–0.69) | 0.226 | |
| Gln/Glu Median (IQR) | 14.94 (10.38–19.61) | 14.68 (8.79–21.93) | 0.868 | |
| Ser/Ala Median (IQR) | 0.44 (0.30–0.51) | 0.39 (0.28–0.47) | 0.122 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Marchena, N.; Marcos, A.; Flores-López, M.; Moreno-Fernández, M.; Requena-Ocaña, N.; Porras-Perales, O.; Torres-Galván, S.; Araos, P.; Serrano, A.; Muga, R.; et al. Plasma Amino Acid Concentrations in Patients with Alcohol and/or Cocaine Use Disorders and Their Association with Psychiatric Comorbidity and Sex. Biomedicines 2022, 10, 1137. https://doi.org/10.3390/biomedicines10051137
García-Marchena N, Marcos A, Flores-López M, Moreno-Fernández M, Requena-Ocaña N, Porras-Perales O, Torres-Galván S, Araos P, Serrano A, Muga R, et al. Plasma Amino Acid Concentrations in Patients with Alcohol and/or Cocaine Use Disorders and Their Association with Psychiatric Comorbidity and Sex. Biomedicines. 2022; 10(5):1137. https://doi.org/10.3390/biomedicines10051137
Chicago/Turabian StyleGarcía-Marchena, Nuria, Alberto Marcos, María Flores-López, Mario Moreno-Fernández, Nerea Requena-Ocaña, Oscar Porras-Perales, Sandra Torres-Galván, Pedro Araos, Antonia Serrano, Roberto Muga, and et al. 2022. "Plasma Amino Acid Concentrations in Patients with Alcohol and/or Cocaine Use Disorders and Their Association with Psychiatric Comorbidity and Sex" Biomedicines 10, no. 5: 1137. https://doi.org/10.3390/biomedicines10051137






