Long-Term Reduction of Bacterial Adhesion on Polyurethane by an Ultra-Thin Surface Modifier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Surface Modification
2.3. Bacterial Incubation and Visualization
2.4. Long Term Incubation
3. Results
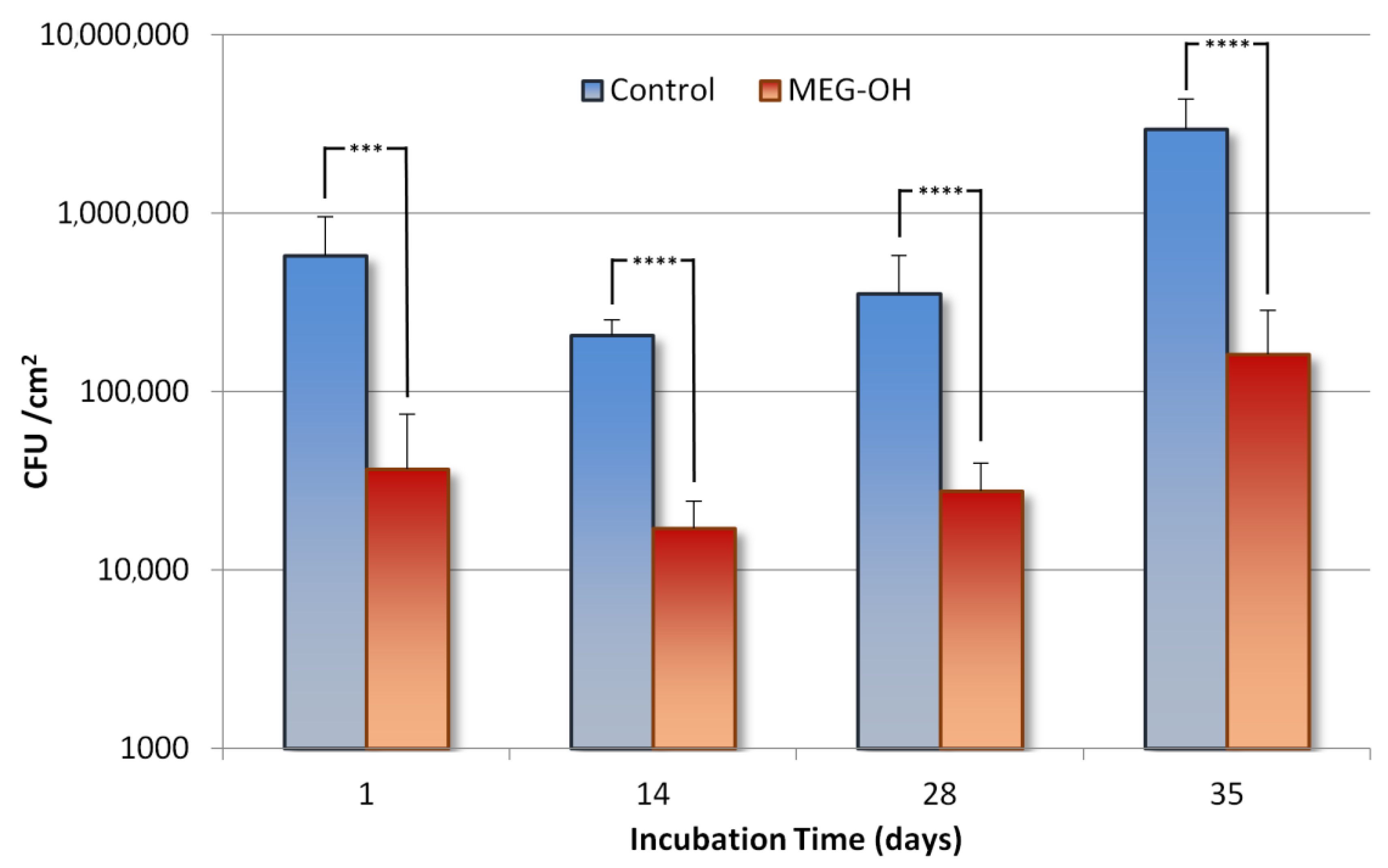
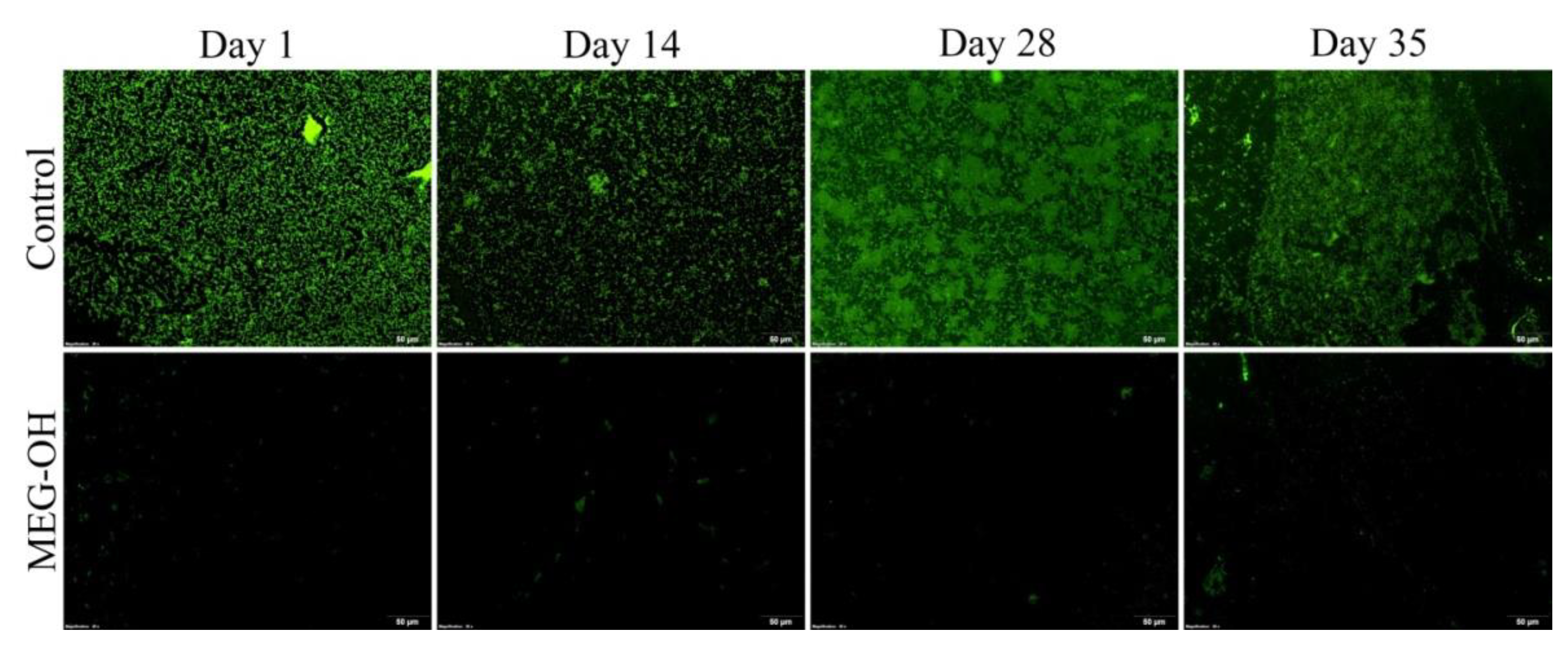
3.1. Reduction of E. coli Fouling
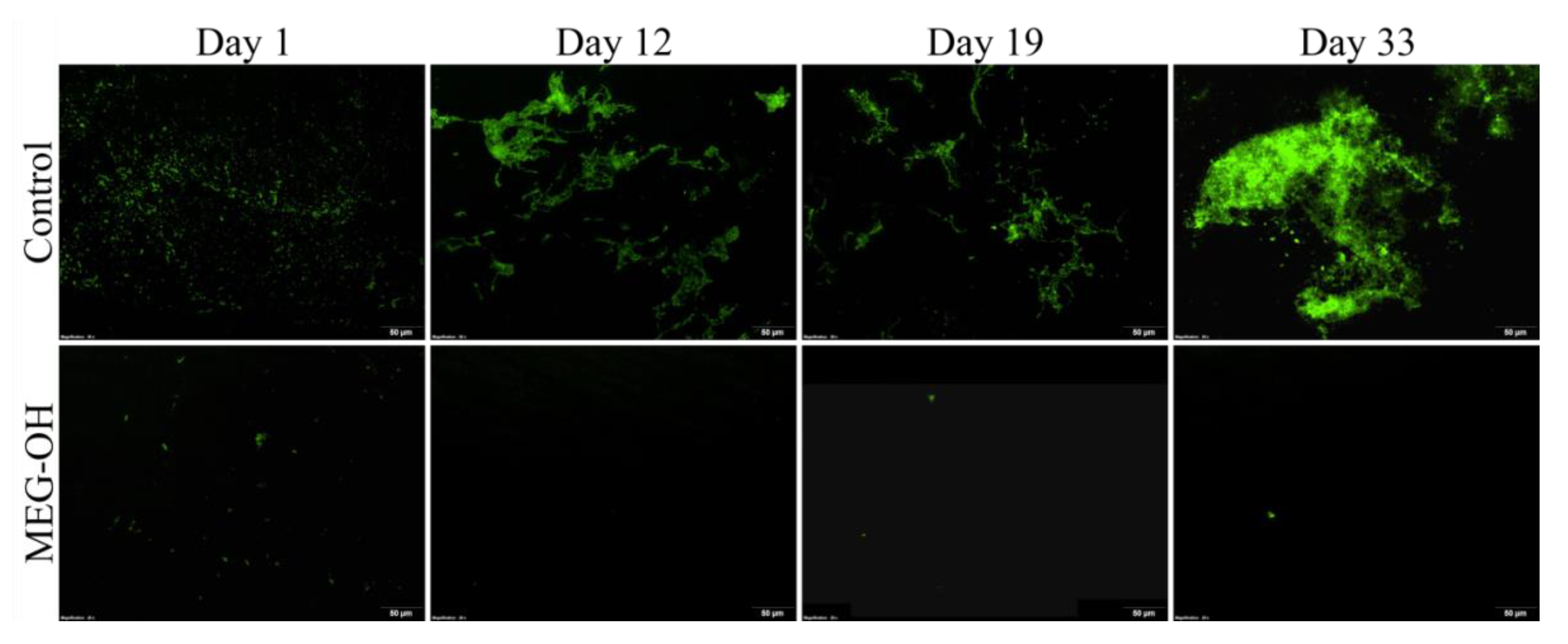
3.2. Reduction of S. aureus Fouling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control 2014, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, E.; Nicolle, L.E.; Coffin, S.E.; Gould, C.; Maragakis, L.L.; Meddings, J.; Pegues, D.A.; Pettis, A.M.; Saint, S.; Yokoe, D.S. Strategies to Prevent Catheter-Associated Urinary Tract Infections in Acute Care Hospitals: 2014 Update. Infect. Control Hosp. Epidemiol. 2014, 35, 464–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.T.; Shirtliff, M.E. Complicated Catheter-Associated Urinary Tract Infections Due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the Proportion of Healthcare-Associated Infections That Are Reasonably Preventable and the Related Mortality and Costs. Infect. Control Hosp. Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.D. The Direct Medical Costs of Healthcare-Associated Infections in US Hospitals and the Benefits of Prevention; Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Coordinating Center for Infectious Diseases, Centers for Disease Control and Prevention: Washington, DC, USA, 2009. [Google Scholar]
- Xing, C.-M.; Meng, F.-N.; Quan, M.; Ding, K.; Dang, Y.; Gong, Y.-K. Quantitative fabrication, performance optimization and comparison of PEG and zwitterionic polymer antifouling coatings. Acta Biomater. 2017, 59, 129–138. [Google Scholar] [CrossRef]
- Konradi, R.; Acikgoz, C.; Textor, M. Polyoxazolines for nonfouling surface coatings—A direct comparison to the gold stand-ard PEG. Macromol. Rapid Commun. 2012, 33, 1663–1676. [Google Scholar] [CrossRef]
- Harris, L.; Tosatti, S.; Wieland, M.; Textor, M.; Richards, R. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(l-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials 2004, 25, 4135–4148. [Google Scholar] [CrossRef]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.B.; Suh, H.; Choi, K.S. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Pasmore, M.; Todd, P.; Smith, S.; Baker, D.; Silverstein, J.; Coons, D.; Bowman, C.N. Effects of ultrafiltration membrane surface properties on Pseudomonas aeruginosa biofilm initiation for the purpose of reducing biofouling. J. Membr. Sci. 2001, 194, 15–32. [Google Scholar] [CrossRef]
- Roosjen, A.; Busscher, H.J.; Norde, W.; van der Mei, H.C. Bacterial factors influencing adhesion of Pseudomonas aeruginosa strains to a poly(ethylene oxide) brush. Microbiology 2006, 152, 2673–2682. [Google Scholar] [CrossRef] [Green Version]
- Nejadnik, M.R.; van der Mei, H.C.; Norde, W.; Busscher, H.J. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials 2008, 29, 4117–4121. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Patel, J.D.; Li, J.; Zhou, G.; Mukherjee, P.K.; McCormick, T.S.; Anderson, J.M.; Ghannoum, M.A. Modification of Surface Properties of Biomaterials Influences the Ability of Candida albicans To Form Biofilms. Appl. Environ. Microbiol. 2005, 71, 8795–8801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Johnson, R.W.; Desai, T.A. Evaluation of the Stability of Nonfouling Ultrathin Poly(ethylene glycol) Films for Silicon-Based Microdevices. Langmuir 2003, 20, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Pidhatika, B.; Rodenstein, M.; Chen, Y.; Rakhmatullina, E.; Muehlebach, A.; Acikgoez, C.; Textor, M.; Konradi, R. Comparative Stability Studies of Poly(2-methyl-2-oxazoline) and Poly(ethylene glycol) Brush Coatings. Biointerphases 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Noguer, A.C.; Olsen, S.M.; Hvilsted, S.; Kiil, S. Long-term stability of PEG-based antifouling surfaces in seawater. J. Coatings Technol. Res. 2016, 13, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Kim, C.; Kwon, D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polymer 1997, 38, 317–323. [Google Scholar] [CrossRef]
- Glastrup, J. Degradation of polyethylene glycol. A study of the reaction mechanism in a model molecule: Tetraethylene glycol. Polym. Degrad. Stabil. 1996, 52, 217–222. [Google Scholar] [CrossRef]
- Kratochvil, M.J.; Welsh, M.A.; Manna, U.; Ortiz, B.J.; Blackwell, H.E.; Lynn, D.M. Slippery Liquid-Infused Porous Surfaces that Prevent Bacterial Surface Fouling and Inhibit Virulence Phenotypes in Surrounding Planktonic Cells. ACS Infect. Dis. 2016, 2, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Keller, N.; Bruchmann, J.; Sollich, T.; Richter, C.; Thelen, R.; Kotz, F.; Schwartz, T.; Helmer, D.; Rapp, B.E. Study of Biofilm Growth on Slippery Liquid-Infused Porous Surfaces Made from Fluoropor. ACS Appl. Mater. Interfaces 2019, 11, 4480–4487. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.; Shen, T.; Chen, H.; Lu, J.; Yang, H.-C.; Li, W. Slippery liquid-infused porous surfaces (SLIPSs): A perfect solution to both marine fouling and corrosion? J. Mater. Chem. A 2020, 8, 7536–7547. [Google Scholar] [CrossRef]
- Mendichi, R.; Schieroni, A.G.; Piovani, D.; Allegrini, D.; Ferrara, M.; Romano, M.R. Comparative Study of Chemical Composition, Molecular and Rheological Properties of Silicone Oil Medical Devices. Transl. Vis. Sci. Technol. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asker, D.; Awad, T.S.; Baker, P.; Howell, P.L.; Hatton, B.D. Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials 2018, 167, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS Appl. Mater. Interfaces 2009, 1, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Pereira, M.O. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Tanford, C. Protein denaturation. In Advances in Protein Chemistry; Anflnsen, C.B., Anson, M.L., Edsall, J.T., Richards, F.M., Eds.; Academic Press: New York, NY, USA, 1968; Volume 23, pp. 121–282. [Google Scholar]
- Klibanov, A.M. Immobilized Enzymes and Cells as Practical Catalysts. Science 1983, 219, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Kheiria, S.; Liua, X.; Thompson, M. Nanoparticles at biointerfaces: Antibacterial activity and nanotoxicology. Colloids Surf. B Biointerfaces 2019, 184, 110550. [Google Scholar] [CrossRef]
- Vasudevan, R.; Kennedy, A.J.; Merritt, M.; Crocker, F.H.; Baney, R.H. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surf. B Biointerfaces 2014, 117, 225–232. [Google Scholar] [CrossRef]
- Parham, S.; Wicaksono, D.H.B.; Bagherbaigi, S.; Lee, S.L.; Nur, H. Antimicrobial treatment of different metal oxide nanoparticles: A critical review. J. Chin. Chem. Soc. 2016, 63, 385–393. [Google Scholar] [CrossRef]
- Qiao, Z.; Yao, Y.; Song, S.; Yin, M.; Luo, J. Silver nanoparticles with pH induced surface charge switchable properties for antibacterial and antibiofilm applications. J. Mater. Chem. B 2018, 7, 830–840. [Google Scholar] [CrossRef]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Franier, B.; Asker, D.; van den Berg, D.; Hatton, B.; Thompson, M. Reduction of microbial adhesion on polyurethane by a sub-nanometer covalently-attached surface modifier. Colloids Surf. B Biointerfaces 2021, 200, 111579. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Yang, D.Y.; Blaszykowski, C.; Thompson, M. Single ether group in a glycol-based ultra-thin layer prevents surface fouling from undiluted serum. Chem. Commun. 2012, 48, 1305–1307. [Google Scholar] [CrossRef]
- Sheikh, S.; Sheng, J.C.-C.; Blaszykowski, C.; Thompson, M. New oligoethylene glycol linkers for the surface modification of an ultra-high frequency acoustic wave biosensor. Chem. Sci. 2010, 1, 271–275. [Google Scholar] [CrossRef]
- Fedorov, K.; Blaszykowski, C.; Sheikh, S.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of thrombogenesis from whole human blood on plastic polymer by ultrathin monoethylene glycol silane adlayer. Langmuir 2014, 30, 3217. [Google Scholar] [CrossRef]
- Fedorov, K.; Jankowski, A.; Sheikh, S.; Blaszykowski, C.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of surface-induced thrombogenesis on poly(vinylchloride). J. Mater. Chem. B 2015, 3, 8623. [Google Scholar] [CrossRef]
- Fedorov, K.; Sheikh, S.; Romaschin, A.; Thompson, M. Enhanced Long-term Antithrombogenicity Instigated by Covalently-Attached Surface Modifier on Biomedical Polymers. Recent Prog. Mater. 2020, 2, 16. [Google Scholar] [CrossRef]
- Pawlowska, N.M.; Fritzsche, H.; Blaszykowski, C.; Sheikh, S.; Vezvaie, M.; Thompson, M. Probing the Hydration of Ultrathin Antifouling Organosilane Adlayers using Neutron Reflectometry. Langmuir 2014, 30, 1199–1203. [Google Scholar] [CrossRef]
- Sheikh, S.; Blaszykowski, C.; Nolan, R.; Thompson, D.; Thompson, M. On the hydration of subnanometric antifouling organosilane adlayers: A molecular dynamics simulation. J. Colloid Interface Sci. 2015, 437, 197–204. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Franier, B.; Asker, D.; Hatton, B.; Thompson, M. Long-Term Reduction of Bacterial Adhesion on Polyurethane by an Ultra-Thin Surface Modifier. Biomedicines 2022, 10, 979. https://doi.org/10.3390/biomedicines10050979
De La Franier B, Asker D, Hatton B, Thompson M. Long-Term Reduction of Bacterial Adhesion on Polyurethane by an Ultra-Thin Surface Modifier. Biomedicines. 2022; 10(5):979. https://doi.org/10.3390/biomedicines10050979
Chicago/Turabian StyleDe La Franier, Brian, Dalal Asker, Benjamin Hatton, and Michael Thompson. 2022. "Long-Term Reduction of Bacterial Adhesion on Polyurethane by an Ultra-Thin Surface Modifier" Biomedicines 10, no. 5: 979. https://doi.org/10.3390/biomedicines10050979





