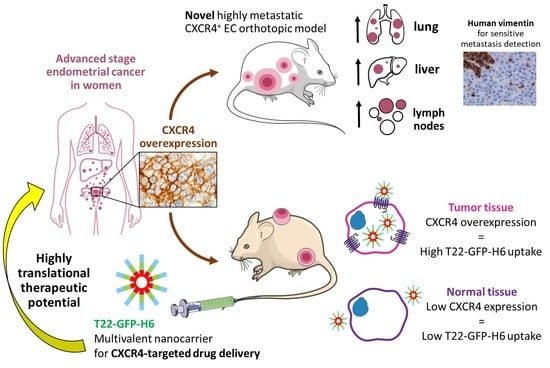
Novel Endometrial Cancer Models Using Sensitive Metastasis Tracing for CXCR4-Targeted Therapy in Advanced Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endometrial Cancer Patient Samples
2.2. Cell Culture
2.2.1. Cell Line Constructs and Stable Cell Line Generation
2.2.2. Lentiviral Transduction
2.2.3. Flow Cytometry
Membrane CXCR4 Assessment
Internalization Assay
2.2.4. Cell Viability Assay
2.2.5. Cell Blocks
2.3. In Vivo Experiments
2.3.1. Orthotopic EC Models
2.3.2. Subcutaneous CXCR4+ EC Models
2.3.3. In Vivo T22-GFP-H6 Biodistribution
2.3.4. Necropsy and Histological Examination
2.4. Bioluminescence Intensity Assessment
2.5. Immunocytochemistry and Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Immunohistochemical Evaluation of CXCR4 Expression in EC Patient Samples
3.2. Generation of CXCR4+ Luciferase+ Human EC Cell Lines
3.3. Development of a Subcutaneous Tumor Model Bearing Human EC Cells in Swiss Nude and Follow-Up Markers of Cancer Cell Growth
3.4. Bioluminescent Follow-Up of Primary Tumor and Metastatic Dissemination in a Novel Orthotopic Model of Advanced EC in NSG Mice
3.5. Marker-Guided Comparison of Metastatic Yield in the EC Intrauterine Orthotopic Models Generated from CXCR4- or CXCR4+ EC Cells in NSG Mice
3.6. In Vitro Uptake of the Fluorescent T22-GFP-H6 Nanocarrier and Its Cytotoxicity in Human EC Cell Lines
3.7. Biodistribution of CXCR4-Targeted Nanocarrier T22-GFP-H6 in a CXCR4+ Subcutaneous EC Model
4. Discussion
4.1. CXCR4 Expression Pattern in EC Patients
4.2. Development of an Aggressive CXCR4+ Advanced EC Metastatic Model
4.3. CXCR4 Overexpression Is Associated with Enhanced Metastatic Dissemination in EC
4.4. Use of Highly Sensitive Human-Vimentin as EC Tumor Cells Marker to Detect Metastatic Foci
4.5. Development of a CXCR4 Subcutaneous Tumor Model and Its Use to Evaluate Targeting of Protein-Based Nanocarriers to CXCR4+ EC Cells
4.6. Future Contribution of the Novel Models for the Development of Targeted Therapies in Advanced EC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund. Endometrial Cancer Statistics 2018. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/endometrial-cancer-statistics (accessed on 4 April 2020).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Y.; Yuan, Z.; Zong, X.; Huo, X.; Cao, D.-Y.; Yang, J.-X.; Shen, K. Fertility-Sparing Treatment in Young Patients with Grade 2 Presumed Stage IA Endometrioid Endometrial Adenocarcinoma. Front. Oncol. 2020, 10, 1437. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; D’Indinosante, M.; Turrini, I.; Giusti, M.; Gullo, G.; Vizzielli, G.; Mattei, A.; Scambia, G.; et al. Fertility Sparing Treatments in Endometrial Cancer Patients: The Potential Role of the New Molecular Classification. Int. J. Mol. Sci. 2021, 22, 12248. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Etrusco, A.; Cucinella, G.; Perino, A.; Chiantera, V.; Laganà, A.S.; Tomaiuolo, R.; Vitagliano, A.; Giampaolino, P.; Noventa, M.; et al. Fertility-Sparing Approach in Women Affected by Stage I and Low-Grade Endometrial Carcinoma: An Updated Overview. Int. J. Mol. Sci. 2021, 22, 11825. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Physician 2016, 93, 468–474. [Google Scholar] [PubMed]
- Santaballa, A.; Matías-Guiu, X.; Redondo, A.; Carballo, N.; Gil, M.; Gómez, C.; Gorostidi, M.; Gutierrez, M.; Gónzalez-Martín, A. SEOM clinical guidelines for endometrial cancer (2017). Clin. Transl. Oncol. 2018, 20, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, S.M.; Cohn, D.E. Treatment of Metastatic Endometrial Cancer. UpToDate 2021, 26, 15–23. Available online: https://www.uptodate.com/contents/treatment-of-metastatic-endometrial-cancer (accessed on 20 December 2021).
- Van Nyen, T.; Moiola, C.P.; Colas, E.; Annibali, D.; Amant, F. Modeling Endometrial Cancer: Past, Present, and Future. Int. J. Mol. Sci. 2018, 19, 2348. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, S.; Llauradó, M.; Castellví, J.; Fernandez, Y.; Alameda, F.; Colás, E.; Ruiz, A.; Doll, A.; Schwartz, S., Jr.; Carreras, R.; et al. Generation and characterization of orthotopic murine models for endometrial cancer. Clin. Exp. Metastasis 2012, 29, 217–227. [Google Scholar] [CrossRef]
- Fedorko, A.M.; Kim, T.H.; Broaddus, R.; Schmandt, R.; Chandramouli, G.V.; Kim, H.I.; Jeong, J.-W.; Risinger, J.I. An immune competent orthotopic model of endometrial cancer with metastasis. Heliyon 2020, 6, e04075. [Google Scholar] [CrossRef]
- Konings, G.F.; Saarinen, N.; Delvoux, B.; Kooreman, L.; Koskimies, P.; Krakstad, C.; Fasmer, K.E.; Haldorsen, I.S.; Zaffagnini, A.; Häkkinen, M.R.; et al. Development of an Image-Guided Orthotopic Xenograft Mouse Model of Endometrial Cancer with Controllable Estrogen Exposure. Int. J. Mol. Sci. 2018, 19, 2547. [Google Scholar] [CrossRef] [Green Version]
- Pillozzi, S.; Fortunato, A.; De Lorenzo, E.; Borrani, E.; Giachi, M.; Scarselli, G.; Arcangeli, A.; Noci, I. Over-Expression of the LH Receptor Increases Distant Metastases in an Endometrial Cancer Mouse Model. Front. Oncol. 2013, 3, 285. [Google Scholar] [CrossRef] [Green Version]
- Winship, A.L.; Van Sinderen, M.; Donoghue, J.; Rainczuk, K.; Dimitriadis, E. Targeting Interleukin-11 Receptor-α Impairs Human Endometrial Cancer Cell Proliferation and Invasion In Vitro and Reduces Tumor Growth and Metastasis In Vivo. Mol. Cancer Ther. 2016, 15, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-Y.; Chiang, Y.-F.; Huang, J.-S.; Huang, T.-C.; Shih, Y.-H.; Wang, K.-L.; Ali, M.; Hong, Y.-H.; Shieh, T.-M.; Hsia, S.-M. Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer. Cancers 2021, 13, 1236. [Google Scholar] [CrossRef]
- Doll, A.; Gonzalez, M.; Abal, M.; Llaurado, M.; Rigau, M.; Colas, E.; Monge, M.; Xercavins, J.; Capella, G.; Diaz, B.; et al. An orthotopic endometrial cancer mouse model demonstrates a role for RUNX1 in distant metastasis. Int. J. Cancer 2009, 125, 257–263. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Popa, M.; Fonnes, T.; Brekke, N.; Kopperud, R.; Visser, N.C.; Rygh, C.B.; Pavlin, T.; Salvesen, H.B.; Mc Cormack, E.; et al. Multimodal Imaging of Orthotopic Mouse Model of Endometrial Carcinoma. PLoS ONE 2015, 10, e0135220. [Google Scholar] [CrossRef] [Green Version]
- Hanekamp, E.E.; Gielen, S.C.; van Oosterhoud, S.A.; Burger, C.W.; Grootegoed, J.; Huikeshoven, F.J.; Blok, L.J. Progesterone receptors in endometrial cancer invasion and metastasis: Development of a mouse model. Steroids 2003, 68, 795–800. [Google Scholar] [CrossRef]
- Kato, M.; Onoyama, I.; Yoshida, S.; Cui, L.; Kawamura, K.; Kodama, K.; Hori, E.; Matsumura, Y.; Yagi, H.; Asanoma, K.; et al. Dual-specificity phosphatase 6 plays a critical role in the maintenance of a cancer stem-like cell phenotype in human endometrial cancer. Int. J. Cancer 2020, 147, 1987–1999. [Google Scholar] [CrossRef] [Green Version]
- Popli, P.; Richters, M.M.; Chadchan, S.B.; Kim, T.H.; Tycksen, E.; Griffith, O.; Thaker, P.H.; Griffith, M.; Kommagani, R. Splicing factor SF3B1 promotes endometrial cancer progression via regulating KSR2 RNA maturation. Cell Death Dis. 2020, 11, 842. [Google Scholar] [CrossRef]
- Unno, K.; Ono, M.; Winder, A.D.; Maniar, K.P.; Paintal, A.S.; Yu, Y.; Wei, J.-J.; Lurain, J.R.; Kim, J.J. Establishment of Human Patient-Derived Endometrial Cancer Xenografts in NOD scid Gamma Mice for the Study of Invasion and Metastasis. PLoS ONE 2014, 9, e116064. [Google Scholar] [CrossRef]
- Liu, P.; Long, P.; Huang, Y.; Sun, F.; Wang, Z. CXCL12/CXCR4 axis induces proliferation and invasion in human endometrial cancer. Am. J. Transl. Res. 2016, 11, 1719. [Google Scholar]
- Teng, F.; Tian, W.-Y.; Wang, Y.-M.; Zhang, Y.-F.; Guo, F.; Zhao, J.; Gao, C.; Xue, F.-X. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J. Hematol. Oncol. 2016, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Buchynska, L.G.; Movchan, O.M.; Iurchenko, N.P. Expression of chemokine receptor CXCR4 in tumor cells and content of CXCL12+-fibroblasts in endometrioid carcinoma of endometrium. Exp. Oncol. 2021, 43, 135–141. [Google Scholar] [PubMed]
- Kircher, M.; Herhaus, P.; Schottelius, M.; Buck, A.K.; Werner, R.A.; Wester, H.-J.; Keller, U.; Lapa, C. CXCR4-directed theranostics in oncology and inflammation. Ann. Nucl. Med. 2018, 32, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felix, A.S.; Edwards, R.; Bowser, R.; Linkov, F. Chemokines and Cancer Progression: A Qualitative Review on the Role of Stromal Cell-derived Factor 1-alpha and CXCR4 in Endometrial Cancer. Cancer Microenviron. 2010, 3, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizokami, Y.; Kajiyama, H.; Shibata, K.; Ino, K.; Kikkawa, F.; Mizutani, S. Stromal cell-derived factor-1?-induced cell proliferation and its possible regulation by CD26/dipeptidyl peptidase IV in endometrial adenocarcinoma. Int. J. Cancer 2004, 110, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, S.; Mangoni, M.; Castiglione, F.; Beltrami, C.; Pieralli, A.; Andersson, K.L.; Fambrini, M.; Taddei, G.L.; Serio, M.; Orlando, C. The CXCR4/CXCL12 axis in endometrial cancer. Clin. Exp. Metastasis 2009, 26, 261–268. [Google Scholar] [CrossRef]
- Lefort, S.; Thuleau, A.; Kieffer, Y.; Sirven, P.; Bieche, I.; Marangoni, E.; Vincent-Salomon, A.; Mechta-Grigoriou, F. CXCR4 inhibitors could benefit to HER2 but not to triple-negative breast cancer patients. Oncogene 2016, 36, 1211–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, L.; Zheng, Y.; Hong, Y.; Wang, M.; Deng, Y.; Wu, Y.; Xu, P.; Yang, S.; Wang, S.; Yao, J.; et al. Comprehensive analysis of the prognostic value and immune function of chemokine-CXC receptor family members in breast cancer. Int. Immunopharmacol. 2020, 87, 106797. [Google Scholar] [CrossRef] [PubMed]
- Krikun, G. The CXL12/CXCR4/CXCR7 axis in female reproductive tract disease: Review. Am. J. Reprod. Immunol. 2018, 80, e13028. [Google Scholar] [CrossRef]
- Walentowicz-Sadlecka, M.; Sadlecki, P.; Bodnar, M.; Marszalek, A.; Walentowicz, P.; Sokup, A.; Wilińska-Jankowska, A.; Grabiec, M. Stromal Derived Factor-1 (SDF-1) and Its Receptors CXCR4 and CXCR7 in Endometrial Cancer Patients. PLoS ONE 2014, 9, e84629. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, Y.; Long, P.; Zhao, S.; Zhang, L. Silencing of CXCR4 and CXCR7 expression by RNA interference suppresses human endometrial carcinoma growth in vivo. Am. J. Transl. Res. 2017, 9, 1896–1904. [Google Scholar]
- Sirohi, V.K.; Popli, P.; Sankhwar, P.; Kaushal, J.B.; Gupta, K.; Manohar, M.; Dwivedi, A. Curcumin exhibits anti-tumor effect and attenuates cellular migration via Slit-2 mediated down-regulation of SDF-1 and CXCR4 in endometrial adenocarcinoma cells. J. Nutr. Biochem. 2017, 44, 60–70. [Google Scholar] [CrossRef]
- Sun, Y.; Yoshida, T.; Okabe, M.; Zhou, K.; Wang, F.; Soko, C.; Saito, S.; Nikaido, T. Isolation of Stem-Like Cancer Cells in Primary Endometrial Cancer Using Cell Surface Markers CD133 and CXCR4. Transl. Oncol. 2017, 10, 976–987. [Google Scholar] [CrossRef] [Green Version]
- Villaverde, A.; Unzueta, U.; Céspedes, M.V.; Ferrer-Miralles, N.; Casanova, I.; Cedano, J.; Corchero, J.L.; Domingo-Espín, J.; Mangues, R.; Vázquez, E. Intracellular CXCR4+ cell targeting with T22-empowered protein-only nanoparticles. Int. J. Nanomed. 2012, 7, 4533–4544. [Google Scholar] [CrossRef] [Green Version]
- Céspedes, M.V.; Unzueta, U.; Álamo, P.; Gallardo, A.; Sala, R.; Casanova, I.; Pavon, M.A.; Mangues, M.A.; Trías, M.; Pousa, A.L.; et al. Cancer-specific uptake of a liganded protein nanocarrier targeting aggressive CXCR4 + colorectal cancer models. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1987–1996. [Google Scholar] [CrossRef]
- Falgàs, A.; Pallarès, V.; Unzueta, U.; Céspedes, M.V.; Arroyo-Solera, I.; Moreno, M.J.; Sierra, J.; Gallardo, A.; Mangues, M.A.; Vázquez, E.; et al. A CXCR4-targeted nanocarrier achieves highly selective tumor uptake in diffuse large B-cell lymphoma mouse models. Haematologica 2019, 105, 741–753. [Google Scholar] [CrossRef] [Green Version]
- Rioja-Blanco, E.; Arroyo-Solera, I.; Álamo, P.; Casanova, I.; Gallardo, A.; Unzueta, U.; Serna, N.; Sánchez-García, L.; Quer, M.; Villaverde, A.; et al. Self-assembling protein nanocarrier for selective delivery of cytotoxic polypeptides to CXCR4+ head and neck squamous cell carcinoma tumors. Acta Pharm. Sin. B 2021, 12, 2578–2591. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Ialongo, C. Understanding the effect size and its measures. Biochem. Med. 2016, 26, 150–163. [Google Scholar] [CrossRef] [Green Version]
- Friel, A.; Sergent, P.; Patnaude, C.; Szotek, P.P.; Oliva, E.; Scadden, D.T.; Seiden, M.V.; Foster, R.; Rueda, B.R. Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle 2008, 7, 242–249. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.-G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Piulats, J.M.; Guerra, E.; Gil-Martín, M.; Roman-Canal, B.; Gatius, S.; Sanz-Pamplona, R.; Velasco, A.; Vidal, A.; Matias-Guiu, X. Molecular approaches for classifying endometrial carcinoma. Gynecol. Oncol. 2016, 145, 200–207. [Google Scholar] [CrossRef]
- Tomiyama, L.; Kamino, H.; Fukamachi, H.; Urano, T. Precise epitope determination of the anti-vimentin monoclonal antibody V9. Mol. Med. Rep. 2017, 16, 3917–3921. [Google Scholar] [CrossRef] [Green Version]
- Pallarès, V.; Unzueta, U.; Falgàs, A.; Sánchez-García, L.; Serna, N.; Gallardo, A.; Morris, G.A.; Alba-Castellón, L.; Álamo, P.; Sierra, J.; et al. An Auristatin nanoconjugate targeting CXCR4+ leukemic cells blocks acute myeloid leukemia dissemination. J. Hematol. Oncol. 2020, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Walenkamp, A.M.E.; Lapa, C.; Herrmann, K.; Wester, H.-J. CXCR4 Ligands: The Next Big Hit? J. Nucl. Med. 2017, 58, 77S–82S. [Google Scholar] [CrossRef] [Green Version]





| Inoculated Cell Line | Liver Mets | Lung Mets | ||||
|---|---|---|---|---|---|---|
| Total Foci | Single Cell Foci | Clustered Cells Foci | Invaded Tissue Area (%) | |||
| Number | Area (µm2) | Number | Number | Area (µm2) | ||
| CXCR4- AN3CA | 10.2 ± 6.7 a | 615.5 ± 429.5 b | 9.4 ± 6.3 | 0.9 ± 0.5 c | 4486.4 ± 2728.0 | 11.5 ± 4.2 d |
| CXCR4+ AN3CA | 24.2 ± 8.3 a | 2536.8 ± 1746.8 b | 15.6 ± 6.5 | 9.7 ± 2.7 c | 5305.0 ± 3517.9 | 26.1 ± 7.0 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Gutiérrez, E.; Céspedes, M.V.; Gallardo, A.; Rioja-Blanco, E.; Pavón, M.À.; Asensio-Puig, L.; Farré, L.; Alba-Castellón, L.; Unzueta, U.; Villaverde, A.; et al. Novel Endometrial Cancer Models Using Sensitive Metastasis Tracing for CXCR4-Targeted Therapy in Advanced Disease. Biomedicines 2022, 10, 1680. https://doi.org/10.3390/biomedicines10071680
Medina-Gutiérrez E, Céspedes MV, Gallardo A, Rioja-Blanco E, Pavón MÀ, Asensio-Puig L, Farré L, Alba-Castellón L, Unzueta U, Villaverde A, et al. Novel Endometrial Cancer Models Using Sensitive Metastasis Tracing for CXCR4-Targeted Therapy in Advanced Disease. Biomedicines. 2022; 10(7):1680. https://doi.org/10.3390/biomedicines10071680
Chicago/Turabian StyleMedina-Gutiérrez, Esperanza, María Virtudes Céspedes, Alberto Gallardo, Elisa Rioja-Blanco, Miquel Àngel Pavón, Laura Asensio-Puig, Lourdes Farré, Lorena Alba-Castellón, Ugutz Unzueta, Antonio Villaverde, and et al. 2022. "Novel Endometrial Cancer Models Using Sensitive Metastasis Tracing for CXCR4-Targeted Therapy in Advanced Disease" Biomedicines 10, no. 7: 1680. https://doi.org/10.3390/biomedicines10071680