A Mouse Model for the Rapid and Binomial Assessment of Putative WNT/β-Catenin Signalling Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Animal Models
2.3. Animal Treatments
2.4. Immunohistochemistry
2.5. Real-Time PCR
2.6. T2 MRI Scan
2.7. Statistical Analysis
3. Results
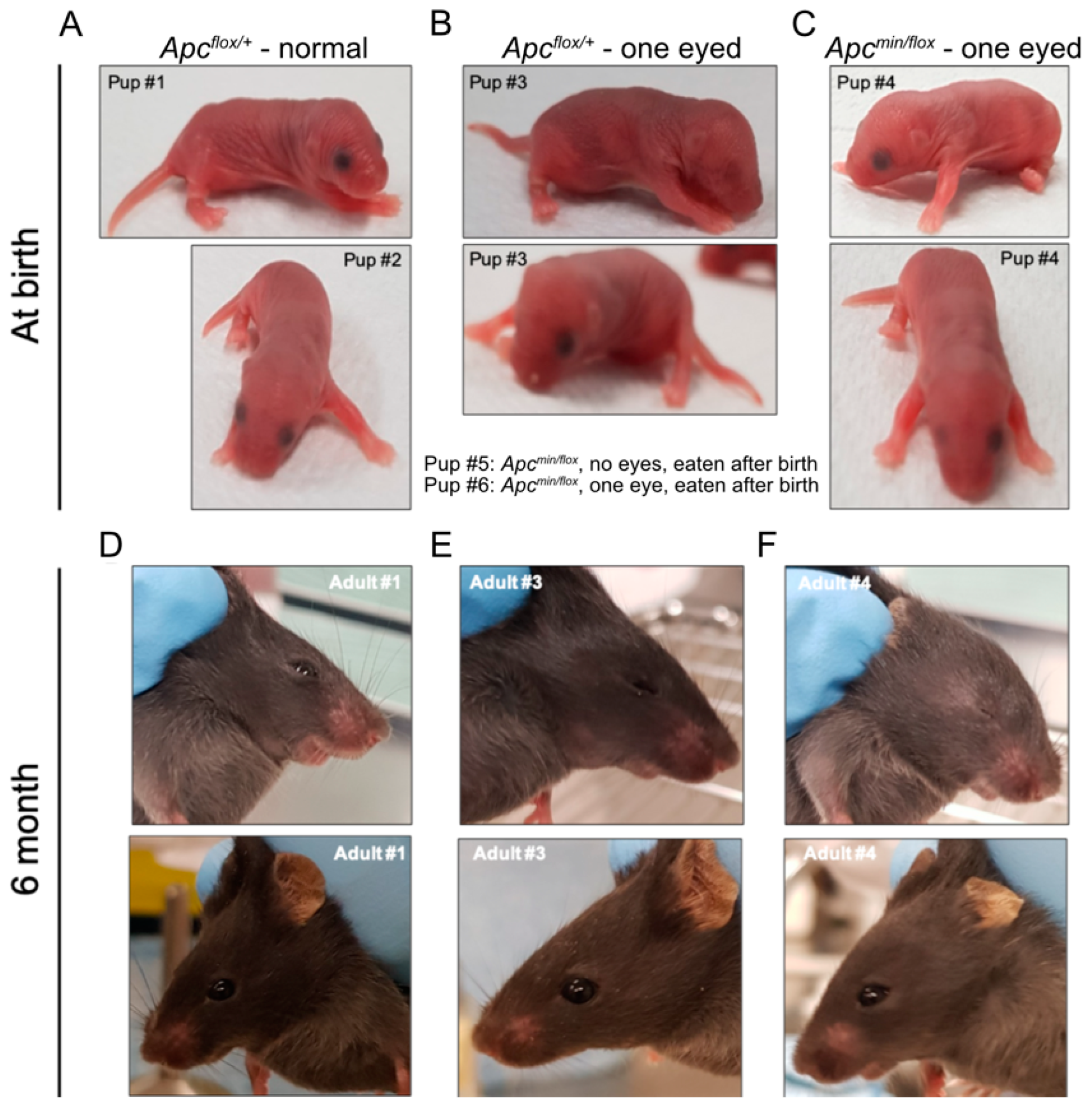
3.1. Administration of TNKSi Attenuates WNT Pathway Activity In Vivo and Partially Rescues Cranial Defects In Vivo
3.2. In Vivo Administration of TNKSi Results in Live Births of Apcmin/flox Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rim, E.Y.; Clevers, H.; Nusse, R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu. Rev. Biochem. 2022, 91, 571–598. [Google Scholar] [CrossRef]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Wiese, K.E.; Nusse, R.; van Amerongen, R. Wnt signalling: Conquering complexity. Development 2018, 145, dev165902. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond beta-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef]
- Morris, A.; Pagare, P.P.; Li, J.; Zhang, Y. Drug discovery efforts toward inhibitors of canonical Wnt/beta-catenin signaling pathway in the treatment of cancer: A composition-of-matter review (2010–2020). Drug Discov. Today 2022, 27, 1115–1127. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef] [PubMed]
- Brinch, S.A.; Amundsen-Isaksen, E.; Espada, S.; Hammarstrom, C.; Aizenshtadt, A.; Olsen, P.A.; Holmen, L.; Hoyem, M.; Scholz, H.; Grodeland, G.; et al. The Tankyrase Inhibitor OM-153 Demonstrates Antitumor Efficacy and a Therapeutic Window in Mouse Models. Cancer Res. Commun. 2022, 2, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.; Chan, E.; Callow, M.; Waaler, J.; Boggs, J.; Blake, R.A.; Magnuson, S.; Sambrone, A.; Schutten, M.; Firestein, R.; et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013, 73, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
- Leenders, R.G.G.; Brinch, S.A.; Sowa, S.T.; Amundsen-Isaksen, E.; Galera-Prat, A.; Murthy, S.; Aertssen, S.; Smits, J.N.; Nieczypor, P.; Damen, E.; et al. Development of a 1,2,4-Triazole-Based Lead Tankyrase Inhibitor: Part II. J. Med. Chem. 2021, 64, 17936–17949. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.; Holsworth, D.D.; Waaler, J.; Wilson, S.R.; Ekblad, B.; Perdreau-Dahl, H.; Dinh, H.; Drewes, G.; Hopf, C.; Morth, J.P.; et al. Structural basis and SAR for G007-LK, a lead stage 1,2,4-triazole based specific tankyrase 1/2 inhibitor. J. Med. Chem. 2013, 56, 3012–3023. [Google Scholar] [CrossRef]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Bugter, J.M.; Fenderico, N.; Maurice, M.M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat. Rev. Cancer 2021, 21, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef]
- Rubinfeld, B.; Albert, I.; Porfiri, E.; Fiol, C.; Munemitsu, S.; Polakis, P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 1996, 272, 1023–1026. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- De Robertis, E.M. Wnt signaling in axial patterning and regeneration: Lessons from planaria. Sci. Signal. 2010, 3, pe21. [Google Scholar] [CrossRef]
- Wieschaus, E.; Nusslein-Volhard, C. The Heidelberg Screen for Pattern Mutants of Drosophila: A Personal Account. Annu. Rev. Cell Dev. Biol. 2016, 32, 1–46. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Fuhrmann, S. Wnt signaling in eye organogenesis. Organogenesis 2008, 4, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Toyama, K.; Shioya, H.; Ito, M.; Hirota, M.; Hasegawa, S.; Matsumoto, H.; Takano, H.; Akiyama, T.; Toyoshima, K.; et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997, 278, 120–123. [Google Scholar] [CrossRef]
- Moser, A.R.; Pitot, H.C.; Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990, 247, 322–324. [Google Scholar] [CrossRef]
- Buchert, M.; Athineos, D.; Abud, H.E.; Burke, Z.D.; Faux, M.C.; Samuel, M.S.; Jarnicki, A.G.; Winbanks, C.E.; Newton, I.P.; Meniel, V.S.; et al. Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo. PLoS Genet. 2010, 6, e1000816. [Google Scholar] [CrossRef] [PubMed]
- Chvedoff, M.; Clarke, M.R.; Irisarri, E.; Faccini, J.M.; Monro, A.M. Effects of housing conditions on food intake, body weight and spontaneous lesions in mice. A review of the literature and results of an 18-month study. Food Cosmet. Toxicol. 1980, 18, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Norum, J.H.; Skarpen, E.; Brech, A.; Kuiper, R.; Waaler, J.; Krauss, S.; Sorlie, T. The tankyrase inhibitor G007-LK inhibits small intestine LGR5(+) stem cell proliferation without altering tissue morphology. Biol. Res. 2018, 51, 3. [Google Scholar] [CrossRef]
- Buchert, M.; Rohde, F.; Eissmann, M.; Tebbutt, N.; Williams, B.; Tan, C.W.; Owen, A.; Hirokawa, Y.; Gnann, A.; Orend, G.; et al. A hypermorphic epithelial beta-catenin mutation facilitates intestinal tumorigenesis in mice in response to compounding WNT-pathway mutations. Dis. Model. Mech. 2015, 8, 1361–1373. [Google Scholar] [CrossRef]
- Haegel, H.; Larue, L.; Ohsugi, M.; Fedorov, L.; Herrenknecht, K.; Kemler, R. Lack of beta-catenin affects mouse development at gastrulation. Development 1995, 121, 3529–3537. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Brinkmann, V.; Erdmann, B.; Birchmeier, C.; Birchmeier, W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 2000, 148, 567–578. [Google Scholar] [CrossRef]
- Moser, A.R.; Shoemaker, A.R.; Connelly, C.S.; Clipson, L.; Gould, K.A.; Luongo, C.; Dove, W.F.; Siggers, P.H.; Gardner, R.L. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev. Dyn. 1995, 203, 422–433. [Google Scholar] [CrossRef]
- Rudloff, S.; Kemler, R. Differential requirements for beta-catenin during mouse development. Development 2012, 139, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Gay, M.; Steiner, S.; Draganova, K.; Zemke, M.; Hoffmans, R.; Cinelli, P.; Aguet, M.; Sommer, L.; Basler, K. Probing transcription-specific outputs of beta-catenin in vivo. Genes. Dev. 2011, 25, 2631–2643. [Google Scholar] [CrossRef] [PubMed]
- Thorne, C.A.; Hanson, A.J.; Schneider, J.; Tahinci, E.; Orton, D.; Cselenyi, C.S.; Jernigan, K.K.; Meyers, K.C.; Hang, B.I.; Waterson, A.G.; et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat. Chem. Biol. 2010, 6, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Faux, M.C.; Weinstock, J.; Gogos, S.; Prato, E.; Azimpour, A.I.; O’Keefe, R.; Cathcart-King, Y.; Garnham, A.L.; Ernst, M.; Preaudet, A.; et al. Combined Treatment with a WNT Inhibitor and the NSAID Sulindac Reduces Colon Adenoma Burden in Mice with Truncated APC. Cancer Res. Commun. 2022, 2, 66–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Chase, H.B. Studies on an Anophthalmic Strain of Mice. III. Results of Crosses with Other Strains. Genetics 1942, 27, 339–348. [Google Scholar] [CrossRef]
- Chase, H.B.; Chase, E.B. Studies on an Anophthalmic Strain of Mice. I. Embryology fo the Eye Region. J. Morphol. 1941, 68, 279–301. [Google Scholar] [CrossRef]
- Tetro, N.; Moushaev, S.; Rubinchik-Stern, M.; Eyal, S. The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res. 2018, 35, 71. [Google Scholar] [CrossRef] [PubMed]
- Ashery-Padan, R.; Marquardt, T.; Zhou, X.; Gruss, P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes. Dev. 2000, 14, 2701–2711. [Google Scholar] [CrossRef]
- Hanson, I.; Van Heyningen, V. Pax6: More than meets the eye. Trends Genet. 1995, 11, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Machon, O.; Kreslova, J.; Ruzickova, J.; Vacik, T.; Klimova, L.; Fujimura, N.; Lachova, J.; Kozmik, Z. Lens morphogenesis is dependent on Pax6-mediated inhibition of the canonical Wnt/beta-catenin signaling in the lens surface ectoderm. Genesis 2010, 48, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Burke, Z.D.; Reed, K.R.; Phesse, T.J.; Sansom, O.J.; Clarke, A.R.; Tosh, D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology 2009, 136, 2316–2324.e3. [Google Scholar] [CrossRef] [PubMed]
- Benhamouche, S.; Decaens, T.; Godard, C.; Chambrey, R.; Rickman, D.S.; Moinard, C.; Vasseur-Cognet, M.; Kuo, C.J.; Kahn, A.; Perret, C.; et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev. Cell 2006, 10, 759–770. [Google Scholar] [CrossRef]
- Niehrs, C. The role of Xenopus developmental biology in unraveling Wnt signalling and antero-posterior axis formation. Dev. Biol. 2022, 482, 1–6. [Google Scholar] [CrossRef]
- Sokol, S.; Christian, J.L.; Moon, R.T.; Melton, D.A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 1991, 67, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Waaler, J.; Machon, O.; von Kries, J.P.; Wilson, S.R.; Lundenes, E.; Wedlich, D.; Gradl, D.; Paulsen, J.E.; Machonova, O.; Dembinski, J.L.; et al. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011, 71, 197–205. [Google Scholar] [CrossRef]


| Inhibitor | Treatment | Outcome |
|---|---|---|
| None | NA | All progeny Apcflox/+ (15/15, born alive, 5 litters) |
| G007-LK | E5–14 | Complete abortion (no embryonic tissue detected at E15; 1 pregnancy) |
| G007-LK | E2–3 | Complete abortion (no embryonic tissue detected at E15; 1 pregnancy) |
| G007-LK | E5–10 | Complete abortion (no embryonic tissue detected at E15; 1 pregnancy) |
| G007-LK | E4–7 | Complete abortion (no embryonic tissue detected at E15; 1 pregnancy) |
| G007-LK | E5–6 | Deformed, non-viable embryos (3/3; E15, 1 litter, genotypes not determined) |
| G007-LK | E5–7 | Rescue of headless phenotype in subset (5/8) of Apcmin/flox embryos (E15, 2 litters, 17 embryos in total: 9 Apcflox/+, 8 Apcmin/flox) |
| G007-LK | E5–7 & E9–10 | Complete abortion (no embryonic tissue detected at E15; 1 pregnancy) |
| G007-LK | E5–7 | Rescue of headless phenotype in subset (8/11) of born pups (3 litters, 19 pups in total: 8 Apcflox/+, 11 Apcmin/flox) |
| Pyrvinium | E5–7 | Rescue of headless phenotype in subset (1/3) of born pups (1 litter, 4 pups in total: 1 Apcflox/+, 3 Apcmin/flox) |
| Born Alive | Stillborn | |||||
|---|---|---|---|---|---|---|
| Drug | Genotype | Two Eyes | One Eye | No Eye | Cranial Defects | Litters (n) |
| none | flox/+ | 15 | 0 | 0 | 0 | 5 |
| none | min/flox | 0 | 0 | 0 | 0 | |
| G007-LK | flox/+ | 7 | 1 | 0 | 0 | 3 |
| G007-LK | min/flox | 0 | 3 | 5 | 3 | |
| Pyrvinium | flox/+ | 1 | 0 | 0 | 0 | 1 |
| Pyrvinium | min/flox | 0 | 0 | 1 | 2 | |
| Treatment | Anophthalmia | Normal | Total |
|---|---|---|---|
| WNT antagonist | 10 | 8 | 18 |
| None | 0 | 15 | 15 |
| Total | 10 | 23 | 33 |
| Study Parameters | |
|---|---|
| Anophthalmia incidence (sporadic), group 1 | 4.3% |
| Anophthalmia incidence (treatment-induced), group 2 | 55.6% |
| Alpha | 0.05 |
| Beta | 0.2 |
| Power | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, J.; O’Keefe, R.; Rigopolous, A.; Carli, A.L.E.; Waaler, J.; Krauss, S.; Ernst, M.; Buchert, M. A Mouse Model for the Rapid and Binomial Assessment of Putative WNT/β-Catenin Signalling Inhibitors. Biomedicines 2023, 11, 2719. https://doi.org/10.3390/biomedicines11102719
Tse J, O’Keefe R, Rigopolous A, Carli ALE, Waaler J, Krauss S, Ernst M, Buchert M. A Mouse Model for the Rapid and Binomial Assessment of Putative WNT/β-Catenin Signalling Inhibitors. Biomedicines. 2023; 11(10):2719. https://doi.org/10.3390/biomedicines11102719
Chicago/Turabian StyleTse, Janson, Ryan O’Keefe, Angela Rigopolous, Annalisa L. E. Carli, Jo Waaler, Stefan Krauss, Matthias Ernst, and Michael Buchert. 2023. "A Mouse Model for the Rapid and Binomial Assessment of Putative WNT/β-Catenin Signalling Inhibitors" Biomedicines 11, no. 10: 2719. https://doi.org/10.3390/biomedicines11102719






