Immunotherapy and Targeted Therapies Efficacy in Thymic Epithelial Tumors: A Systematic Review
Abstract
:1. Introduction
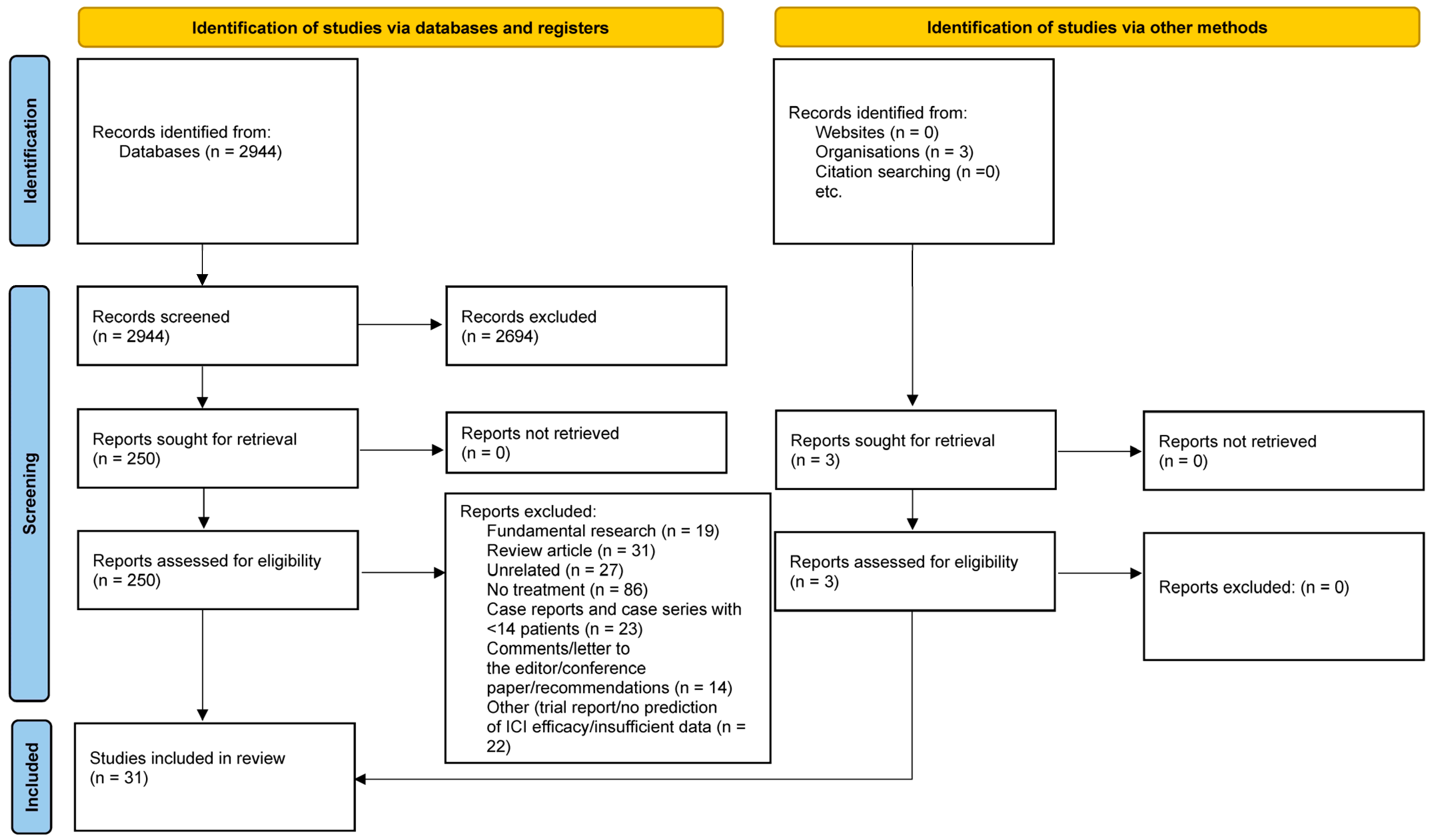
2. Material and Methods
- (1)
- Phase II/III clinical trials and retrospective series (>14 patients according to Simon’s design) [34,35] assessing ICI in TET and reporting at least one of the following clinical outcomes:
- progression-free survival (PFS), defined as the time from randomization to disease progression or death from any cause;
- overall survival (OS), defined as the time from randomization until death from any cause;
- objective response rate (ORR), defined as the proportion of patients who achieved an objective response (partial or complete according to the Response Evaluation Criteria in Solid Tumors (RECIST));
- all grade or grade ≥ 3 treatment-related adverse events.
- (2)
- Phase I/II/III clinical trials and retrospective series (>14 patients according to Simon’s design) assessing targeted therapies against an oncogenic driver mutation or translocation (EGFR, cKIT, KRAS, ALK, BRAF, PDGFR, HER2, MET etc.).
- (3)
- Experimental cohort studies investigating any of the following:
- −
- TME of TET, % of PD-L1 expression in TET or tumor mutational burden (TMB) AND prediction of ICI efficacy.
Data Synthesis
3. Results
4. Discussion
4.1. Immune Checkpoint Inhibitors in TET
4.2. Targeted Therapies in TET
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. The Literature Search Strategy
- Subject: immunotherapy and targeted therapies for thymic epithelial tumors
- Medline via l’interface OvidSP (Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid Medline® Daily and Ovid Medline® 1946-present)
- P = exp Thymus Neoplasms/ OR thymus neoplasm*.ti,ab,kw OR thymus cancer*.ti,ab,kw OR Thymus Carcinoma*.ti,ab,kw OR thymus tumour*.ti,ab,kw OR thymus tumor*.ti,ab,kw OR Thymoma*.ti,ab,kw OR thymic neoplasm*.ti,ab,kw OR thymic cancer*.ti,ab,kw OR thymic tumour*.ti,ab,kw OR thymic tumor*.ti,ab,kw OR Thymic Carcinoma*.ti,ab,kw OR Thymic Epithelial Tumor*.ti,ab,kw OR Thymic Epithelial Tumour*.ti,ab,kw
- I = exp Angiogenesis Modulating Agents/ OR exp Immunotherapy/ OR immunotherap*.ti,ab,kw OR immunization*.ti,ab,kw OR immunosuppression*.ti,ab,kw OR radioimmunotherap*.ti,ab,kw OR vaccin*.ti,ab,kw OR Cixutumumab.ti,ab,kw,nm OR anti-IGF-1R antibody A12.ti,ab,kw,nm OR Tivantinib.ti,ab,kw,nm OR ARQ 197.ti,ab,kw,nm OR Vorinostat/ OR Vorinostat.ti,ab,kw,nm OR Zolinza.ti,ab,kw,nm OR Ramucirumab.ti,ab,kw,nm OR Ipilimumab/ OR Ipilimumab.ti,ab,kw,nm OR Yervoy.ti,ab,kw,nm OR Anti-CTLA-4.ti,ab,kw,nm OR Tremelimumab.ti,ab,kw,nm OR ticilimumab.ti,ab,kw,nm OR Nivolumab/ OR Nivolumab.ti,ab,kw,nm OR Opdivo.ti,ab,kw,nm OR Pembrolizumab.ti,ab,kw,nm OR lambrolizumab.ti,ab,kw,nm OR Keytruda.ti,ab,kw,nm OR Atezolizumab.ti,ab,kw,nm OR MPDL3280A.ti,ab,kw,nm OR Durvalumab.ti,ab,kw,nm OR Avelumab.ti,ab,kw,nm OR Amatuximab.ti,ab,kw,nm OR MORAb-009.ti,ab,kw,nm OR SS1P.ti,ab,kw,nm OR anetumab ravtansine.ti,ab,kw,nm OR BAY 94-9343.ti,ab,kw,nm OR BNC105P.ti,ab,kw,nm OR BNC-105P.ti,ab,kw,nm OR ADI-PEG20.ti,ab,kw,nm OR pegylated arginine deiminase.ti,ab,kw,nm OR interleukin*.ti,ab,kw,nm OR interferon*.ti,ab,kw,nm OR EZH.ti,ab,kw,nm OR enhancer of zeste homolog.ti,ab,kw,nm OR Immune Checkpoint Inhibitors/ OR Immune Checkpoint Inhibitor*.ti,ab,kw,nm OR Immune Checkpoint Blockade.ti,ab,kw,nm OR PD L1.ti,ab,kw,nm OR PD 1 Inhibitor*.ti,ab,kw,nm OR Tumor Microenvironment/ OR Tumor Microenvironment*.ti,ab,kw OR Tumour Microenvironment*.ti,ab,kw OR Cancer Microenvironment*.ti,ab,kw OR Epidermal Growth Factor/ OR Epidermal Growth Factor.ti,ab,kw OR "HER2/Neu".ti,ab,kw,nm OR "anti-HER-2/neu".ti,ab,kw,nm OR exp Vascular Endothelial Growth Factors/ OR Vascular Endothelial Growth Factor*.ti,ab,kw,nm OR VEGFs.ti,ab,kw,nm OR exp Fibroblast Growth Factors/ OR Fibroblast Growth Factor*.ti,ab,kw,nm OR bFGF.ti,ab,kw,nm OR Tyrosine Protein Kinase Inhibitors/ OR TKI.ti,ab,kw,nm OR Tyrosine kinase inhibitor*.ti,ab,kw,nm OR CTLA-4 Antigen/ OR CTLA 4.ti,ab,kw,nm OR druggable molecular anomal*.ti,ab,kw OR immune-mediated adverse effect*.ti,ab,kw OR genetic marker*.ti,ab,kw OR immune microenvironment.ti,ab,kw OR PI3K.ti,ab,kw,nm
- = 1724 (6/02/2023) – 1715 (after duplicate removal)
- SciVerse Scopus
- P = TITLE-ABS-KEY(“thymus neoplasm*” OR “thymus cancer*” OR “Thymus Carcinoma*” OR “thymus tumour*” OR “thymus tumor*” OR Thymoma* OR “thymic neoplasm*” OR "thymic cancer*” OR “thymic tumour*” OR “thymic tumor*” OR “Thymic Carcinoma*” OR “Thymic Epithelial Tumor*” OR “Thymic Epithelial Tumour*”)
- I = TITLE-ABS-KEY(immunotherap* OR immunization* OR immunosuppression* OR radioimmunotherap* OR vaccin* OR Cixutumumab OR “anti-IGF-1R antibody A12” OR Tivantinib OR “ARQ 197” OR Vorinostat OR Zolinza OR Ramucirumab OR Ipilimumab OR Yervoy OR “Anti-CTLA-4” OR Tremelimumab OR ticilimumab OR Nivolumab OR Opdivo OR Pembrolizumab OR lambrolizumab OR Keytruda OR Atezolizumab OR “MPDL3280A” OR Durvalumab OR Avelumab OR Amatuximab OR MORAb-009 OR SS1P OR “anetumab ravtansine” OR “BAY 94-9343” OR BNC105P OR BNC-105P OR ADI-PEG20 OR “pegylated arginine deiminase” OR interleukin* OR interferon* OR EZH OR “enhancer of zeste homolog” OR “Immune Checkpoint Inhibitor*” OR “Immune Checkpoint Blockade” OR “PD L1” OR “PD 1 Inhibitor*” OR “Tumor Microenvironment*” OR “Tumour Microenvironment*” OR “Cancer Microenvironment*” OR “Epidermal Growth Factor” OR “HER2/Neu” OR “anti-HER-2/neu” OR “Vascular Endothelial Growth Factor*” OR VEGFs OR “Fibroblast Growth Factor*” OR bFGF OR TKI OR “Tyrosine kinase inhibitor*” OR “CTLA 4” OR “druggable molecular anomal*” OR “immune-mediated adverse effect*” OR “genetic marker*” OR “immune microenvironment” OR PI3K)
- = 2747 (6/2/2023)
- = 2944 (6/2/2023) merged
References
- Wright, C.D. Management of thymomas. Crit Rev. Oncol. Hematol. 2008, 65, 109–120. [Google Scholar]
- Marx, A.; Chan, J.K.; Coindre, J.-M.; Detterbeck, F.; Girard, N.; Harris, N.L.; Jaffe, E.S.; Kurrer, M.O.; Marom, E.M.; Moreira, A.L.; et al. The 2015 World Health Organization classification of tumors of the thymus: Continuity and changes. J. Thorac. Oncol. 2015, 10, 1383–1395. [Google Scholar]
- Ko, R.; Shukuya, T.; Okuma, Y.; Tateishi, K.; Imai, H.; Iwasawa, S.; Miyauchi, E.; Fujiwara, A.; Sugiyama, T.; Azuma, K.; et al. Prognostic factors and efficacy of first-line chemotherapy in patients with advanced thymic carcinoma: A retrospective analysis of 286 patients from NEJ023 study. Oncologist 2018, 23, 1210–1217. [Google Scholar]
- Berghmans, T.; Durieux, V.; Holbrechts, S.; Jungels, C.; Lafitte, J.-J.; Meert, A.-P.; Moretti, L.; Ocak, S.; Roelandts, M.; Girard, N. Systemic treatments for thymoma and thymic carcinoma: A systematic review. Lung Cancer 2018, 126, 25–31. [Google Scholar]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S.; ESMO Guidelines Committee. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S6), v40–v55. [Google Scholar]
- Ak, N.; Aydiner, A. Nivolumab treatment for metastatic thymic epithelial tumors. J. Oncol. Pharm. Pract. 2021, 27, 1710–1715. [Google Scholar] [CrossRef]
- Zander, T.; Aebi, S.; Rast, A.C.; Zander, A.; Winterhalder, R.; Brand, C.; Diebold, J.; Gautschi, O. Response to Pembrolizumab in a patient with relapsing thymoma. J. Thorac. Oncol. 2016, 11, e147–e149. [Google Scholar] [CrossRef]
- Uchida, N.; Fujita, K.; Okamura, M.; Nakatani, K.; Mio, T. The clinical benefits of immune checkpoint inhibitor for thymic carcinomas approximately experience of single public hospital in Japan approximately. Respir. Med. Case Rep. 2019, 26, 39–41. [Google Scholar]
- Katsuya, Y.; Horinouchi, H.; Seto, T.; Umemura, S.; Hosomi, Y.; Satouchi, M.; Nishio, M.; Kozuki, T.; Hida, T.; Sukigara, T.; et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur. J. Cancer 2019, 113, 78–86. [Google Scholar]
- Yang, Y.; Ding, L.; Wang, P. Dramatic response to anti-PD-1 therapy in a patient of squamous cell carcinoma of thymus with multiple lung metastases. J. Thorac. Dis. 2016, 8, E535–E537. [Google Scholar] [CrossRef]
- Yang, P.-C.; Guo, J.-C.; Hsieh, M.-S.; Lin, C.-C.; Hsu, C.-H. Response to nivolumab as salvage therapy in a patient with thymic carcinoma. J. Thorac. Oncol. 2018, 13, e36–e39. [Google Scholar] [CrossRef] [PubMed]
- Girard, N. Immune checkpoints in thymic epithelial tumors: Challenges and opportunities. Immuno-Oncol. Technol. 2019, 3, 8–14. [Google Scholar]
- Nivolumab in Patients with Type B3 Thymoma and Thymic Carcinoma (NIVOTHYM). Available online: https://clinicaltrials.gov/ct2/show/NCT03134118?term=NCT03134118&draw=2&rank=1 (accessed on 15 May 2023).
- Palmieri, G.; Marino, M.; Salvatore, M.; Budillon, A.; Meo, G.; Caraglia, M.; Montella, L. Cetuximab is an active treatment of metastatic and chemorefractory thymoma. Front. Biosci. 2007, 12, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Farina, G.; Garassino, M.C.; Gambacorta, M.; La Verde, N.; Gherardi, G.; Scanni, A. Response of thymoma to cetuximab. Lancet Oncol. 2007, 8, 449–450. [Google Scholar] [CrossRef]
- Christodoulou, C.; Murray, S.; Dahabreh, J.; Petraki, K.; Nikolakopoulou, A.; Mavri, A.; Skarlos, D. Response of malignant thymoma to erlotinib. Ann. Oncol. 2008, 19, 1361–1362. [Google Scholar] [CrossRef]
- Pedersini, R.; Vattemi, E.; Lusso, M.R.; Mazzoleni, G.; Ebner, H.; Graiff, C. Erlotinib in advanced well-differentiated thymic carcinoma with overexpression of EGFR: A case report. Tumori J. 2008, 94, 849–852. [Google Scholar] [CrossRef]
- Nakagiri, T.; Funaki, S.; Kadota, Y.; Takeuchi, Y.; Shiono, H.; Akashi, A.; Okumura, M. Does gefitinib have effects on EGFR mutation-positive thymoma? Case report of thymoma recurrence. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 674–676. [Google Scholar] [CrossRef]
- Giaccone, G.; Rajan, A.; Ruijter, R.; Smit, E.; van Groeningen, C.; Hogendoorn, P.C. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J. Thorac. Oncol. 2009, 4, 1270–1273. [Google Scholar] [CrossRef]
- Ströbel, P.; Hartmann, M.; Jakob, A.; Mikesch, K.; Brink, I.; Dirnhofer, S.; Marx, A. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N. Engl. J. Med. 2004, 350, 2625–2626. [Google Scholar] [CrossRef]
- Buti, S.; Donini, M.; Sergio, P.; Garagnani, L.; Schirosi, L.; Passalacqua, R.; Rossi, G. Impressive response with imatinib in a heavily pretreated patient with metastatic c-KIT mutated thymic carcinoma. J. Clin. Oncol. 2011, 29, e803–e805. [Google Scholar] [CrossRef]
- Bisagni, G.; Rossi, G.; Cavazza, A.; Sartori, G.; Gardini, G.; Boni, C. Long lasting response to the multikinase inhibitor bay 43–9006 (Sorafenib) in a heavily pretreated metastatic thymic carcinoma. J. Thorac. Oncol. 2009, 4, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Carter, C.A.; Berman, A.; Cao, L.; Kelly, R.J.; Thomas, A.; Khozin, S.; Chavez, A.L.; Bergagnini, I.; Scepura, B.; et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: A multicentre, open-label, phase 2 trial. Lancet Oncol. 2014, 15, 191–200. [Google Scholar] [PubMed]
- Wheler, J.J.; Hong, D.; Swisher, S.G.; Falchook, G.S.; Tsimberidou, A.M.; Helgason, T.; Naing, A.; Stephen, B.; Janku, F.; Stephens, P.J.; et al. Thymoma patients treated in a phase I clinic at MD Anderson Cancer Center: Responses to mTOR inhibitors and molecular analyses. Oncotarget 2013, 4, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Rajan, A.; Berman, A.; Kelly, R.J.; Szabo, E.; Lopez-Chavez, A.; Trepel, J.; Lee, M.-J.; Cao, L.; Espinoza-Delgado, I.; et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J. Clin. Oncol. 2011, 29, 2052–2059. [Google Scholar] [CrossRef]
- Azad, A.; Herbertson, R.A.; Pook, D.; White, S.; Mitchell, P.L.; Tebbutt, N.C. Motesanib diphosphate (AMG 706), an oral angiogenesis inhibitor, demonstrates clinical efficacy in advanced thymoma. Acta Oncol. 2009, 48, 619–621. [Google Scholar] [CrossRef]
- Ströbel, P.; Bargou, R.; Wolff, A.; Spitzer, D.; Manegold, C.; Dimitrakopoulou-Strauss, A.; Strauss, L.; Sauer, C.; Mayer, F.; Hohenberger, P.; et al. Sunitinib in metastatic thymic carcinomas: Laboratory findings and initial clinical experience. Br. J. Cancer 2010, 103, 196–200. [Google Scholar] [CrossRef]
- Chuah, C.; Lim, T.H.; Lim, A.S.T.; Tien, S.L.; Lim, C.H.; Soong, R.; Lee, F.; Linn, Y.C.; Goh, Y.T.; Cheah, F.K.; et al. Dasatinib induces a response in malignant thymoma. J. Clin. Oncol. 2006, 24, e56–e68. [Google Scholar] [CrossRef]
- Neuhaus, T.; Luyken, J. Long lasting efficacy of sorafenib in a heavily pretreated patient with thymic carcinoma. Target. Oncol. 2012, 7, 247–251. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar]
- Agrafiotis, A.C.; Siozopoulou, V.; Hendriks, J.M.H.; Pauwels, P.; Koljenovic, S.; Van Schil, P.E. Tumor Microenvironment in Thymic Epithelial Tumors: A Narrative Review. Cancers 2022, 14, 6082. [Google Scholar]
- Ohm, B.; Jungraithmayr, W. Balancing the Risk of Adverse Events against the Efficacy of Immunotherapy in Advanced Thymic Epithelial Tumors. Cancers 2023, 15, 289. [Google Scholar]
- Masaoutis, C.; Palamaris, K.; Kokkali, S.; Levidou, G.; Theocharis, S. Unraveling the Immune Microenvironment of Thymic Epithelial Tumors: Implications for Autoimmunity and Treatment. Int. J. Mol. Sci. 2022, 23, 7864. [Google Scholar] [CrossRef]
- Simon, R. Design, Analysis and Reporting of Cancer Clinical Trials. In Biopharmaceutical Statistics for Drug Development; Peace, K.E., Ed.; Marcel Dekker: New York, NY, USA, 1987. [Google Scholar]
- Simon, R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials 1989, 10, 1–10. [Google Scholar] [PubMed]
- Giaccone, G.; Kim, C.; Thompson, J.; McGuire, C.; Kallakury, B.; Chahine, J.J.; Manning, M.; Mogg, R.; Blumenschein, W.M.; Tan, M.T.; et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol. 2018, 19, 347–355. [Google Scholar] [PubMed]
- Giaccone, G.; Kim, C. Durable Response in Patients with Thymic Carcinoma Treated with Pembrolizumab After Prolonged Follow-Up. J. Thorac. Oncol. 2021, 16, 483–485. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.S.; Ku, B.M.; Choi, Y.-L.; Cristescu, R.; Han, J.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: An open-label phase II trial. J. Clin. Oncol. 2019, 37, 2162–2170. [Google Scholar] [CrossRef]
- Wang, W.; Lin, G.; Hao, Y.; Guan, Y.; Zhang, Y.; Xu, C.; Wang, Q.; Wang, D.; Jiang, Z.; Cai, J.; et al. Treatment outcomes and prognosis of immune checkpoint inhibitors therapy in patients with advanced thymic carcinoma: A multicentre retrospective study. Eur. J. Cancer 2022, 174, 21–30. [Google Scholar]
- Conforti, F.; Zucali, P.A.; Pala, L.; Catania, C.; Bagnardi, V.; Sala, I.; Della Vigna, P.; Perrino, M.; Zagami, P.; Corti, C.; et al. Avelumab plus axitinib in unresectable or metastatic type B3 thymomas and thymic carcinomas (CAVEATT): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 1287–1296. [Google Scholar] [CrossRef]
- Girard, N.; Aix, S.P.; Cedres, S.; Berghmans, T.; Burgers, S.; Toffart, A.-C.; Popat, S.; Janssens, A.; Gervais, R.; Hochstenbag, M.; et al. Efficacy and safety of nivolumab for patients with pre-treated type B3 thymoma and thymic carcinoma: Results from the EORTC-ETOP NIVOTHYM phase II trial. ESMO Open 2023, 8, 101576. [Google Scholar] [CrossRef]
- Perrino, M.; De Pas, T.; Bozzarelli, S.; Giordano, L.; De Vincenzo, F.; Conforti, F.; Digiacomo, N.; Cordua, N.; D’Antonio, F.; Borea, F.; et al. Resound Trial: A phase 2 study of regorafenib in patients with thymoma (type B2-B3) and thymic carcinoma previously treated with chemotherapy. Cancer 2022, 128, 719–726. [Google Scholar] [CrossRef]
- Remon, J.; Girard, N.; Mazieres, J.; Dansin, E.; Pichon, E.; Greillier, L.; Dubos, C.; Lindsay, C.R.; Besse, B. Sunitinib in patients with advanced thymic malignancies: Cohort from the French RYTHMIC network. Lung Cancer 2016, 97, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Rajan, A.; Berman, A.; Tomita, Y.; Brzezniak, C.; Lee, M.-J.; Lee, S.; Ling, A.; Spittler, A.J.; Carter, C.A.; et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: An open-label phase 2 trial. Lancet Oncol. 2015, 16, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Antonarelli, G.; Corti, C.; Zucali, P.A.; Perrino, M.; Manglaviti, S.; Russo, G.L.; Varano, G.M.; Salvini, P.; Curigliano, G.; Catania, C.; et al. Continuous sunitinib schedule in advanced platinum refractory thymic epithelial neoplasms: A retrospective analysis from the ThYmic MalignanciEs (TYME) Italian collaborative group. Eur. J. Cancer 2022, 174, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Abu Zaid, M.I.; Radovich, M.; Althouse, S.; Liu, H.; Spittler, A.J.; Solzak, J.; Badve, S.; Loehrer, P.J., Sr. A phase II study of buparlisib in relapsed or refractory thymomas. Front. Oncol. 2022, 12, 891383. [Google Scholar] [CrossRef]
- Guan, Y.; Gu, X.; Si, J.; Xiang, J.; Wei, J.; Hao, Y.; Wang, W.; Sun, Y. The efficacy of small molecule anti-angiogenic drugs in previously treated Thymic carcinoma. BMC Cancer 2023, 23, 16. [Google Scholar]
- Gubens, M.A.; Burns, M.; Perkins, S.M.; Pedro-Salcedo, M.S.; Althouse, S.K.; Loehrer, P.J.; Wakelee, H.A. A phase II study of saracatinib (AZD0530), a Src inhibitor, administered orally daily to patients with advanced thymic malignancies. Lung Cancer 2015, 89, 57–60. [Google Scholar]
- Zucali, P.A.; De Pas, T.; Palmieri, G.; Favaretto, A.; Chella, A.; Tiseo, M.; Caruso, M.; Simonelli, M.; Perrino, M.; De Vincenzo, F.; et al. Phase II Study of Everolimus in Patients with Thymoma and Thymic Carcinoma Previously Treated with Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2018, 36, 342–349. [Google Scholar] [CrossRef]
- Kurup, A.; Burns, M.; Dropcho, S.; Pao, W.; Loehrer, P.J. Phase II study of gefitinib treatment in advanced thymic malignancies. J. Clin. Oncol. 2005, 23, 7068. [Google Scholar] [CrossRef]
- Palmieri, G.; Marino, M.; Buonerba, C.; Federico, P.; Conti, S.; Milella, M.; Petillo, L.; Evoli, A.; Lalle, M.; Ceribelli, A.; et al. Imatinib mesylate in thymic epithelial malignancies. Cancer Chemother. Pharmacol. 2012, 69, 309–315. [Google Scholar] [CrossRef]
- Besse, B.; Garassino, M.C.; Rajan, A.; Novello, S.; Mazieres, J.; Weiss, G.J.; Kocs, D.M.; Barnett, J.M.; Davite, C.; Crivori, P.; et al. Efficacy of milciclib (PHA-848125AC), a pan-cyclin d-dependent kinase inhibitor, in two phase II studies with thymic carcinoma (TC) and B3 thymoma (B3T) patients. J. Clin. Oncol. 2018, 36, 8519. [Google Scholar] [CrossRef]
- Sato, J.; Satouchi, M.; Itoh, S.; Okuma, Y.; Niho, S.; Mizugaki, H.; Murakami, H.; Fujisaka, Y.; Kozuki, T.; Nakamura, K.; et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): A multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Bedano, P.M.; Perkins, S.; Burns, M.; Kessler, K.; Nelson, R.; Schneider, B.P.; Risley, L.; Dropcho, S.; Loehrer, P.J. A phase II trial of erlotinib plus bevacizumab in patients with recurrent thymoma or thymic carcinoma. J. Clin. Oncol. 2008, 26, 19087. [Google Scholar] [CrossRef]
- Hou, X.; Lin, S.; Liu, Y.; Wang, K.; Yu, Z.; Jia, J.; Yu, J.; Zheng, W.; Bai, J.; Chang, L.; et al. Analysis of the tumor microenvironment and mutation burden identifies prognostic features in thymic epithelial tumors. Am. J. Cancer Res. 2022, 12, 2387–2396. [Google Scholar] [PubMed]
- Su, Y.; Ou, Y.; Chen, Y.; Ma, X. Construction of immune-related LncRNAs classifier to predict prognosis and immunotherapy response in thymic epithelial tumors. Biosci. Rep. 2022, 42, BSR20220317. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Byun, C.S.; Bae, M.K.; Lee, C.Y.; Park, I.K.; Kim, D.J.; Chung, K.Y.; Lee, J.G. Prognostic effect of stromal lymphocyte infiltration in thymic carcinoma. Lung Cancer 2011, 74, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kitano, S.; Motoi, N.; Ino, Y.; Yamamoto, N.; Watanabe, S.; Ohe, Y.; Hiraoka, N. CD20+ tumor-infiltrating immune cells and CD204+ M2 macrophages are associated with prognosis in thymic carcinoma. Cancer Sci. 2020, 111, 1921–1932. [Google Scholar] [CrossRef]
- Blessin, N.C.; Spriestersbach, P.; Li, W.; Mandelkow, T.; Dum, D.; Simon, R.; Hube-Magg, C.; Lutz, F.; Viehweger, F.; Lennartz, M.; et al. Prevalence of CD8+ cytotoxic lymphocytes in human neoplasms. Cell. Oncol. 2020, 43, 421–430. [Google Scholar]
- Bocchialini, G.; Schiefer, A.I.; Müllauer, L.; Thanner, J.; Bauer, J.; Thaler, F.; Laggner, M.; Veraar, C.; Klepetko, W.; Hötzenecker, K.; et al. Tumour immune microenvironment in resected thymic carcinomas as a predictor of clinical outcome. Br. J. Cancer 2022, 127, 1162–1171. [Google Scholar]
- Kim, K.H.; Cho, J.; Ku, B.M.; Koh, J.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Cheon, J.; Min, Y.J.; Park, S.-H.; et al. The First-week Proliferative Response of Peripheral Blood PD-1+CD8+ T Cells Predicts the Response to Anti-PD-1 Therapy in Solid Tumors. Clin. Cancer Res. 2019, 25, 2144–2154. [Google Scholar]
- Kim, K.H.; Hur, J.Y.; Cho, J.; Ku, B.M.; Koh, J.; Koh, J.Y.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; et al. Immune-related adverse events are clustered into distinct subtypes by T-cell profiling before and early after anti-PD-1 treatment. OncoImmunology 2020, 9, 1722023. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Guan, Y.; Huang, Y.; Lin, J.; Chen, L.; Li, J.; Chen, G.; Pan, L.K.; Xia, X.; et al. Prevalence of PRKDC mutations and association with response to immune checkpoint inhibitors in solid tumors. Mol. Oncol. 2020, 14, 2096–2110. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Z.; Zhao, P.; Li, W. Prognostic and immune regulating roles of YIF1B in Pan-Cancer: A potential target for both survival and therapy response evaluation. Biosci. Rep. 2020, 40, BSR20201384. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [PubMed]
- Arbour, K.C.; Naidoo, J.; Steele, K.E.; Ni, A.; Moreira, A.L.; Rekhtman, N.; Robbins, P.B.; Karakunnel, J.; Rimner, A.; Huang, J.; et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS ONE 2017, 12, e0182665. [Google Scholar]
- Tateo, V.; Manuzzi, L.; De Giglio, A.; Parisi, C.; Lamberti, G.; Campana, D.; Pantaleo, M. Immunobiology of Thymic Epithelial Tumors: Implications for Immunotherapy with Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 9056. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti–PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Madeddu, D.; Falco, A.; Bocchialini, G.; Goldoni, M.; Sogni, F.; Armani, G.; Lagrasta, C.A.; Lorusso, B.; Mangiaracina, C.; et al. Low PD-1 expression in cytotoxic CD8 + tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin. Cancer Res. 2018, 24, 407–420. [Google Scholar]
- Yokoyama, S.; Miyoshi, H.; Nakashima, K.; Shimono, J.; Hashiguchi, T.; Mitsuoka, M.; Takamori, S.; Akagi, Y.; Ohshima, K. Prognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinoma. Clin. Cancer Res. 2016, 22, 4727–4734. [Google Scholar] [CrossRef]
- Graab, P.; Bock, C.; Weiss, K.; Hirth, A.; Koller, N.; Braner, M.; Jung, J.; Loehr, F.; Tampé, R.; Behrends, C.; et al. Lysosomal targeting of the ABC transporter TAPL is determined by membrane-localized charged residues. J. Biol. Chem. 2019, 294, 7308–7323. [Google Scholar] [PubMed]
- Petrini, I.; Meltzer, P.S.; Kim, I.-K.; Lucchi, M.; Park, K.-S.; Fontanini, G.; Gao, J.; A Zucali, P.; Calabrese, F.; Favaretto, A.; et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat. Genet. 2014, 46, 844–849. [Google Scholar]
- Yoh, K.; Nishiwaki, Y.; Ishii, G.; Goto, K.; Kubota, K.; Ohmatsu, H.; Niho, S.; Nagai, K.; Saijo, N. Mutational status of EGFR and KIT in thymoma and thymic carcinoma. Lung Cancer 2008, 62, 316–320. [Google Scholar] [PubMed]
- Yamaguchi, H.; Soda, H.; Kitazaki, T.; Tsukamoto, K.; Hayashi, T.; Kohno, S. Thymic carcinoma with epidermal growth factor receptor gene mutations. Lung Cancer 2006, 52, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Sasaki, H.; Kawano, O.; Endo, K.; Haneda, H.; Yukiue, H.; Kobayashi, Y.; Yano, M.; Fujii, Y. Expression and mutation statuses of epidermal growth factor receptor in thymic epithelial tumors. Jpn. J. Clin. Oncol. 2006, 36, 351–356. [Google Scholar] [PubMed]
- Henley, J.D.; Cummings, O.W.; Loehrer, P.J., Sr. Tyrosine kinase receptor expression in thymomas. J. Cancer Res. Clin. Oncol. 2004, 130, 222–224. [Google Scholar] [CrossRef]
- Pan, C.C.; Chen, P.C.; Chiang, H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J. Pathol. 2004, 202, 375–381. [Google Scholar] [CrossRef]
- Girard, N.; Shen, R.; Guo, T.; Zakowski, M.F.; Heguy, A.; Riely, G.J.; Huang, J.; Lau, C.; Lash, A.E.; Ladanyi, M.; et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin. Cancer Res. 2009, 15, 6790–6799. [Google Scholar]
- Cimpean, A.M.; Raica, M.; Encica, S.; Cornea, R.; Bocan, V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann. Anat. Anat. Anz. 2008, 190, 238–245. [Google Scholar]
- Sasaki, H.; Yukiue, H.; Kobayashi, Y.; Nakashima, Y.; Moriyama, S.; Kaji, M.; Kiriyama, M.; Fukai, I.; Yamakawa, Y.; Fujii, Y. Elevated serum vascular endothelial growth factor and basic fıbroblast growth factor levels in patients with thymic epithelial neoplasms. Surg. Today 2001, 31, 1038–1040. [Google Scholar] [CrossRef]
- Girard, N.; Teruya-Feldstein, J.; Payabyab, E.C.; Riely, G.J.; Rusch, V.W.; Kris, M.G.; Zakowski, M.F. Insulin-like growth factor-1 receptor expression in thymic malignancies. J. Thorac. Oncol. 2010, 5, 1439–1446. [Google Scholar]
- Zucali, P.A.; Petrini, I.; Lorenzi, E.; Merino, M.; Cao, L.; Di Tommaso, L.; Lee, H.S.; Incarbone, M.; Walter, B.A.; Simonelli, M.; et al. Insulin-like growth factor-1 receptor and phosphorylated AKT-serine 473 expression in 132 resected thymomas and thymic carcinomas. Cancer 2010, 116, 4686–4695. [Google Scholar]
- Steele, N.L.; Plumb, J.A.; Vidal, L.; Tjørnelund, J.; Knoblauch, P.; Rasmussen, A.; Ooi, C.E.; Buhl-Jensen, P.; Brown, R.; Evans, T.R.J.; et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 804–810. [Google Scholar] [PubMed]
- Petrini, I.; Zucali, P.A.; Lee, H.S.; Pineda, M.A.; Meltzer, P.S.; Walter-Rodriguez, B.; Roncalli, M.; Santoro, A.; Wang, Y.; Giaccone, G. Expression and mutational status of c-kit in thymic epithelial tumors. J. Thorac. Oncol. 2010, 5, 1447–1453. [Google Scholar] [PubMed]
- Hirai, F.; Edagawa, M.; Shimamatsu, S.; Toyozawa, R.; Toyokawa, G.; Nosaki, K.; Yamaguchi, M.; Seto, T.; Twakenoyama, M.; Ichinose, Y. c-kit mutation-positive advanced thymic carcinoma successfully treated as a mediastinal gastrointestinal stromal tumor: A case report. Mol. Clin. Oncol. 2016, 4, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, I.S.; Govindan, R.; Javidan-Nejad, C.; Pfeifer, J.D.; Cottrell, C.E. Stabilization of disease after targeted therapy in a thymic carcinoma with KIT mutation detected by clinical next-generation sequencing. J. Thorac. Oncol. 2014, 9, e12–e16. [Google Scholar] [PubMed]
- Imbimbo, M.; Vitali, M.; Fabbri, A.; Ottaviano, M.; Pasello, G.; Petrini, I.; Palmieri, G.; Berardi, R.; Zucali, P.; Ganzinelli, M.; et al. RELEVENT Trial: Phase II Trial of Ramucirumab, Carboplatin, and Paclitaxel in Previously Untreated Thymic Carcinoma/B3 Thymoma with Area of Carcinoma. Clin. Lung Cancer 2018, 19, e811–e814. [Google Scholar] [CrossRef]
| First Author | Year of Publication | Country | Drug | Action | Type of Study | N Patients | M/F | Median Age (Years/Range) |
|---|---|---|---|---|---|---|---|---|
| Giaccone [36] | 2018 | USA | Pembrolizumab | PD-1 inhibitor | Phase II | 40 | 28/12 (70%/30%) | 57 (25–80) |
| Katsuya (PRIMER) [9] | 2019 | Japan (multi center) | Nivolumab | PD-1 inhibitor | Phase II | 15 | 12/3 (80%/20%) | 55 (34–70) |
| Cho [38] | 2019 | South Korea (singe center) | Pembrolizumab | PD-1 inhibitor | Phase II | 33 | 33/11 (63.6%/36.4%) | 57 (26–78) |
| Conforti (CAVEATT) [40] | 2022 | Italy (multi center) | Avelumab+axitinib | PD-1 inhibitor+anti-angiogenic agent | Phase II | 32 | 19/13 (59%/41%) | 62 (49–71) |
| Wang [39] | 2022 | China | Nivolumab, pembrolizumab, sintilimab, camrelizumab, tislelizumab, toripalimab | PD-1 inhibitors | Retrospective multicentric study | 77 | 48/29 (62.3%/37.7%) | 55 (19–84) |
| Girard (NIVOTHYM) [41] | 2023 | International multi center | Nivolumab | PD-1 inhibitor | Phase II | 55 (49 eligible) | 35/20 (64%/36%) | 58 (32–82) |
| First Author | Median FU Duration (Months) | Primary Endpoint | Histology | Stage | Previous Treatment | ORR | Median PFS (Months) | Median OS (Months) | Grade 3–4 Treatment-Related AE |
|---|---|---|---|---|---|---|---|---|---|
| Giaccone [36] | 20 | ORR | TC | III (3%), IVa (15%), IVb (82%) | Surgery, radiotherapy, chemotherapy | 22.5% (95% CI 10.8–38.5) | 4.2 (95% CI 2.9–10.3) | 24.9 (15.5–not reached) | Increased AST and ALT (5/13%] patients each, 6 (15%) patients developed severe autoimmune toxicity, including two (5%) patients with myocarditis |
| Katsuya (PRIMER) [9] | 14.1 | ORR | TC | Stage III (1/15) IV (14/15) | Surgery, radiotherapy, chemotherapy | 0 (95% CI: 0–21.8) | 3.8 (95% CI 1.9–7.0) | 14.1 (95% CI 11.1-not estimable) | 1/15 (AST increase) |
| Cho [38] | 14.9 | ORR | 7 Thy 26 TC | IVa 48.5%, IVb 51.5% | Surgery, radiotherapy, chemotherapy | 21.2% (overall) (10.7 to 37.8) | 6.1 (5.3 to 6.9) | Not reported | 5 (71.4%) Thymoma and 4 (15.4%) TC: including hepatitis (4; 12.1%), myocarditis (3; 9.1%), myasthenia gravis (2; 6.1%), thyroiditis (1; 3.0%), antineutrophil cytoplasmic antibody–associated glomerulonephritis (1; 3.0%), colitis (1; 3.0%), subacute myoclonus (1; 3.0%) |
| Conforti (CAVEATT) [40] | 22.4 | ORR | B3 Thy (3), TC (27), Mixed B3/TC (2) | IVa 3 (9%), IVb 29 (91%) | At least one line of platinum-based chemotherapy | 34% (90% CI 21–50) | 7.5 (90% CI 3.7–10.0) | 26.6 (90% CI 17.0–30.0) | 6/32 (19%) |
| Wang [39] | - | - | TC | III (1.3%), IVa (53.2%), IVb (45.5%) | Surgery, radiotherapy, chemotherapy | 36.4% | 8.6 (95% CI 4.024–13.109) | 35.4 (95% CI 27.628–43.239) | 12/77 (15.6%), Grade III most common 4 (5.2%) elevated liver function tests, Grade IV 1 (1.3%) skin rash |
| Girard (NIVOTHYM) [41] | 13.3 | PFS rate at 6 months (PFSR-6) | B3 Thy (10), TC (43), other (2) | No M-K stage reported (Not amenable to curative-intent radical treatment) | Prior radical treatment | 12% (95% CI 5% to 25%) | 6.0 (95% CI 3.1–10.4) | 21.3 (95% CI 11.6-not estimable) | G 3/4 in 31 (57%), AEs of grade 4 included 1 neutropenia, 1 immune-mediated transaminitis, 2myocarditis |
| First Author | Year of Publication | Country | Drug | Action | Type of Study | Number of Patients (M/F) | Age |
|---|---|---|---|---|---|---|---|
| Perrino (Resound trial) [42] | 2022 | Italy (2 centers) | Regorafenib | VEGFR-PDGFR-FGFR inhibitor | Phase II | 19 (8/11) | NR |
| Guan [47] | 2023 | China (single center) | Apatinib/anlotinib | VEGFRs, KIT, PDGFRs TKI | Retrospective study | 17 (10/7) | 59 (35–73) |
| Remon [43] | 2016 | France (multicentre) | Sunitinib | VEGFRs, KIT, PDGFRs TKI | Prospective cohort | 28 (19/9) | 50 |
| Thomas [44] | 2015 | USA (2 centres) | Sunitinib | VEGFRs, KIT, PDGFRs TKI | Phase II | 41 (23/18) | 57.5 (31–81) |
| Antonarelli [45] | 2022 | Italy (multicentre) | Sunitinib | VEGFRs, KIT, PDGFRs TKI | Retrospective study | 20 (10/10) | 59 (51–63) |
| Abu Zaid [46] | 2022 | USA (single center) | Buparlisib | Pan-PI3K inhibitor | Phase II | 14 (4/10) | 58 (23–74) |
| Gubens [48] | 2015 | USA (2 centres) | Saracatinib | Src inhibitor | Phase II | 21 (11/10) | 54 (18–84) |
| Rajan [23] | 2014 | USA (multicentre) | Cixutumumab | IGF-1R inhibitor | Phase II | 49 (26/23) | 52 (26–86) |
| Zucali [49] | 2018 | Italy (multicentre) | Everolimus | mTOR inhibitor | Phase II | 51 (29/22) | 55 (36–80) |
| Giaccone [25] | 2011 | USA (2 centres) | Belinostat | Pan-HDAC inhibitor | Phase II | 41 (20/21) | 53 (23–83) |
| Kurup [50] | 2018 | USA (2 centres) | Gefitinib | EGFR inhibitor | Phase II | 26 (11/15) | NR |
| Bedano [54] | 2008 | USA (single center) | Erlotinib/bevacizumab | EGFR inhibitor/VEGFR inhibitor | Phase II | 18 (8/10) | NR |
| Palmieri [51] | 2011 | Italy (single center) | Imatinib | BCR-ABL TKI | Phase II | 15 (10/5) | 51 (42–54) |
| Besse [52] | 2018 | France (multicentre) | Milciclib | Pan-cyclin d-dependent kinase inhibitor | Phase II (CDKO-125A-006) | 72 (NR) | NR |
| Besse [52] | 2018 | France (multicentre) | Milciclib | Pan-cyclin d-dependent kinase inhibitor | Phase II (CDKO-125A-007) | 30 (NR) | NR |
| Sato (REMORA) [53] | 2020 | Japan (multicentre) | Lenvatinib | Multi-targeted inhibitor of VEGFR, FGFR, RET, c-Kit, and other kinases | Phase II | 42 (29/13) | 55.5 (49–65) |
| First Author | Drug | Median FU Duration (Months) | Primary Endpoint | Histology | Stage | Previous Treatment | Median PFS (Months) | Median OS (Months) | ORR | Grade 3–4 Treatment-Related AE |
|---|---|---|---|---|---|---|---|---|---|---|
| Perrino (Resound trial) [42] | Regorafenib | 39.1 | 8 weeks PFS rate | 6 B2/5 B3/8 TC | NR | Platinum-containing chemotherapy | 9.6 (95% CI, 3.6–12.8% months) | 33.8 (95% CI, 10.2%-not reached) | n/a | 52.6% (Hypertension 10.5%, increase in lipase value 5.3%) |
| Guan [47] | Apatinib/anlotinib | 46 | n/a | TC | Stage IV | Surgery, chemotherapy | Total 7.9 (6.5–9.3), Apatinib 7 (5.0–9.0), anlotinib 8 (2.7–3.3) | Total 47.0 (35.4–58.6), apatinib 47 (43.7–50.2) | Total 23%, apatinib 30.8%, anlotinib 0%) | Hypertension (3, 23.1%), proteinuria, hand-foot syndrome ( both 2, 15.4%) |
| Remon [43] | Sunitinib | n/a | n/a | 20 TC, 8 T | Stage III and IV | Up to four lines of systemic treatments | Whole population 3.7 (5.4 T, 3.3 TC) | Whole population 15.4 (not reached T, 12.3 TC) | Total 22.2%, Thymomas 28.6%, TC 20% | 28.6% (Stomatitis, asthenia, diarrhoea, decline in LVEF) |
| Thomas [44] | Sunitinib | 17 | Investigator-assessed best tumour response | 25 TC, 16 thymoma | NR | At least one prior platinum-containing chemotherapy | TC: 7.2 (3·4–15·2), thymoma: 8·5 (2.8–11.3) | TC: not reached, thymoma: 15.5 (12.6-undefined) | n/a | Lymphocytopenia (8, 20%), fatigue (8, 20%), oral mucositis (8, 20%). 5 (13%) decreases in LVEF |
| Antonarelli [45] | Sunitinib | n/a | Median PFS, ORR, median DOR, major treatment-related AEs | 12 thymic carcinoma, 6 B3, and 2 B2 thymoma | Stage IV | Platinum refractory | Overall 7.3 (4.5–10.3): 7.3 (4.4-NA) thymoma and 6.8 (2.8–10.3) TC | Not reported | 31.6% (12.5%-56.5%) | 30% (Asthenia/fatigue 10%) |
| Abu Zaid [46] | Buparlisib | 16.6 | ORR | B2 21%/B3 71% | Stage IV | Surgery, radiotherapy, chemotherapy | 11.1 (2.9–18.8) | 22.5 (10.7–31.3) | 7% | Dyspnea (21%), rash (14%), elevated transaminases (14%), cough (7%), pneumonitis (7%), anxiety (7%), fatigue (7%) and hyperglycemia (7%) |
| Gubens [48] | Saracatinib | Not reported | ORR | 12 thymoma, 9 TC | At least one prior chemotherapy | All: 2.5 (1.7–5.7), thymoma 5.3 (1.7–7.8), TC 0.9 (0.9–4.0) | All: 23.1 (7.3–37.5), thymoma 37.5 (12.3-not estimable), TC 6.7 (2.5, 15.0) | 0% | Hypophosphatemia 3 (14%), pleural effusion 1 (5%), anemia 1 (5%), hyponatremia 1 (5%), hypoalbuminemia 1 (5%), neutropenia 1 (5%) | |
| Rajan [23] | Cixutumumab | 24 | ORR | 37 thymomas, 12 TC | NR | At least one prior platinum-containing chemotherapy | TC: TTP 1.7 (0.9–2.7)/thymoma: TTP 9.9 (7.3–12.8) | TC: OS 8.4 (4.7–12.8)/thymoma: OS 27.5 (15.0- undefined) | Total 10% (3–22%), thymomas 14% (5–29%, TC 0% (0–26%) | Hyperglycemia (5/10%), lipase elevation (3/6%), weight loss, tumor pain, and hyperuricemia (2 each/4%) |
| Zucali [49] | Everolimus | 25.7 | DCR | Thymoma 32, TC 19 | Stage III and IV | Systemic therapies | 10.1 (6.0–14.2): thymoma 16.6 (9.8–29.8), TC 5.6 (2.6–8.5) | 25.7 (16.1-not evaluable): thymoma not reached, TC 14.7 (3.5–24.0) | 11.70% | Fourteen patients (28%). Liver toxicity (8%), neutropenia (4%), and metabolic disorders (4%) |
| Giaccone [25] | Belinostat | Not reported | ORR | 25 Thymoma, 16 TC | Stage IV | Surgery, radiotherapy, chemotherapy | Median time to progression 5.8 | Thymomas not reached, TC 12.4 | Thymomas 8% (2.3–25.9), TC 0% (0–19.4%) | 32.5%, QTc prolongation 12.5% |
| Kurup [50] | Gefitinib | Not reported | ORR | 19 Thymomas 7 TC | Stage IV | Systemic therapies | TTP 4 months | NR | 3.80% | 23%, dyspnea 11.5%, fatigue 3.8%, Anemia/thrombocytopenia 3.8%, myocardial infarction 3.8% |
| Bedano [54] | Erlotinib/bevacizumab | Not reported | NR | 11 Thymoma, 7 TC | NR | NR | NR | Not reached | 0% | 38.8%, rash 11.1%, dyspnea 11.1%, fatigue 5.5%, pericardial tamponade 5.5%, aortic insufficiency 5.5% |
| Palmieri [51] | Imatinib | Not reported | NR | 12 Thymoma, 3 TC | NR | At least one prior chemotherapy | 3 (2–4) | Not reached | 0% | None |
| Besse [52] | Milciclib | Not reported | PFS-3 | B3 Thymoma 27.8%, TC 72.2% | NR | One prior chemotherapy | 6.83 | 24.18 | 3.70% | Neutropenia (8.4%), creatinine, amylase, lipase increase (5.6%), nausea and asthenia (8.3%) |
| Besse [52] | Milciclib | Not reported | PFS-3 | B3 Thymoma 56.7%, TC 43.3% | NR | Multiple chemotherapies | 9.76 | Not reached | 4.20% | Neutropenia (8.4%), creatinine, amylase, lipase increase (5.6%), nausea and asthenia (8.3%) |
| Sato (REMORA) [53] | Lenvatinib | 15.5 | ORR | TC | I-IVb (majority stage IVa and IVb) | At least one platinum-based chemotherapy | 9.3 (7.7–13.9) | Not reached (16.1-not reached) | 38% (25.6–52%) | Hypertension 64%, palmar-plantar erythrodysaesthesia syndrome (7%) |
| First Author (Year of Publication) | Patients/Tumor Specimens | Histology | TME |
|---|---|---|---|
| Shim (2011) [57] | 32 | TC | High intensities of stromal CD4+ cells and stromal CD20+ lymphocytes are significantly associated with improved survival in TC |
| Kim (2019) [61] | 31 | 6 Thymomas–25 TC | A higher fold-change in the percentage of Ki-67+ cells among PD-1+CD8+ T cells 7 days after the first dose (Ki-67D7/D0) significantly predicted DCB and prolonged PFS |
| Blessin (2020) [59] | 27 | Thymoma (among other types of cancers) | The quantity of TILs influences the likelihood of response to immune checkpoint inhibitors |
| Kim (2020) [62] | 31 | 6 Thymomas–25 TC | Patients with severe irAEs presented a significantly lower fold increase in the frequency of effector regulatory T (eTreg) cells after anti-PD-1 treatment, a higher ratio of T helper-17 (Th17) and T helper-1 cells at baseline, and a higher percentage of Ki-67+ cells among PD-1+ CD8+ T cells posttreatment |
| Sato (2020) [58] | 42 | TC | High mean numbers of stromal CD8+, CD20+, and FOXP3+ cells were significantly associated with favorable prognosis, while high CD204+ cell density tended to be correlated with poor prognosis |
| Liu (2020) [64] | n/a | Thymoma–TC (among other types of cancers) | A positive relationship between YIF1B expression and immune cell infiltration; YIF1B expression positively correlated with TMB, microsatellite instability, and methylation in some cancer types |
| Chen (2020) [63] | Not reported | TC (among other types of cancers) | PRKDC mutation is one of the significant factors linked to increased TMB, inflamed TME, and greater responsiveness to ICI |
| Bocchialini (2022) [60] | 39 | TC | Higher total density of CD3+ and CD8+ TILs in early stages, lower density of CD3+ and CD8+ TILs in advanced TC stages compared to early stages, high densities of stromal CD4+ TILs and CD8+ TILs were associated with improved freedom from recurrence (FFR) and cause-specific survival (CSS), high density of FoxP3+ TILs was associated with improved FFR |
| Su (2022) [56] | 20 | Not specified | Higher expression levels of AC138207.2, AC148477.2, AL450270.1 and SNHG8 as well as lower expression levels of AC004466.3, and HOXB-AS1 in TETs samples compared with normal controls; more immunotherapy responders in the low-risk IRL subgroup |
| Hou (2022) [55] | 21 | 15 Thymomas–6 TC | Higher immune score, higher immune cell infiltration level, and T cell diversity in thymoma; higher stromal score, significantly lower expression of HMGB1 (a pro-inflammatory cytokine-related gene), which is associated with a dismal prognosis, and higher mutation burden in TC |
| NCT Number | Status | Histology | Intervention | Phase | Enrollment |
|---|---|---|---|---|---|
| NCT03463460 | Recruiting | Thymic carcinoma | Pembrolizumab/sunitinib malate | II | 40 |
| NCT03694002 | Active, not recruiting | Thymic carcinoma | Ramucirumab/carboplatine/paclitaxel | II | 66 |
| NCT04417660 | Recruiting | Thymoma | M7824 | II | 38 |
| NCT01621568 | Active, not recruiting | Thymoma | Sunitinib | II | 56 |
| NCT05104736 | Recruiting | Thymoma/thymic carcinoma | PT-112 | II | 53 |
| NCT01306045 | Active, not recruiting | Thymic carcinoma | AZD6244/MK-2206/lapatinib/erlotinib/sunitinib | II | 647 |
| NCT04667793 | Recruiting | Thymoma/thymic carcinoma | Toripalimab and chemotherapy | II | 15 |
| NCT03663764 | Active, not recruiting | Thymoma/thymic carcinoma | Thymosin a1 | II | 57 |
| NCT05683886 | Recruiting | Thymoma/thymic carcinoma | KC1036 | II | 30 |
| NCT01025089 | Active, not recruiting | Thymoma/thymic carcinoma | Cetuximab, cisplatin, doxorubicin and cyclophosphamide | II | 18 |
| NCT03076554 | Recruiting | Thymoma/thymic carcinoma | Avelumab | II | 55 |
| NCT05832827 | Not yet recruiting | Thymic carcinoma | MK-3475/lenvatinib/carboplatin/paclitaxel | II | 35 |
| NCT03583086 | Active, not recruiting | Thymic carcinoma | VEGFR/ PDGFR dual kinase inhibitor X-82—nivolumab | I/II | 88 |
| NCT03134118 | Active, not recruiting | Thymoma type B3/thymic carcinoma | Nivolumab | II | 55 |
| NCT04925947 | Recruiting | Thymic carcinoma | KN046 | II | 29 |
| NCT04321330 | Active, not recruiting | Thymic carcinoma | Atezolizumab | II | 34 |
| NCT04710628 | Recruiting | Metastatic thymic carcinoma/thymoma Type B3 | Pembrolizumab/lenvatinib | II | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrafiotis, A.C.; Brandão, M.; Berghmans, T.; Durieux, V.; Jungels, C. Immunotherapy and Targeted Therapies Efficacy in Thymic Epithelial Tumors: A Systematic Review. Biomedicines 2023, 11, 2722. https://doi.org/10.3390/biomedicines11102722
Agrafiotis AC, Brandão M, Berghmans T, Durieux V, Jungels C. Immunotherapy and Targeted Therapies Efficacy in Thymic Epithelial Tumors: A Systematic Review. Biomedicines. 2023; 11(10):2722. https://doi.org/10.3390/biomedicines11102722
Chicago/Turabian StyleAgrafiotis, Apostolos C., Mariana Brandão, Thierry Berghmans, Valérie Durieux, and Christiane Jungels. 2023. "Immunotherapy and Targeted Therapies Efficacy in Thymic Epithelial Tumors: A Systematic Review" Biomedicines 11, no. 10: 2722. https://doi.org/10.3390/biomedicines11102722





