Decoding Hidden Messengers: Proteomic Profiling of Exosomes in Mammary Cancer Research
Abstract
:1. Introduction
2. Breast Cancer Biomarkers
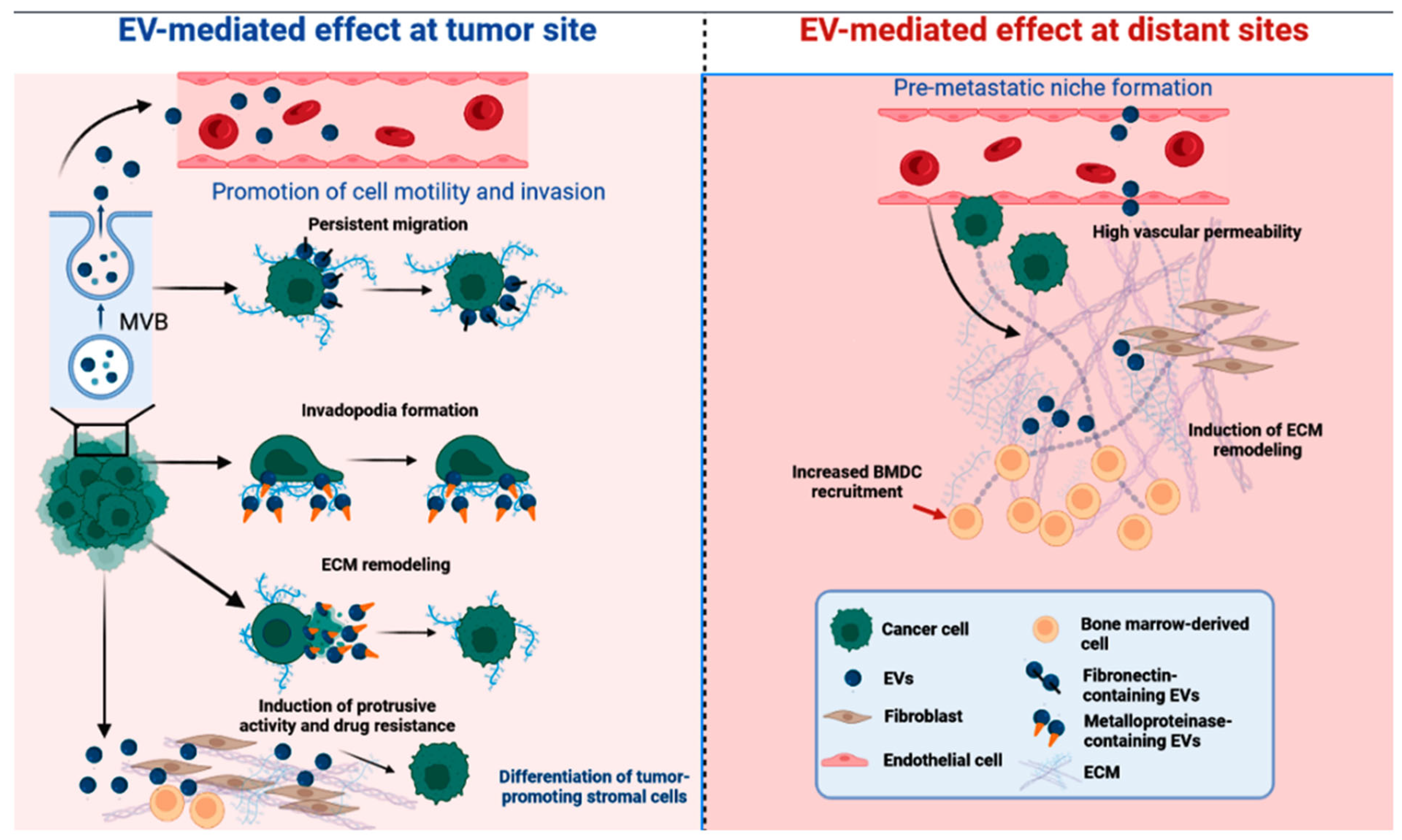
3. Small Extracellular Vesicles in Cancer Intercellular Communication
4. SEVs Shuttle Cargo Proteins That Regulate Tumorigenesis and Show Diagnostics and Prognostics Potential
5. Mammary Canine SEV Proteomic Studies Are Scarce
6. SEV Proteomics May Identify Novel Breast Cancer Biomarkers
| Author | Proteins | Isolation | Pathology | Applicability | Source |
|---|---|---|---|---|---|
| Khan et al. (2014) [36] | BIRC5 and splice variants | UC | TNBC, ER, and PR positive | Diagnosis, prognosis, and treatment | Tumor |
| Harris et al. (2015) [37] | Several proteins related with adhesion/motility/cytoskeleton, proteases, transporters, cell surface receptor, stress response proteins, small GTPases, metabolic enzymes, and RNA binding | UC | TNBC | Treatment | Cells in vitro |
| Several proteins related with tetraspanin, adhesion, cell surface receptor, transporter, stress response proteins, budding vesicles, trafficking/transport, calcium binding, and small GTPase | UC | Luminal | |||
| Blomme et al. (2016) [38] | MYOF | UC | Luminal and TNBC | Treatment | Cells in vitro |
| Vardaky et al. (2016) [40] | POSTN | UC | TNBC cells and patients with LN metastasis | Diagnosis | Cells in vitro and tumor samples |
| Hurwitz et al. (2016) [42] | POSTN | ExtraPEG and UC | TNBC | Diagnosis | Cells in vitro |
| RFTN1, FBLN7, and SERPINE1 | Metastatic breast cancer | ||||
| Moon et al. (2016) [43]; Lee et al. (2017) [45] | DEL-1 | UC | Early-stage breast cancer, several subtypes | Diagnosis | Cells in vitro and plasma samples |
| Gangoda et al. (2017) [46] | CP and MTDH | UC | TNBC, highly metastatic | Diagnosis | Cells in vitro |
| Maji et al. (2017) [47] | ANXA2 | UC | Metastatic breast cancer cells | Diagnosis and treatment | Cells in vitro |
| Rontogianni et al. (2019) [48] | EPHA2, DNAJA1, PABPC1, and NRP1 | UC | TNBC | Diagnosis | Cells in vitro and serum samples |
| HER2, GRB7, EIF3H, and ARFGEF2 | HER2 | ||||
| Jordan et al. (2020) [20] | Several proteins related with spliceosome, transcription factors, ribosomal proteins, tRNA ligases, proteasome units; pyrophosphatase, annexin, LAMP-1, EEA1, NUMA1, VTN, collagen, filamin proteins, and EDIL3 | Sepharose CL-2B SEC | TNBC | Diagnosis | Cells in vitro and serum samples |
| Proteins from adherin family members, laminin proteins, proteoglycans, SDC1, EPCAM, b-catenin, collagen, CD109, RARRES1, PTGFRN, FAT1, S100A14, AREG, calcium-binding proteins, serine proteases, and cholesterol- and lipoprotein-binding proteins | Ductal carcinoma in situ | ||||
| Dalla et al. (2020) [49] | Actin cytoplasmic 1, PKM, GAPDH, HSP60, ATP1B3, and VDAC2 | UC | Shared among MCF-7, MDA-MB-231, and T47D | Diagnosis | Cells in vitro |
| Risha et al. (2020) [50] | GLUT1, GPC3 and ADAM10 | UC | MDA-MB-231 | Diagnosis and prognosis | Cells in vitro |
| Vinik et al. (2020) [51] | EGFR, FAK, fibronectin, p38_pT180_Y182, N-cadherin, E2F1, PARP, MEK1, Aurora-B, p90RSK_pT573, S6_pS240_S244 | SEC | Stage I | Diagnosis, and treatment | Serum samples |
| C-Raf, fibronectin, heregulin, FAK, MEK, β-actin, N-cadherin, FoxO3a_PS318_S231, P-cadherin, PDHK1, TAZ | Stage IIA | ||||
| Li et al. (2021) [52] | CD151 | UC | TNBC | Diagnosis | Serum samples |
| Patwardhan et al. (2021) [53] | THBS1 | ExoEnrich | TNBC | Treatment | Cells in vitro |
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef] [PubMed]
- Lakhtakia, R. A Brief History of Breast Cancer: Part I: Surgical domination reinvented. Sultan Qaboos Univ. Med. J. 2014, 14, e166-9. [Google Scholar]
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef]
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Alimirzaie, S.; Bagherzadeh, M.; Akbari, M.R. Liquid biopsy in breast cancer: A comprehensive review. Clin. Genet. 2019, 95, 643–660. [Google Scholar] [CrossRef]
- Freitas, A.J.A.D.; Causin, R.L.; Varuzza, M.B.; Calfa, S.; Hidalgo Filho, C.M.T.; Komoto, T.T.; Souza, C.D.P.; Marques, M.M.C. Liquid biopsy as a tool for the diagnosis, treatment, and monitoring of breast cancer. Int. J. Mol. Sci. 2022, 23, 9952. [Google Scholar] [CrossRef]
- Halvaei, S.; Daryani, S.; Eslami-S, Z.; Samadi, T.; Jafarbeik-Iravani, N.; Bakhshayesh, T.O.; Majidzadeh-A, K.; Esmaeili, R. Exosomes in cancer liquid biopsy: A focus on breast cancer. Mol. Ther. Nucleic Acids 2018, 10, 131–141. [Google Scholar] [CrossRef]
- Tay, T.K.Y.; Tan, P.H. Liquid biopsy in breast cancer: A focused review. Arch. Pathol. Lab. Med. 2021, 145, 678–686. [Google Scholar] [CrossRef]
- da Costa, A.; Lenze, D.; Hummel, M.; Kohn, B.; Gruber, A.D.; Klopfleisch, R. Identification of six potential markers for the detection of circulating canine mammary tumour cells in the peripheral blood identified by microarray analysis. J. Comp. Pathol. 2012, 146, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, J.; Hu, G. Biomarker studies in early detection and prognosis of breast cancer. Adv. Exp. Med. Biol. 2017, 1026, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Gam, L.-H. Breast cancer and protein biomarkers. World J. Exp. Med. 2012, 2, 86–91. [Google Scholar] [CrossRef]
- Loke, S.Y.; Lee, A.S.G. The future of blood-based biomarkers for the early detection of breast cancer. Eur. J. Cancer 2018, 92, 54–68. [Google Scholar] [CrossRef]
- Celis, J.E.; Gromov, P.; Cabezón, T.; Moreira, J.M.A.; Ambartsumian, N.; Sandelin, K.; Rank, F.; Gromova, I. Proteomic characterization of the interstitial fluid perfusing the breast tumor microenvironment: A novel resource for biomarker and therapeutic target discovery. Mol. Cell. Proteom. 2004, 3, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Buxton, I.L.O. Evolution of medical approaches and prominent therapies in breast cancer. Cancers 2022, 14, 2450. [Google Scholar] [CrossRef]
- Antonyak, M.A.; Li, B.; Boroughs, L.K.; Johnson, J.L.; Druso, J.E.; Bryant, K.L.; Holowka, D.A.; Cerione, R.A. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4852–4857. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Jordan, K.R.; Hall, J.K.; Schedin, T.; Borakove, M.; Xian, J.J.; Dzieciatkowska, M.; Lyons, T.R.; Schedin, P.; Hansen, K.C.; Borges, V.F. Extracellular vesicles from young women’s breast cancer patients drive increased invasion of non-malignant cells via the Focal Adhesion Kinase pathway: A proteomic approach. Breast Cancer Res. 2020, 22, 128. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Jabalee, J.; Towle, R.; Garnis, C. The role of extracellular vesicles in cancer: Cargo, function, and therapeutic implications. Cells 2018, 7, 93. [Google Scholar] [CrossRef]
- Wang, H.-X.; Gires, O. Tumor-derived extracellular vesicles in breast cancer: From bench to bedside. Cancer Lett. 2019, 460, 54–64. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Klose, P.; Weise, C.; Bondzio, A.; Multhaup, G.; Einspanier, R.; Gruber, A.D. Proteome of metastatic canine mammary carcinomas: Similarities to and differences from human breast cancer. J. Proteome Res. 2010, 9, 6380–6391. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Bonnet, A.; Herráez, P.; Martín de las Mulas, J.; Rodríguez, F.; Déniz, J.M.; Espinosa de los Monteros, A. Expression of 14-3-3 σ protein in normal and neoplastic canine mammary gland. Vet. J. 2011, 190, 345–351. [Google Scholar] [CrossRef]
- Jagarlamudi, K.K.; Westberg, S.; Rönnberg, H.; Eriksson, S. Properties of cellular and serum forms of thymidine kinase 1 (TK1) in dogs with acute lymphocytic leukemia (ALL) and canine mammary tumors (CMTs): Implications for TK1 as a proliferation biomarker. BMC Vet. Res. 2014, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.C.; Shrivastava, S.; Saxena, S.; Kumar, N.; Maiti, S.K.; Mishra, B.P.; Singh, R.K. Surface plasmon resonance immunosensor for label-free detection of BIRC5 biomarker in spontaneously occurring canine mammary tumours. Sci. Rep. 2019, 9, 13485. [Google Scholar] [CrossRef]
- Fhaikrue, I.; Srisawat, W.; Nambooppha, B.; Pringproa, K.; Thongtharb, A.; Prachasilchai, W.; Sthitmatee, N. Identification of potential canine mammary tumour cell biomarkers using proteomic approach: Differences in protein profiles among tumour and normal mammary epithelial cells by two-dimensional electrophoresis-based mass spectrometry. Vet. Comp. Oncol. 2020, 18, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Tamarindo, G.H.; Novais, A.A.; Chuffa, L.G.A.; Zuccari, D.A.P.C. Metabolic alterations in canine mammary tumors. Animals 2023, 13, 2757. [Google Scholar] [CrossRef]
- Park, H.-M.; Kim, H.; Kim, D.W.; Yoon, J.-H.; Kim, B.-G.; Cho, J.-Y. Common plasma protein marker LCAT in aggressive human breast cancer and canine mammary tumor. BMB Rep. 2020, 53, 664–669. [Google Scholar] [CrossRef]
- Cordeiro, Y.G.; Mulder, L.M.; van Zeijl, R.J.M.; Paskoski, L.B.; van Veelen, P.; de Ru, A.; Strefezzi, R.F.; Heijs, B.; Fukumasu, H. Proteomic Analysis Identifies FNDC1, A1BG, and Antigen Processing Proteins Associated with Tumor Heterogeneity and Malignancy in a Canine Model of Breast Cancer. Cancers 2021, 13, 5901. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.H.-C.; Chang, S.-C.; Huang, Y.; Liu, H.-P. Serum Level of Tumor-Overexpressed AGR2 Is Significantly Associated with Unfavorable Prognosis of Canine Malignant Mammary Tumors. Animals 2021, 11, 2923. [Google Scholar] [CrossRef]
- Gast, M.-C.W.; Schellens, J.H.M.; Beijnen, J.H. Clinical proteomics in breast cancer: A review. Breast Cancer Res. Treat. 2009, 116, 17–29. [Google Scholar] [CrossRef]
- Hondermarck, H.; Tastet, C.; El Yazidi-Belkoura, I.; Toillon, R.-A.; Le Bourhis, X. Proteomics of breast cancer: The quest for markers and therapeutic targets. J. Proteome Res. 2008, 7, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Latterich, M.; Abramovitz, M.; Leyland-Jones, B. Proteomics: New technologies and clinical applications. Eur. J. Cancer 2008, 44, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Bennit, H.F.; Turay, D.; Perez, M.; Mirshahidi, S.; Yuan, Y.; Wall, N.R. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer 2014, 14, 176. [Google Scholar] [CrossRef]
- Harris, D.A.; Patel, S.H.; Gucek, M.; Hendrix, A.; Westbroek, W.; Taraska, J.W. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS ONE 2015, 10, e0117495. [Google Scholar] [CrossRef]
- Blomme, A.; Fahmy, K.; Peulen, O.; Costanza, B.; Fontaine, M.; Struman, I.; Baiwir, D.; de Pauw, E.; Thiry, M.; Bellahcène, A.; et al. Myoferlin is a novel exosomal protein and functional regulator of cancer-derived exosomes. Oncotarget 2016, 7, 83669–83683. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, B.; Zhao, C.; Ba, Z.; Xu, H.; Yan, X.; Liu, W.; Zhu, B.; Wang, L.; Ren, C. Myoferlin, a multifunctional protein in normal cells, has novel and key roles in various cancers. J. Cell. Mol. Med. 2019, 23, 7180–7189. [Google Scholar] [CrossRef]
- Vardaki, I.; Ceder, S.; Rutishauser, D.; Baltatzis, G.; Foukakis, T.; Panaretakis, T. Periostin is identified as a putative metastatic marker in breast cancer-derived exosomes. Oncotarget 2016, 7, 74966–74978. [Google Scholar] [CrossRef]
- Dorafshan, S.; Razmi, M.; Safaei, S.; Gentilin, E.; Madjd, Z.; Ghods, R. Periostin: Biology and function in cancer. Cancer Cell Int. 2022, 22, 315. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Rider, M.A.; Bundy, J.L.; Liu, X.; Singh, R.K.; Meckes, D.G. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 2016, 7, 86999–87015. [Google Scholar] [CrossRef]
- Moon, P.-G.; Lee, J.-E.; Cho, Y.-E.; Lee, S.J.; Jung, J.H.; Chae, Y.S.; Bae, H.-I.; Kim, Y.-B.; Kim, I.-S.; Park, H.Y.; et al. Identification of Developmental Endothelial Locus-1 on Circulating extracellular vesicles as a Novel Biomarker for Early Breast Cancer Detection. Clin. Cancer Res. 2016, 22, 1757–1766. [Google Scholar] [CrossRef]
- Lin, T.-C.; Yang, C.-H.; Cheng, L.-H.; Chang, W.-T.; Lin, Y.-R.; Cheng, H.-C. Fibronectin in cancer: Friend or foe. Cells 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, J.; Jung, J.H.; Park, H.Y.; Moon, P.-G.; Chae, Y.S.; Baek, M.-C. Exosomal Del-1 as a Potent Diagnostic Marker for Breast Cancer: Prospective Cohort Study. Clin. Breast Cancer 2021, 21, e748–e756. [Google Scholar] [CrossRef] [PubMed]
- Gangoda, L.; Liem, M.; Ang, C.-S.; Keerthikumar, S.; Adda, C.G.; Parker, B.S.; Mathivanan, S. Proteomic Profiling of Exosomes Secreted by Breast Cancer Cells with Varying Metastatic Potential. Proteomics 2017, 17, 1600370. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol. Cancer Res. 2017, 15, 93–105. [Google Scholar] [CrossRef]
- Rontogianni, S.; Synadaki, E.; Li, B.; Liefaard, M.C.; Lips, E.H.; Wesseling, J.; Wu, W.; Altelaar, M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun. Biol. 2019, 2, 325. [Google Scholar] [CrossRef]
- Dalla, P.V.; Santos, J.; Milthorpe, B.K.; Padula, M.P. Selectively-Packaged Proteins in Breast Cancer extracellular vesicles Involved in Metastasis. Int. J. Mol. Sci. 2020, 21, 4990. [Google Scholar] [CrossRef]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef] [PubMed]
- Vinik, Y.; Ortega, F.G.; Mills, G.B.; Lu, Y.; Jurkowicz, M.; Halperin, S.; Aharoni, M.; Gutman, M.; Lev, S. Proteomic analysis of circulating extracellular vesicles identifies potential markers of breast cancer progression, recurrence, and response. Sci. Adv. 2020, 6, eaba5714. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Yang, S.; Pi, H.; Li, Z.; Yao, P.; Zhang, Q.; Wang, Q.; Shen, P.; Li, X.; et al. Proteomic Landscape of Exosomes Reveals the Functional Contributions of CD151 in Triple-Negative Breast Cancer. Mol. Cell. Proteom. 2021, 20, 100121. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.; Mahadik, P.; Shetty, O.; Sen, S. ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1. Biomaterials 2021, 279, 121185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novais, A.A.; Tamarindo, G.H.; Chuffa, L.G.d.A.; Zuccari, D.A.P.d.C. Decoding Hidden Messengers: Proteomic Profiling of Exosomes in Mammary Cancer Research. Biomedicines 2023, 11, 2839. https://doi.org/10.3390/biomedicines11102839
Novais AA, Tamarindo GH, Chuffa LGdA, Zuccari DAPdC. Decoding Hidden Messengers: Proteomic Profiling of Exosomes in Mammary Cancer Research. Biomedicines. 2023; 11(10):2839. https://doi.org/10.3390/biomedicines11102839
Chicago/Turabian StyleNovais, Adriana Alonso, Guilherme Henrique Tamarindo, Luiz Gustavo de Almeida Chuffa, and Debora Aparecida Pires de Campos Zuccari. 2023. "Decoding Hidden Messengers: Proteomic Profiling of Exosomes in Mammary Cancer Research" Biomedicines 11, no. 10: 2839. https://doi.org/10.3390/biomedicines11102839







