SARS-CoV-2 Affects Thyroid and Adrenal Glands: An 18F-FDG PET/CT Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
- −
- Positivity to nasopharyngeal swab with real time polymerase chain reaction (RT-PCR) test for SARS-CoV-2 between 2020 and 2022;
- −
- Patients admitted in low intensity departments or intensive care unit (ICU);
- −
- Availability of at least one 18F-FDG PET/CT scan during the active SARS-CoV-2 infection performed in order to assess the extent of the disease.
- −
- Patients with pre-existing structural and/or functional alterations of thyroid and adrenal glands;
- −
- Patients who previously received chemotherapy or biologic therapies for oncologic reasons;
- −
- Pregnancy or nursing women;
- −
- Age < 18 years.
2.2. The 18F-FDG PET/CT Studies
2.3. Analysis of 18F-FDG PET/CT Studies
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Thyroid and Adrenal Gland Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [PubMed]
- Hossain, M.F.; Hasana, S.; Mamun, A.A.; Uddin, M.S.; Wahed, M.I.I.; Sarker, S.; Behl, T.; Ullah, I.; Begum, Y.; Bulbul, I.J.; et al. COVID-19 outbreak: Pathogenesis, current therapies, and potentials for future management. Front. Pharmacol. 2020, 11, 563478. [Google Scholar]
- Giovanella, L.; Ruggeri, R.M.; Ovčariček, P.P.; Campenni, A.; Treglia, G.; Deandreis, D. Prevalence of thyroid dysfunction in patients with COVID-19: A systematic review. Clin. Transl. Imaging 2021, 9, 233–240. [Google Scholar] [PubMed]
- Marazuela, M.; Giustina, A.; Puig-Domingo, M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev. Endocr. Metab. Disord. 2020, 21, 495–507. [Google Scholar]
- Parolin, M.; Parisotto, M.; Zanchetta, F.; Sartorato, P.; De Menis, E. Coronaviruses and endocrine system: A systematic review on evidence and shadows. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1242–1251. [Google Scholar]
- Clarke, S.A.; Abbara, A.; Dhillo, W.S. Impact of COVID-19 on the Endocrine System: A Mini-review. Endocrinology 2021, 163, bqab203. [Google Scholar]
- Chen, M.; Zhou, W.; Xu, W. Thyroid function analysis in 50 patients with COVID-19: A retrospective study. Thyroid 2021, 31, 8–11. [Google Scholar] [CrossRef]
- Khoo, B.; Tan, T.; Clarke, S.A.; Mills, E.G.; Patel, B.; Modi, M.; Phylactou, M.; Eng, P.C.; Thurston, L.; Alexander, E.C.; et al. Thyroid function before, during, and after COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e803–e811. [Google Scholar]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935. [Google Scholar]
- Muller, I.; Cannavaro, D.; Dazzi, D.; Covelli, D.; Mantovani, G.; Muscatello, A.; Ferrante, E.; Orsi, E.; Resi, V.; Longari, V.; et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020, 8, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Lania, A.; Sandri, M.T.; Cellini, M.; Mirani, M.; Lavezzi, E.; Mazziotti, G. Thyrotoxicosis in patients with COVID-19: The THYRCOV study. Eur. J. Endocrinol. 2020, 183, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Salat, M.; Urgell, E.; Chico, A. SARS-COV-2 as a trigger for autoimmune disease: Report of two cases of Graves’ disease after COVID-19. J. Endocrinol. Investig. 2020, 43, 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Blanco, S.; Pla-Peris, B.; Marazuela, M. COVID-19: A cause of recurrent Graves’ hyperthyroidism? J. Endocrinol. Investig. 2021, 44, 387–388. [Google Scholar] [CrossRef]
- Morita, S.; Takagi, T.; Inaba, H.; Furukawa, Y.; Kishimoto, S.; Uraki, S.; Shimo, N.; Takeshima, K.; Uraki, S.; Doi, K.; et al. Effect of SARS-CoV-2 BNT162b2 mRNA vaccine on thyroid autoimmunity: A twelve-month follow-up study. Front. Endocrinol. 2023, 14, 1058007. [Google Scholar] [CrossRef]
- Muller, I.; Consonni, D.; Crivicich, E.; Di Marco, F.; Currò, N.; Salvi, M. Increased risk of Thyroid Eye Disease following Covid-19 Vaccination. J. Clin. Endocrinol. Metab. 2023, 25, dgad501. [Google Scholar]
- Mainieri, F.; Chiarelli, F.; Betterle, C.; Bernasconi, S. Graves’ disease after COVID mRNA vaccination for the first time diagnosed in adolescence-case report. Cause and effect relationship or simple coincidence? J. Pediatr. Endocrinol. Metab. 2023, 36, 993–997. [Google Scholar] [CrossRef]
- Kumar, R.; Guruparan, T.; Siddiqi, S.; Sheth, R.; Jacyna, M.; Naghibi, M.; Vrentzou, E. A case of adrenal infarction in a patient with COVID 19 infection. BJR Case Rep. 2020, 6, 20200075. [Google Scholar] [CrossRef]
- Elkhouly, M.M.N.; Elazzab, A.A.; Moghul, S.S. Bilateral adrenal hemorrhage in a man with severe COVID-19 pneumonia. Radiol. Case Rep. 2021, 16, 1438–1442. [Google Scholar]
- Sharrack, N.; Baxter, C.T.; Paddock, M.; Uchegbu, E. Adrenal haemorrhage as a complication of COVID-19 infection. BMJ Case Rep. 2020, 13, 5–8. [Google Scholar] [CrossRef]
- Álvarez-Troncoso, J.; Zapatero Larrauri, M.; Montero Vega, M.D.; Vallano, R.G.; Pelaez, E.P.; Rojas-Marcos, P.M.; Martin-Luengo, F.; Del Campo, P.L.; Gil, C.R.H.; Esteban, E.T.; et al. Case report: COVID-19 with bilateral adrenal hemorrhage. Am. J. Trop. Med. Hyg. 2020, 103, 1156–1157. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.; Athar, S.; Gaba, W.H. New onset adrenal insufficiency in a patient with COVID-19. BMJ Case Rep. 2021, 14, e237690. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M. COVID-19 and the endocrine system: Exploring the unexplored. J. Endocrinol. Investig. 2020, 43, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Wheatland, R. Molecular mimicry of ACTH in SARS-implications for corticosteroid treatment and prophylaxis. Med. Hypotheses 2004, 63, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xu, B.; Guan, W.; Xu, D.; Li, F.; Ren, R.; Zhu, X.; Gao, Y.; Jiang, L. The Adrenal Cortex, an Underestimated Site of SARS-CoV-2 Infection. Front. Endocrinol. 2021, 11, 593179. [Google Scholar] [CrossRef]
- Piticchio, T.; Le Moli, R.; Tumino, D.; Frasca, F. Relationship between betacoronaviruses and the endocrine system: A new key to understand the COVID-19 pandemic—A comprehensive review. J. Endocrinol. Investig. 2021, 44, 1553–1570. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Stragapede, A.; Solimando, A.G.; Albanese, F.; Capobianco, M.; Giagulli, V.A.; Guastamacchia, E.; de Pergola, G.; Vacca, A. COVID-19 and the Endocrine System: A Comprehensive Review on the Theme. J. Clin. Med. 2021, 10, 2920. [Google Scholar] [CrossRef]
- Brender, E.; Lynm, C.; Glass, R.M. JAMA patient page. Adrenal insufficiency. JAMA 2005, 294, 2528. [Google Scholar] [CrossRef]
- Broersen, L.H.; Pereira, A.M.; Jørgensen, J.O.; Dekkers, O.M. Adrenal Insufficiency in Corticosteroids Use: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2171–2180. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent symptoms after Covid-19: Qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv. Res. 2020, 20, 1144. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, M.; Nel, J.; Blumberg, L.; Madhi, S.A.; Dryden, M.; Stevens, W. Long-COVID: An evolving problem with an extensive impact. S. Afr. Med. J. 2020, 111, 10–12. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelma, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Clarke, S.A.; Phylactou, M.; Patel, B.; Mills, E.G.; Muzi, B.; Izzi-Engbeaya, C.; Choudhury, S.; Khoo, B.; Meeran, K.; Comninos, A.N.; et al. Normal adrenal and thyroid function in patients who survive COVID-19 infection. J. Clin. Endocrinol. Metab. 2021, 106, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Salzano, C.; Saracino, G.; Cardillo, G. Possible adrenal involvement in long COVID syndrome. Medicina 2021, 57, 1087. [Google Scholar] [CrossRef] [PubMed]
- Olivari, L.; Riccardi, N.; Rodari, P.; Buonfrate, D.; Diodato, S.; Formenti, F.; Angheben, A.; Salgarello, M. Accidental diagnosis of COVID-19 pneumonia after 18F FDG PET/CT: A case series. Clin. Transl. Imaging 2020, 8, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Bertagna, F.; Bertoli, M.; Bosio, G.; Lucchini, S.; Motta, F.; Panarotto, M.B.; Peli, A.; Camoni, L.; Bengel, F.M.; et al. Incidental findings suggestive of COVID-19 in asymptomatic patients undergoing nuclear medicine procedures in a high-prevalence region. J. Nucl. Med. 2020, 61, 632–636. [Google Scholar] [CrossRef]
- Setti, L.; Kirienko, M.; Dalto, S.C.; Bonacina, M.; Bombardieri, E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1649–1656. [Google Scholar] [CrossRef]
- Qin, C.; Liu, F.; Yen, T.C.; Lan, X. 18F-FDG PET/CT findings of COVID-19: A series of four highly suspected cases. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1281–1286. [Google Scholar] [CrossRef]
- Colandrea, M.; Gilardi, L.; Travaini, L.L.; Fracassi, S.L.V.; Funicelli, L.; Grana, C.M. 18F-FDG PET/CT in asymptomatic patients with COVID-19: The submerged iceberg surfaces. Jpn. J. Radiol. 2020, 38, 1007–1011. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, J.; Chen, L.; Fu, C.; Kang, Y.; Zhang, W.; Fakhri, G.E.; Gu, J.; Shao, F.; Wang, M. Inflammatory response in lungs and extrapulmonary sites detected by [18F] fluorodeoxyglucose PET/CT in convalescing COVID-19 patients tested negative for coronavirus. Eur. J. Nucl. Med. Mol. Imaging 2021, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Lauri, C.; Bianchi, M.P.; Pelliccia, S.; Lenza, A.; Tetti, S.; Martini, M.L.; Franchi, G.; Trapasso, F.; De Biase, L.; et al. [18F]FDG PET/CT in Patients Affected by SARS-CoV-2 and Lymphoproliferative Disorders and Treated with Tocilizumab. J. Pers. Med. 2022, 12, 1839. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Lauri, C.; Colandrea, M.; Di Girolamo, M.; Chiodo, E.; Grana, C.M.; Campagna, G.; Aceti, A. Lymphopenia in patients affected by SARS-CoV-2 infection is caused by margination of lymphocytes in large bowel: An [18F]FDG PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2022, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, A.A.; Searle, J.; Hagan, I.; Lyburn, I.D. COVID-19 vaccination induced axillary nodal uptake on [18F] FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2655–2656. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, A.A.; Searle, J.; Hopkins, R.; Lyburn, I.D. False-positive axillary lymph nodes on FDG PET/CT resulting from COVID-19 immunization. Clin. Nucl. Med. 2021, 46, 1004–1005. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.; Nakhoda, S.K.; Li, Y.; Jian, Q.Y. COVID-19 vaccine–related local FDG uptake. Clin. Nucl. Med. 2021, 46, 439–441.7. [Google Scholar] [CrossRef]
- Eifer, M.; Eshet, Y. Imaging of COVID-19 vaccination at FDG PET/CT. Radiology 2021, 299, E248. [Google Scholar] [CrossRef]
- McIntosh, L.J.; Bankier, A.A.; Vijayaraghavan, G.R.; Licho, R.; Rosen, M.P. COVID-19 vaccination-related uptake on FDG PET/CT: An emerging dilemma and suggestions for management. Am. J. Roentgenol. 2021, 217, 975–983. [Google Scholar] [CrossRef]
- Moghimi, S.; Wilson, D.; Martineau, P. FDG PET Findings Post–COVID Vaccinations: Signs of the Times? Clin. Nucl. Med. 2021, 46, 437–438. [Google Scholar] [CrossRef]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Signore, A. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Goto, R.; Okada, K.; Kinomura, S.; Fukuda, H. A bone marrow F-18 FDG uptake exceeding the liver uptake may indicate bone marrow hyperactivity. Ann. Nucl. Med. 2009, 23, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Hwang, S.H.; Jung, S.M.; Lee, S.-W.; Park, Y.-B.; Yun, M.; Song, J.J. Evaluation of spleen glucose metabolism using 18F-FDG PET/CT in patients with febrile autoimmune disease. J. Nucl. Med. 2017, 58, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Hwang, S.H.; Jung, S.M.; Lee, S.-W.; Park, Y.-B.; Yun, M.; Song, J.J. The clinical utility of splenic fluorodeoxyglucose uptake for diagnosis and prognosis in patients with macrophage activation syndrome. Medicine 2017, 96, e7901. [Google Scholar] [CrossRef] [PubMed]
- Boursier, C.; Duval, X.; Mahida, B.; Hoen, B.; Goehringer, F.; Selton-Suty, C.; Chevalier, E.; Roch, V.; Lamiral, Z.; Bourdon, A.; et al. Hypermetabolism of the spleen or bone marrow is an additional albeit indirect sign of infective endocarditis at FDG-PET. J. Nucl. Cardiol. 2021, 28, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.J.; Schisterman, E.F. The Youden Index and the optimal cut-point corrected for measurement error. Biom. J. 2005, 47, 428–441. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Mukhtar, N.; Aljomaiah, A.; Aljamei, H.; Bakhsh, A.; Alsudani, N.; Elsayed, T.; Alrashidi, N.; Fadel, R.; Alqahtani, E.; et al. The Impact of COVID-19 Viral Infection on the Hypothalamic-Pituitary-Adrenal Axis. Endocr. Pract. 2021, 27, 83–89. [Google Scholar] [CrossRef]
- Siejka, A.; Barabutis, N. Adrenal insufficiency in the COVID-19 era. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E784–E785. [Google Scholar] [CrossRef]
- Akbas, E.M.; Akbas, N. COVID-19, adrenal gland, glucocorticoids, and adrenal insufficiency. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2021, 165, 1–7. [Google Scholar] [CrossRef]
- Kanczkowski, W.; Evert, K.; Stadtmüller, M.; Haberecker, M.; Laks, L.; Chen, L.S.; Frontzek, K.; Pablik, J.; Hantel, C.; Beuschlein, F.; et al. COVID-19 targets human adrenal glands. Lancet Diabetes Endocrinol. 2022, 10, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Daraei, M.; Hasibi, M.; Abdollahi, H.; Mirabdolhagh Hazaveh, M.; Zebaradst, J.; Hajinoori, M.; Asadollahi-Amin, A. Possible role of hypothyroidism in the prognosis of COVID-19. Intern. Med. J. 2020, 50, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodríguez, Y.; Zapata, E.; Ramírez-Santana, C.; Anaya, J.M. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J. Infect. Dis. 2022, 225, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Mongioì, L.M.; Barbagallo, F.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; La Vignera, S.; Calogero, A.E. Possible long-term endocrine-metabolic complications in COVID-19: Lesson from the SARS model. Endocrine 2020, 68, 467–470. [Google Scholar] [CrossRef]
- Albano, D.; Treglia, G.; Giovanella, L.; Giubbini, R.; Bertagna, F. Detection of thyroiditis on PET/CT imaging: A systematic review. Hormones 2020, 19, 341–349. [Google Scholar] [CrossRef]
- Van Leer, B.; van Snick, J.H.; Londema, M.; Nijsten, M.W.N.; Kasalak, O.; Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Pillay, J. [18F]FDG-PET/CT in mechanically ventilated critically ill patients with COVID-19 ARDS and persistent inflammation. Clin. Transl. Imaging 2023, 11, 297–306. [Google Scholar] [CrossRef]
- Frara, S.; Allora, A.; Castellino, L.; di Filippo, L.; Loli, P.; Giustina, A. COVID-19 and the pituitary. Pituitary 2021, 24, 465–481. [Google Scholar] [CrossRef]
- Sollini, M.; Morbelli, S.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Chiola, S.; Gelardi, F.; Chiti, A. Long COVID hallmarks on [18F] FDG-PET/CT: A case-control study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3187–3197. [Google Scholar] [CrossRef]
- Bülbül, O.; Göksel, S.; Demet, N.A.K. Effect of Coronavirus Disease 2019 on Fluorine-18 fluorodeoxyglucose Uptake of Endocrine Organs. Cumhur. Med. J. 2023, 45, 81–86. [Google Scholar] [CrossRef]
- Annunziata, S.; Bauckneht, M.; Albano, D.; Argiroffi, G.; Calabro, D.; Abenavoli, E.; Linguanti, F.; Laudicella, R.; Young Committee of the Italian Association of Nuclear Medicine (AIMN). Impact of the COVID-19 pandemic in nuclear medicine departments: Preliminary report of the first international survey. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2090–2099. [Google Scholar] [CrossRef]
- Freudenberg, L.S.; Paez, D.; Giammarile, F.; Cerci, J.; Modiselle, M.; Pascual, T.N.B.; El-Haj, N.; Orellana, P.; Pynda, Y.; Carrio, I.; et al. Global impact of COVID-19 on nuclear medicine departments: An international survey in April 2020. J. Nucl. Med. 2020, 61, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, L.S.; Dittmer, U.; Herrmann, K. Impact of COVID-19 on nuclear medicine in Germany, Austria and Switzerland: An international survey in April 2020. Nuklearmedizin 2020, 59, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, S.; Albano, D.; Laudicella, R.; Bauckneht, M.; Young Committee of the Italian Association of Nuclear Medicine (AIMN). Surveys on COVID-19 in nuclear medicine: What happened and what we learned. Clin. Transl. Imaging 2020, 8, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Pal, R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 2020, 68, 251–252. [Google Scholar] [CrossRef]
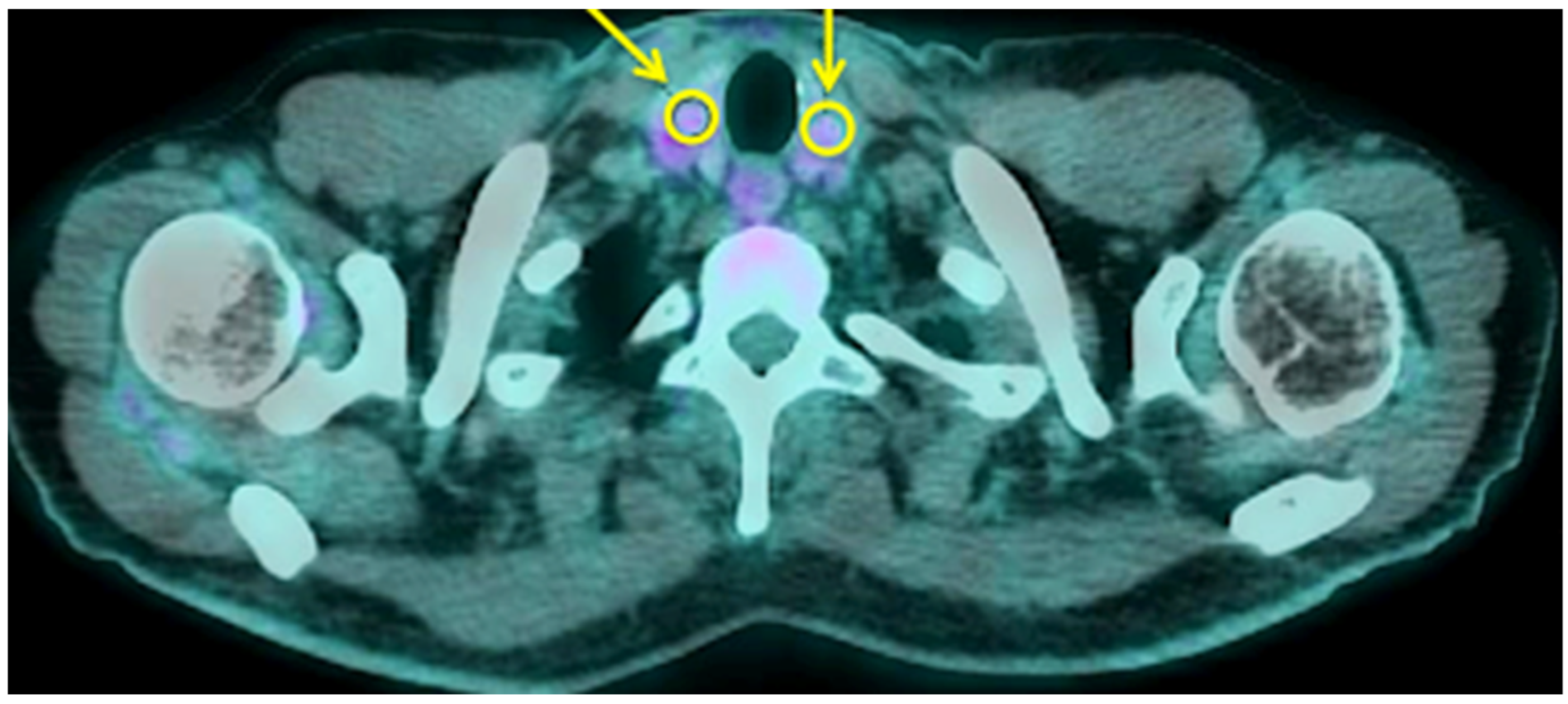
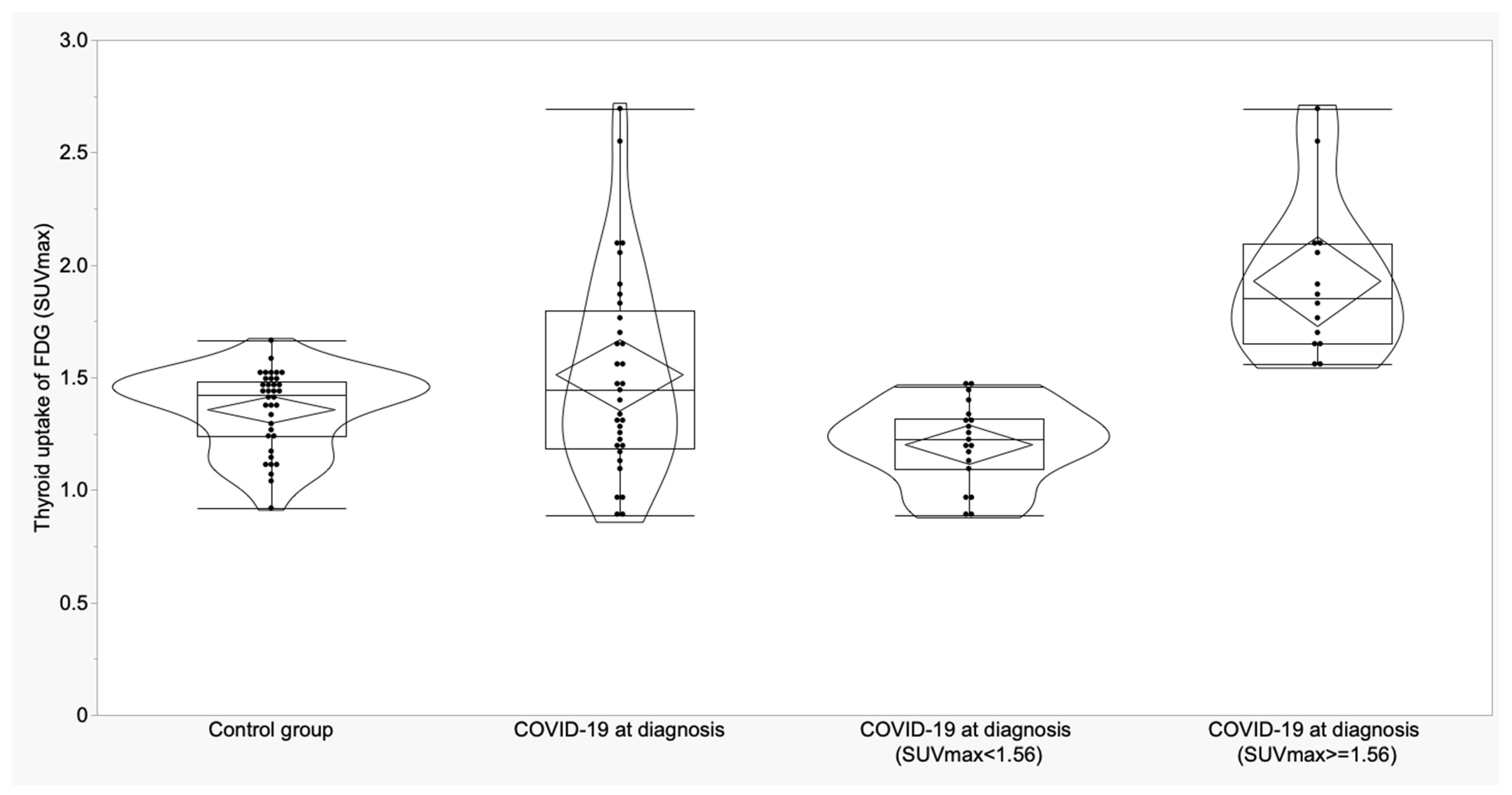
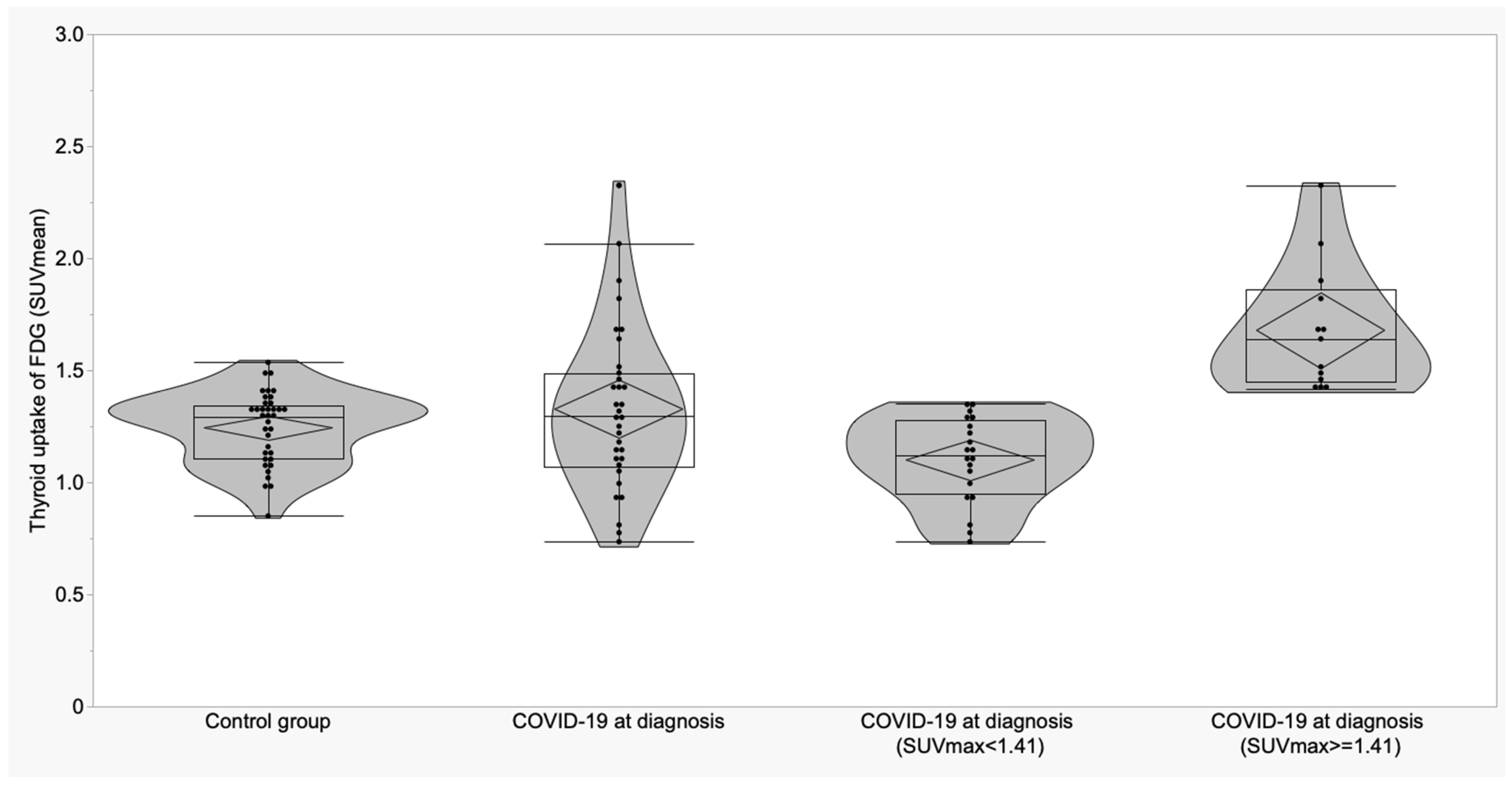

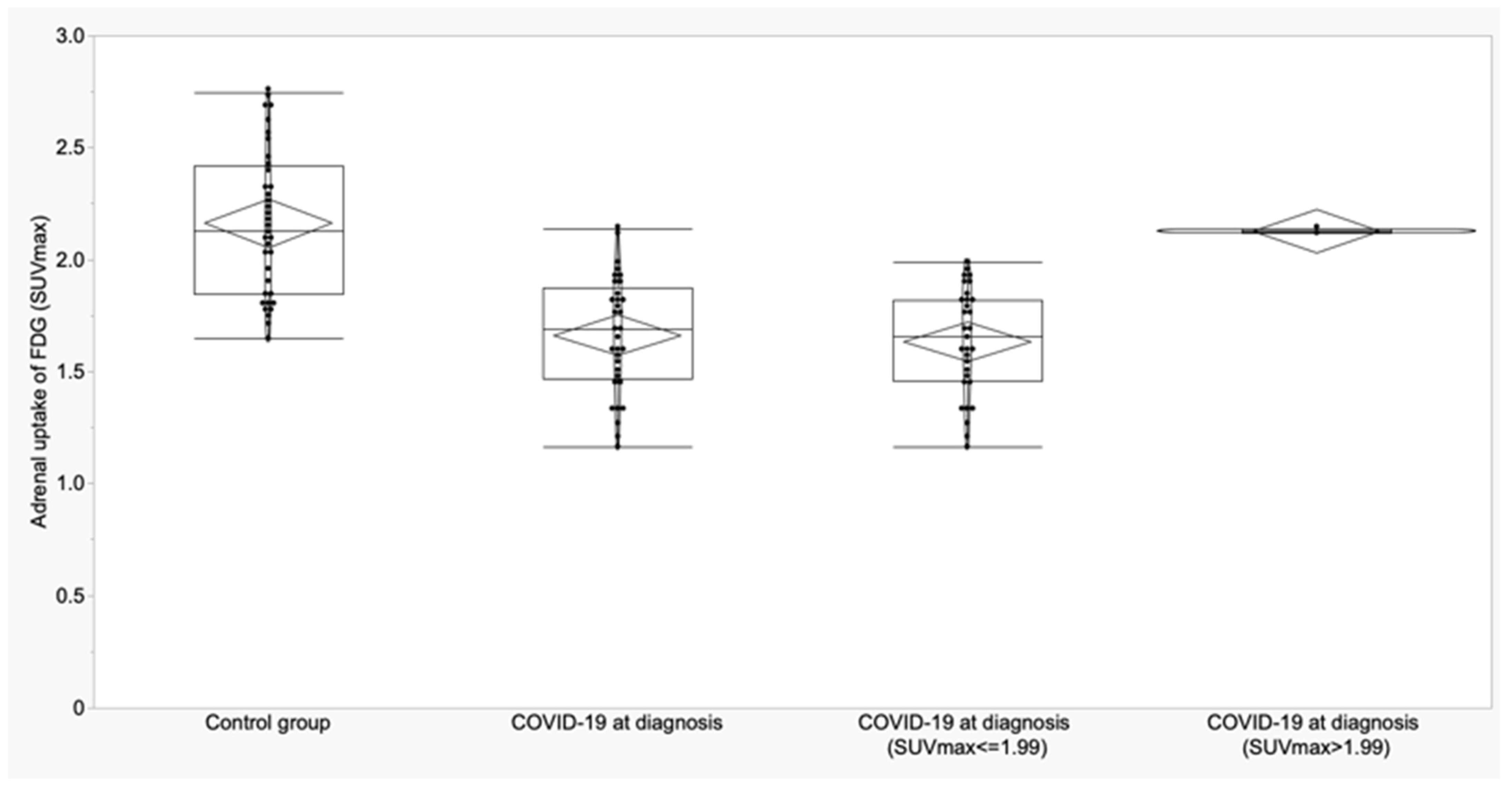
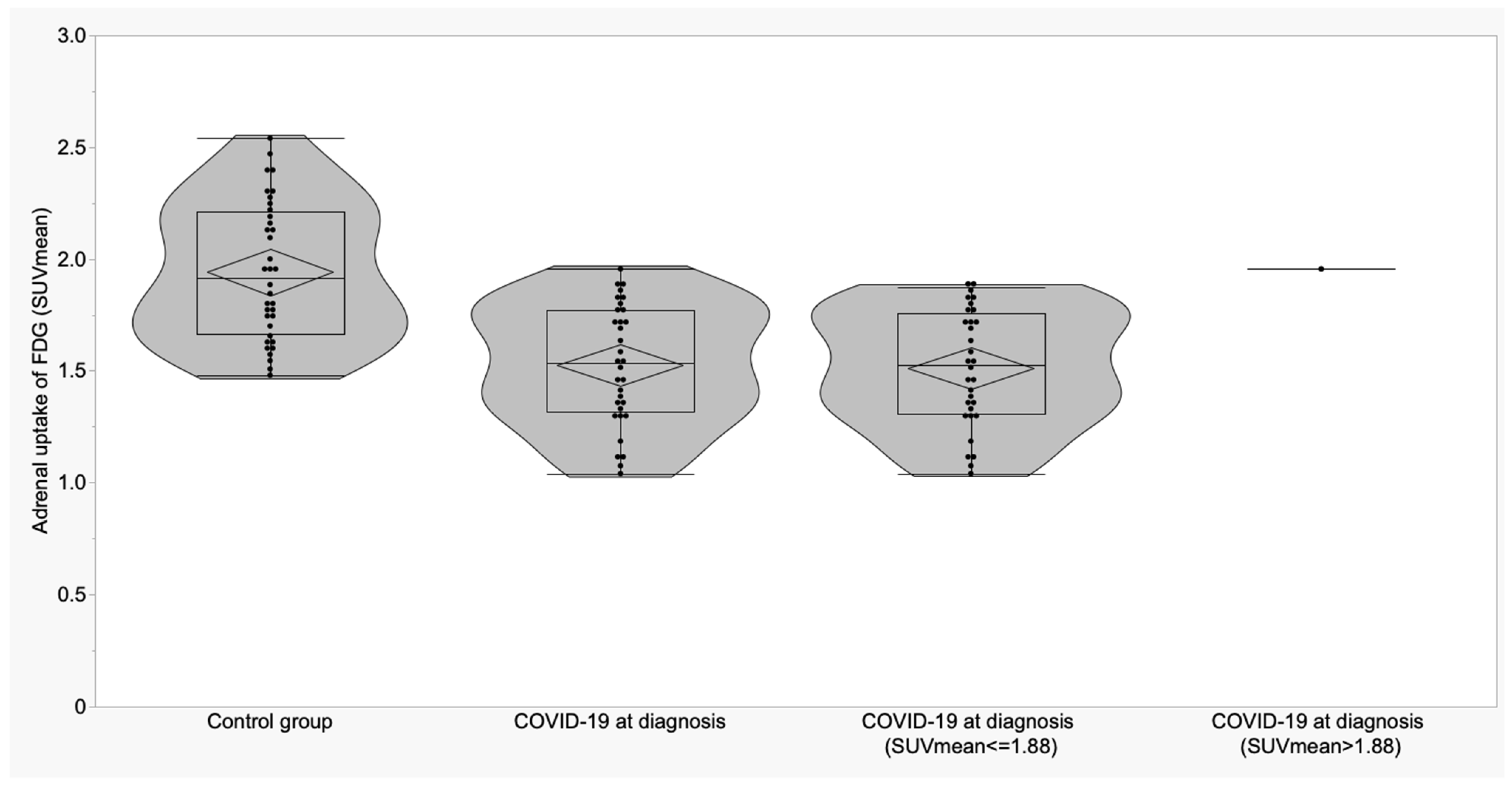
| Variable | A Control Subjects (n = 36) Mean ± SD (95% CI) | B COVID-19 at Diagnosis (n = 28) Mean ± SD (95% CI) | C COVID-19 ICU (n = 5) Mean ± SD (95% CI) | p | p (A vs. B) | p (A vs. C) | p (B vs. C) |
|---|---|---|---|---|---|---|---|
| Thyroid (SUVmax) | 1.36 ± 0.18 (1.30 to 1.42) | 1.55 ± 0.47 (1.37 to 1.73) | 1.31 ± 0.24 (1.02 to 1.61) | 0.06 | -- | -- | -- |
| Thyroid (SUVmean) | 1.24 ± 0.16 (1.19 to 1.30) | 1.35 ± 0.38 (1.21 to 1.50) | 1.18 ± 0.22 (0.90 to 1.45) | 0.19 | -- | -- | -- |
| Adrenal (SUVmax) | 2.16 ± 0.32 (2.05 to 2.27) | 1.65 ± 0.26 (1.55 to 1.75) | 1.73 ± 0.26 (1.40 to 2.06) | <0.0001 | <0.0001 | 0.003 | 0.58 |
| Adrenal (SUVmean) | 1.94 ± 0.30 (1.84 to 2.04) | 1.51 ± 0.27 (1.40 to 1.61) | 1.64 ± 0.23 (1.35 to 1.93) | <0.0001 | <0.0001 | 0.03 | 0.34 |
| Thyroid Uptake | Control Group (n = 36) Mean ± SD (95% CI) | COVID-19 after Recovery (n = 7) Mean ± SD (95% CI) | p |
|---|---|---|---|
| SUVmax | 1.36 ± 0.18 (1.30 to 1.42) | 1.65 ± 0.30 (1.37 to 1.93) | 0.009 |
| SUVmean | 1.24 ± 0.16 (1.19 to 1.30) | 1.46 ± 0.22 (1.25 to 1.66) | 0.004 |
| Adrenal Glands’ Uptake | Control Group (n = 36) Mean ± SD (95% CI) | COVID-19 after Recovery (n = 7) Mean ± SD (95% CI) | p |
|---|---|---|---|
| SUVmax | 2.16 ± 0.32 (2.05 to 2.27) | 1.66 ± 0.26 (1.57 to 1.75) | <0.0001 |
| SUVmean | 1.94 ± 0.30 (1.84 to 2.04) | 1.53 ± 0.26 (1.43 to 1.62) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauri, C.; Campagna, G.; Glaudemans, A.W.J.M.; Slart, R.H.J.A.; van Leer, B.; Pillay, J.; Colandrea, M.; Grana, C.M.; Stigliano, A.; Signore, A. SARS-CoV-2 Affects Thyroid and Adrenal Glands: An 18F-FDG PET/CT Study. Biomedicines 2023, 11, 2899. https://doi.org/10.3390/biomedicines11112899
Lauri C, Campagna G, Glaudemans AWJM, Slart RHJA, van Leer B, Pillay J, Colandrea M, Grana CM, Stigliano A, Signore A. SARS-CoV-2 Affects Thyroid and Adrenal Glands: An 18F-FDG PET/CT Study. Biomedicines. 2023; 11(11):2899. https://doi.org/10.3390/biomedicines11112899
Chicago/Turabian StyleLauri, Chiara, Giuseppe Campagna, Andor W. J. M. Glaudemans, Riemer H. J. A. Slart, Bram van Leer, Janesh Pillay, Marzia Colandrea, Chiara Maria Grana, Antonio Stigliano, and Alberto Signore. 2023. "SARS-CoV-2 Affects Thyroid and Adrenal Glands: An 18F-FDG PET/CT Study" Biomedicines 11, no. 11: 2899. https://doi.org/10.3390/biomedicines11112899





