Advances and Perspectives in Relation to the Molecular Basis of Diabetic Retinopathy—A Review
Abstract
:1. Aim
2. Introduction
3. Risk Factors
4. Pathophysiology
5. Molecular Biomarkers
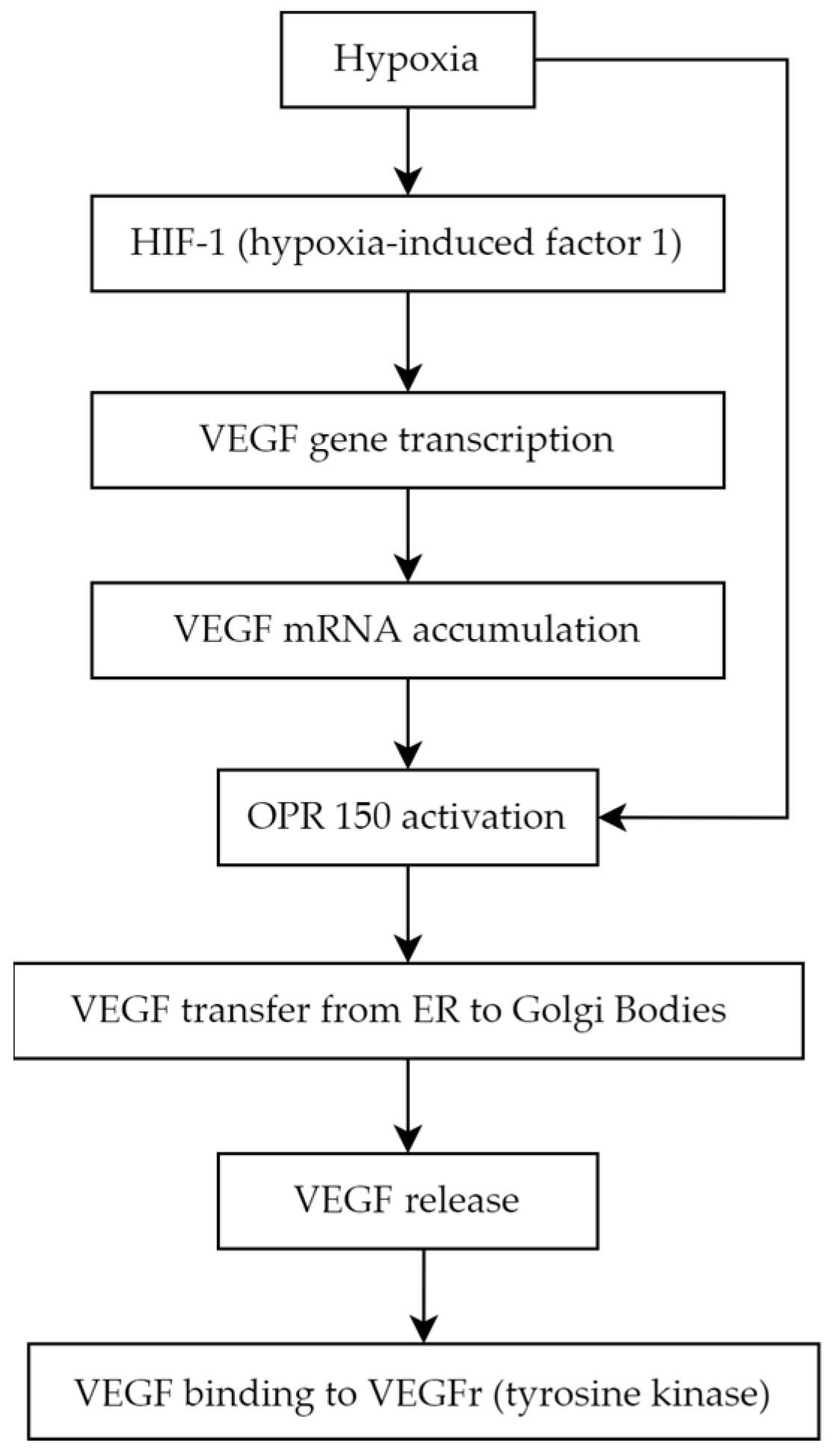
5.1. Vascular Endothelial Growth Factor
5.2. Asymmetric Dimethylarginine
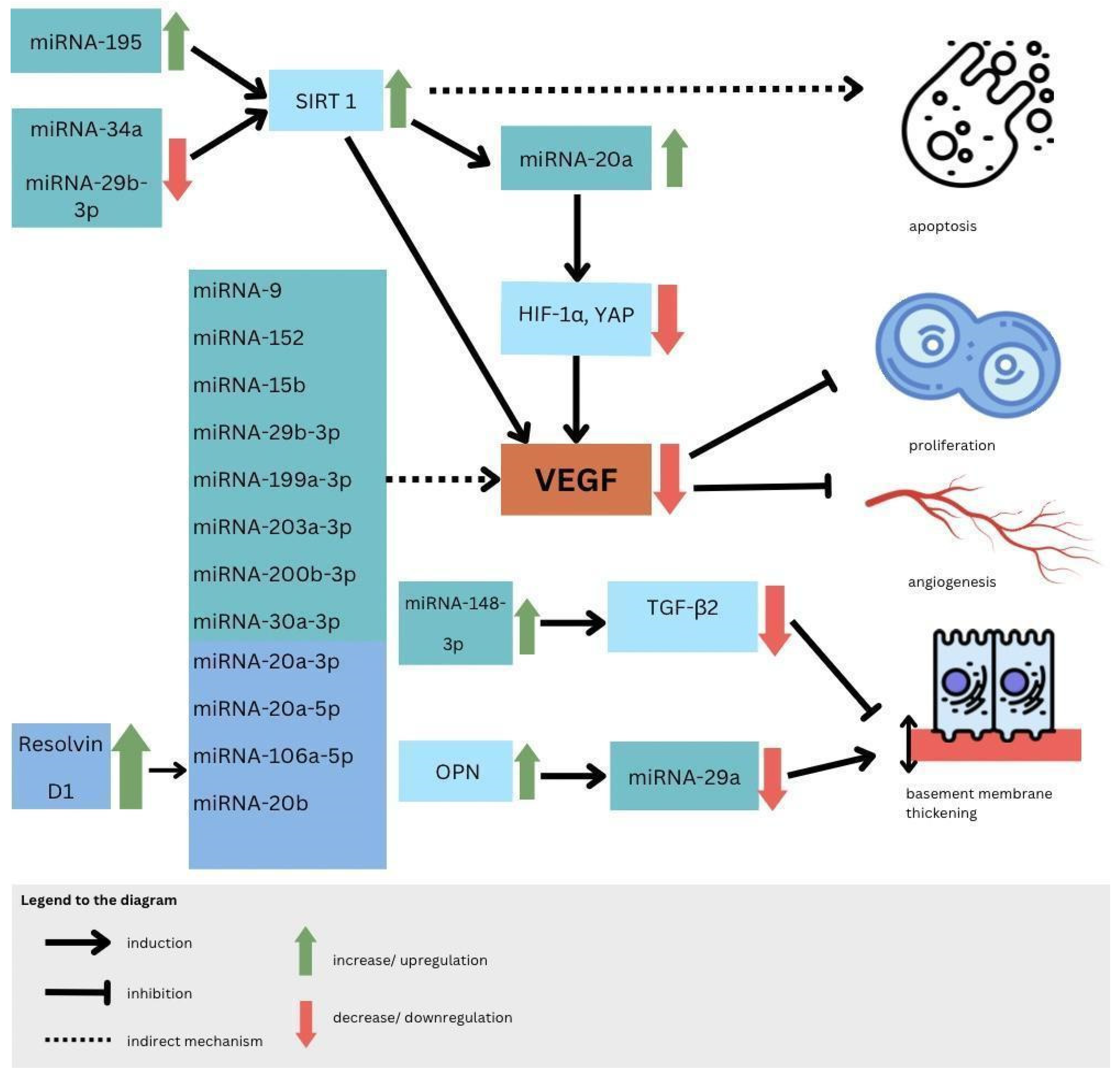
5.3. MicroRNAs
5.4. Endothelin-1
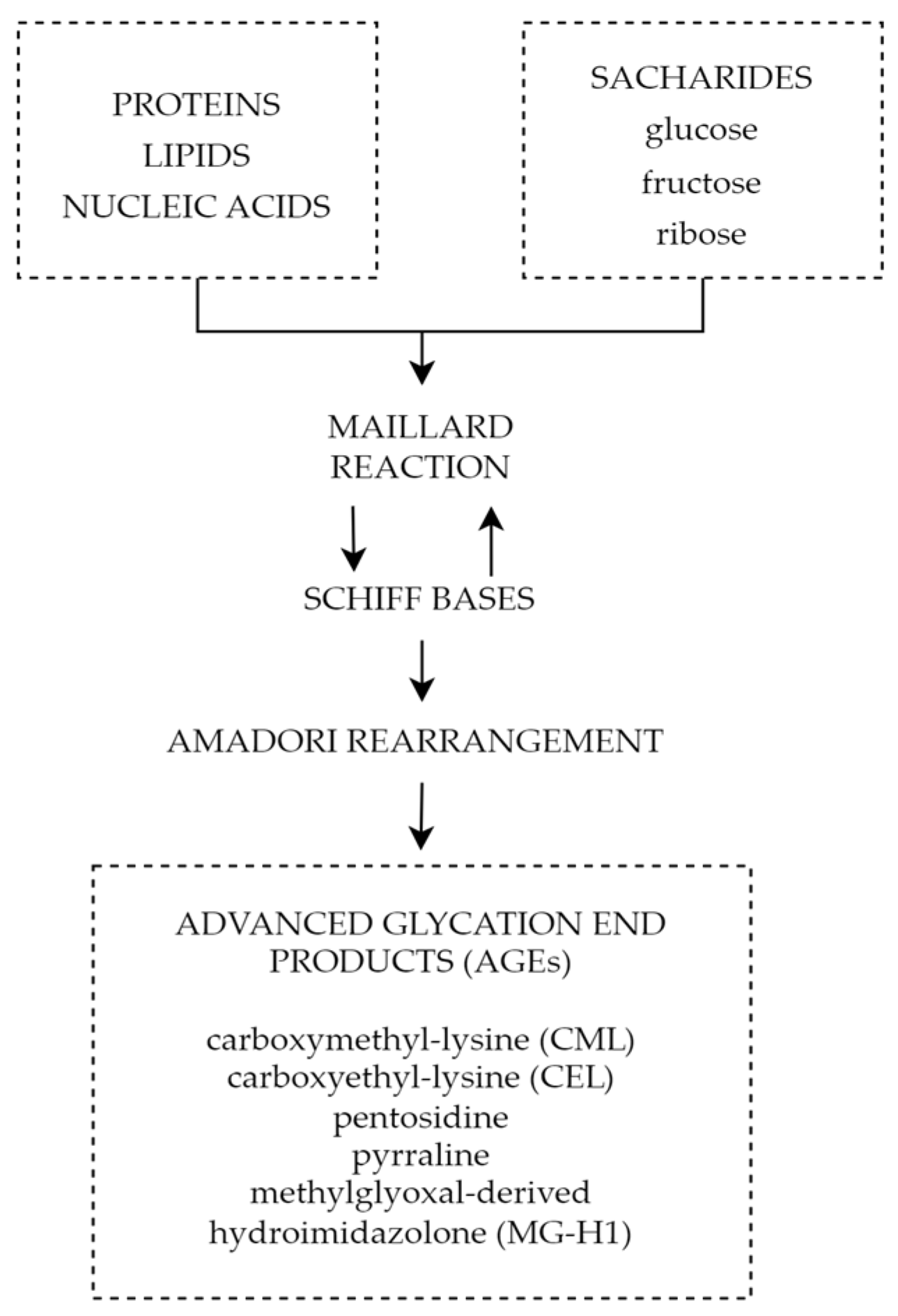
5.5. Advanced Glycation End Products
6. Discussion
7. Conclusions
8. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. International Diabetes Federation—Facts & Figures. Idf.org. Published 12 September 2021. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 15 May 2023).
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Modjtahedi, B.S.; Wu, J.; Luong, T.Q.; Gandhi, N.K.; Fong, D.S.; Chen, W. Severity of Diabetic Retinopathy and the Risk of Future Cerebrovascular Disease, Cardiovascular Disease, and All-Cause Mortality. Ophthalmology 2021, 128, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Liu, T.Y.A. The Impact of COVID-19 on Diabetic Retinopathy Monitoring and Treatment. Curr. Diabetes Rep. 2021, 21, 40. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Sagarra, R.; Hervás, E.; Blasco, R.; Molina, J.; Moreno, E.F.; Garcia-Curto, E. Impact of the COVID-19 Pandemic on the Metabolic Control of Diabetic Patients in Diabetic Retinopathy and Its Screening. J. Clin. Med. 2022, 11, 7121. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Vision & Eye Health Surveillance System (VEHSS), Vision Health Initiative. Available online: https://www.cdc.gov/visionhealth/vehss/project/index.html (accessed on 26 October 2023).
- Galiero, R.; Pafundi, P.C.; Nevola, R.; Rinalida, L. The Importance of Telemedicine during COVID-19 Pandemic: A Focus on Diabetic Retinopathy. J. Diabetes Res. 2020, 2020, 9036847. [Google Scholar] [CrossRef]
- Robles, R.; Patel, N.; Neag, E.; Mittal, A.; Markatia, Z.; Ameli, K.; Lin, B. A Systematic Review of Digital Ophthalmoscopes in Medicine. Clin. Ophthalmol. 2023, 17, 2957–2965. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Pafundi, P.C.; Gelso, A.; Bono, C. Telemedicine for screening diabetic retinopathy: The NO BLIND Italian multicenter study. Diabetes Metab. Res. Rev. 2019, 35, e3113. [Google Scholar] [CrossRef]
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef]
- Chew, E.Y.; Davis, M.D.; Danis, R.P.; Locato, J.F. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014, 121, 2443–2451. [Google Scholar] [CrossRef]
- Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Eye Study Group; The Action to Control Cardiovascular Risk in Diabetes Follow-On (ACCORDION) Study Group. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) follow-on study. Diabetes Care 2016, 39, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Tomić, M.; Gverović Antunica, A.; Ljubić, S.; Salopek Rabatić, J.; Karabatić, M. Body mass index: A risk factor for retinopathy in type 2 diabetic patients. Mediat. Inflamm. 2013, 2013, 436329. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Wu, W.C. Dyslipidemia and diabetic retinopathy. Rev. Diabet. Stud. 2013, 10, 121–132. [Google Scholar] [CrossRef]
- Kohner, E.M.; Aldington, S.J.; Stratton, I.M.; Manley, S.E.; Holman, R.R.; Matthews, D.R.; Turner, R.C. United Kingdom Prospective Diabetes Study 30. Diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch. Ophthalmol. 1998, 116, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Sharrett, A.R.; Klein, B.E.; Moss, S.E.; Folsom, A.R.; Wong, T.Y.; Brancati, F.L.; Hubbard, L.D.; Couper, D.; ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: The Atherosclerosis Risk in Communities study. Ophthalmology 2002, 109, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Marino, E.K.; Kuller, L.H.; Polak, J.F.; Tracy, R.P.; Gottdiener, J.S.; Burke, G.L.; Hubbard, L.D.; Boineau, R. The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. Br. J. Ophthalmol. 2002, 86, 84–90. [Google Scholar] [CrossRef] [PubMed]
- van Leiden, H.A.; Dekker, J.M.; Moll, A.C.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Stehouwer, C.D.; Polak, B.C. Blood pressure, lipids, and obesity are associated with retinopathy: The Hoorn study. Diabetes Care 2002, 25, 1320–1325. [Google Scholar] [CrossRef]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Peto, P. Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Y.; Yang, W.; Gao, X.; Han, X.; Ji, L. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: A meta-analysis. Endocrine 2018, 62, 299–306. [Google Scholar] [CrossRef]
- Rasmussen, K.L.; Laugesen, C.S.; Ringholm, L.; Vestgaard, M.; Damm, P.; Mathiesen, E.R. Progression of diabetic retinopathy during pregnancy in women with type 2 diabetes. Diabetologia 2010, 53, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Ansari, P.; Tabasumma, N.; Snigdha, N.N.; Siam, N.H.; Panduru, R.V.N.R.S.; Azam, S.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022, 3, 159–175. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Santos, J.M.; Mishra, M. Epigenetic modifications and diabetic retinopathy. Biomed. Res. Int. 2013, 2013, 635284. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.N.; Ulbig, M.W. Diabetic retinopathy: Early diagnosis and effective treatment. Dtsch. Arztebl. Int. 2010, 107, 75–83. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Ahmed, E.; Carrasquillo, K.G.; Amano, S.; Hida, T.; Oguchi, Y.; Adamis, A.P. VEGF164 is proinflammatory in the diabetic retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2155–2162. [Google Scholar] [CrossRef]
- Williams, B.; Baker, A.Q.; Gallacher, B.; Lodwick, D. Angiotensin II increases vascular permeability factor gene expression by human vascular smooth muscle cells. Hypertension 1995, 25, 913–917. [Google Scholar] [CrossRef]
- Viswanath, K.; McGavin, D.D. Diabetic retinopathy: Clinical findings and management. Community Eye Health 2003, 16, 21–24. [Google Scholar]
- Roy, S.; Kim, D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2021, 82, 100903. [Google Scholar] [CrossRef]
- Kern, T.S.; Huang, S.S. Vascular damage in diabetic retinopathy. Ocul. Dis. Mech. Manag. 2010, 2010, 506–513. [Google Scholar]
- Bakri, S.J.; Kaiser, P.K. Chapter 21—Diabetic Retinopathy. In Retinal Imaging; Huang, D., Kaiser, P.K., Lowder, C.Y., Elias, I., Traboulsi, E.I., Eds.; Mosby: Maryland Heights, MO, USA, 2006; pp. 233–240. [Google Scholar]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9.23.1–9.23.17. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, I.M.; Rezzola, S.; Cancarini, A.; Russo, A.; Costagliola, C.; Semeraro, F.; Presta, M. Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Prog. Retin. Eye Res. 2019, 72, 100756. [Google Scholar] [CrossRef]
- Homsi, J.; Daud, A.I. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control 2007, 14, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor receptor-2: Its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2005, 94, 751–756. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Stuttfeld, E.; Ballmer-Hofer, K. Structure and function of VEGF receptors. IUBMB Life 2009, 61, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M. Molecular Biology of the VEGF and the VEGF Receptor Family. Semin. Thromb. Hemost. 2000, 26, 561–570. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L. VEGF receptor signal transduction—A brief update. Vasc. Pharmacol. 2016, 86, 14–17. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Harpal, K. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Barbieri, A.; Vigano, I.; Band, F. Short-and Long-Term Expression of Vegf: A Temporal Regulation of a Key Factor in Diabetic Retinopathy. Front. Pharmacol. 2021, 12, 707909. [Google Scholar] [CrossRef]
- Hirano, T.; Toriyama, Y.; Iesato, Y.; Imai, A.; Murata, T. Changes in plasma vascular endothelial growth factor level after intravitreal injection of Bevacizumab, Aflibercept, or Ranibizumab for diabetic macular edema. Retina 2018, 38, 1801–1808. [Google Scholar] [CrossRef]
- Wu, F.; Phone, A.; Lamy, R.; Chen, Y. Correlation of Aqueous, Vitreous, and Plasma Cytokine Levels in Patients with Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, T. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3549–3560. [Google Scholar] [CrossRef]
- Bonfiglio, V.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Pizzo, C.; Reibaldi, M.; Russo, A.; Fallico, M.; Ortisi, E.; Pignatelli, F.; et al. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. Int. J. Mol. Sci. 2020, 21, 9558. [Google Scholar] [CrossRef]
- Nalini, M.; Raghavulu, B.V.; Annapurna, A.; Chandl, V. Correlation of various serum biomarkers with the severity of diabetic retinopathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S451–S454. [Google Scholar] [CrossRef]
- Nakhleh, E.; Abu, Y.; Nafez, M.; Abu, T.; Ala MAbojaradeh, A.S.; Al-Akily, E.M.; Abdo, L.; Emoush, O. Relationship between Serum Vascular Endothelial Growth Factor Levels and Stages of Diabetic Retinopathy and Other Biomarkers. J. Ophthalmol. 2020, 2020, 8480193. [Google Scholar] [CrossRef]
- Ahuja, S.; Saxena, S.; Akduman, L.; Khanna, V. Serum vascular endothelial growth factor is a biomolecular biomarker of severity of diabetic retinopathy. Int. J. Retin. Vitr. 2019, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.J.; Zunaina, E.; Norfadzillah, A.J.; Lewin, A.S. Evaluation of vascular endothelial growth factor levels in tears and serum among diabetic patients. PLoS ONE 2019, 14, e0221481. [Google Scholar] [CrossRef] [PubMed]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Gverović Antunica, A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem. Medica 2020, 30, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Majidreza, S.; Alizadeh, M.; Saeid, A. The tear VEGF and IGFBP3 in healthy and diabetic retinopathy. Int. J. Diabetes Dev. Ctries. 2019, 40, 93–98. [Google Scholar] [CrossRef]
- Mei, C.; Pan, L.; Xu, W.; Li, Z. An ultrasensitive reusable aptasensor for noninvasive diabetic retinopathy diagnosis target on tear biomarker. Sens. Actuators B Chem. 2021, 345, 130398. [Google Scholar] [CrossRef]
- Wang, J.Y.; Kwon, J.S.; Hsu, S.M.; Chuang, H.S. Sensitive tear screening of diabetic retinopathy with dual biomarkers enabled using a rapid electrokinetic patterning platform. Lab A Chip 2020, 20, 356–362. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Okada, A.; Matsui, H.; Obata, R. Recent trends in anti-vascular endothelial growth factor intravitreal injections: A large claims database study in Japan. Jpn. J. Ophthalmol. 2023, 67, 109–118. [Google Scholar] [CrossRef]
- Grillo, M.A.; Colombatto, S. Arginine revisited: Minireview article. Amino Acids 2004, 26, 345–351. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Yonem, A.; Duran, C.; Unal, M.; Ipcioglu, O.M.; Ozcan, O. Plasma apelin and asymmetric dimethylarginine levels in type 2 diabetic patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2009, 84, 219–223. [Google Scholar] [CrossRef]
- Narayanan, P.S.; Rojas, M.; Suwanpradid, J.; Toque, H.A.; Caldwell, W.R.; Caldwell, R.B. Arginase in retinopathy. Prog. Retin. Eye Res. 2013, 36, 260–280. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Pereira, A.M.; Seica, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef]
- Forstermann, U.; Closs, E.I.; Pollock, J.S.; Nakane, M.; Schwarz, P.; Gath, I.; Kleinert, H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994, 23, 1121–1131. [Google Scholar] [CrossRef]
- Toutouzas, K.; Riga, M.; Stefanadi, E.; Stefanadis, C. Asymmetric dimethylarginine (ADMA) and other endogenous nitric oxide synthase (NOS) inhibitors as an important cause of vascular insulin resistance. Horm. Metab. Res. 2008, 40, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Bode-Boger, S.M.; Scalera, F.; Martens-Lobenhoffer, J. Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc. Med. 2005, 10, 65–71. [Google Scholar] [CrossRef]
- Sirman, Y.V.; Savytskyi, I.V. Study of endothelial dysfunction and asymmetric dimethylarginine levels. J. Educ. Health Sport 2019, 9, 395–412. [Google Scholar] [CrossRef]
- Leiper, J.M.; Vallance, P. The synthesis and metabolism of asymmetric dimethylarginine (ADMA). Eur. J. Clin. Pharmacol. 2006, 62, 33–38. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine metabolism revisited. J. Nutr. 2016, 146, 2579–2586. [Google Scholar] [CrossRef]
- Trocha, M.; Merwid-Lad, A.; Szuba, A.; Sozanski, T.; Magdalan, J.; Szelag, A. Asymmetric dimethylarginine synthesis and degradation under physiological and pathological conditions. Adv. Clin. Exp. Med. 2010, 19, 233–243. [Google Scholar]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Munzel, T. ADMA and oxidative stress. Atheroscler. Suppl. 2003, 4, 41–51. [Google Scholar] [CrossRef]
- Cardounel, A.J.; Cui, H.; Samouilov, A.; Johnson, W.; Kearns, P.; Tsai, A.-L.; Berka, V.; Zweier, J.L. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J. Biol. Chem. 2007, 282, 879–887. [Google Scholar] [CrossRef]
- Jian, Q.; Wu, Y.; Zhang, F. Metabolomics in diabetic retinopathy: From potential biomarkers to molecular basis of oxidative stress. Cells 2022, 11, 3005. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.S.; Rivera, E.; Warden, C.; Harlow, P.A.; Mitchell, S.L.; Calcutt, M.W.; Samuels, D.C.; Brantley, M.A. Plasma arginine and citrulline are elevated in diabetic retinopathy. Am. J. Ophthalmol. 2022, 235, 154–162. [Google Scholar] [CrossRef]
- Sumarriva, K.; Uppal, K.; Ma, C.; Herren, D.J.; Wang, Y.; Chocron, I.M.; Warden, C.; Mitchell, S.L.; Burgess, G.L.; Goodale, M.P.; et al. Arginine and carnitine metabolites are altered in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3119–3126. [Google Scholar] [CrossRef]
- Dag, U.; Caglayan, M.; Alakus, M.F.; Oncul, H. The relationship between reduced choroidal thickness due to high plasma asymmetrical dimethylarginine level and increased severity of diabetic retinopathy. Arq. Bras. Oftalmol. 2023, 86, 27–32. [Google Scholar] [CrossRef]
- Lamprou, S.; Koletsos, N.; Mintziori, G.; Anyfanti, P.; Trakatelli, C.; Kotsis, V.; Gkaliagkousi, E.; Triantafyllou, A. Microvascular and endothelial dysfunction in prediabetes. Life 2023, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Krasnicki, P.; Proniewska-Skretek, E.; Dmuchowska, D.A.; Dobrzycki, S.; Mariak, Z. Asymmetric dimethylarginine (ADMA) as a marker of blood flow disturbances in ocular circulation in patients with type 2 diabetes and coronary artery disease. Mag. Lek. Okulisty 2009, 3, 325–331. [Google Scholar]
- Tousoulis, D.; Kampoli, A.-M.; Stefanadis, C. Diabetes mellitus and vascular endothelial dysfunction: Current perspectives. Curr. Vasc. Pharmacol. 2012, 10, 19–32. [Google Scholar] [CrossRef]
- Stepien, E.; Szuscik, I.; Tokarz, A.; Enguita, F.J.; Solnica, B.; Zurakowski, A.; Malecki, M. The role of microparticles in pathomechanisms of diabetic retinopathy—Analysis of intercellular communication mechanisms in endothelial aging. Case control study in patients with metabolic syndrome, diabetes type 1 and type 2. J. Med. Sci. 2014, 83, 322–327. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Zhou, T.; Li, G.; Li, M.-Y.; Xiong, X.-M.; Wu, M.-T.; Jiang, J.-L. Asymmetric dimethylarginine aggravates blood-retinal barrier breakdown of diabetic retinopathy via inhibition of intercellular communication in retinal pericytes. Amino Acids 2019, 51, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Chen, W.; He, K.; Ma, H.; Ma, B.; Zhao, P.; Tian, L. Relationship between serum asymmetric dimethylarginine level and microvascular. Bio. Med. Res. Int. 2019, 2019, 2941861. [Google Scholar] [CrossRef]
- Alpay, A.; Ozcan, O.; Ugurbas, S.C.; Ugurbas, S.H. Investigated of vitreous and serum asymmetric dimethylarginine levels in diabetic. Res. Sq. 2019, 2019. [Google Scholar] [CrossRef]
- Sugai, M.; Ohta, A.; Ogata, Y.; Nakanishi, M.; Ueno, S.; Kawata, T.; Saito, N.; Tanaka, Y. Asymmetric dimethylarginine (ADMA) in the aqueous humor of diabetic patients. Endocr. J. 2007, 54, 303–309. [Google Scholar] [CrossRef]
- Abhary, S.; Kasmeridis, N.; Burdon, K.P.; Kuot, A.; Whiting, M.J.; Yew, W.P.; Petrovsky, N.; Craig, J.E. Diabetic retinopathy is associated with elevated serum asymmetric and symmetric dimethylarginines. Diabetes Care 2009, 32, 2084–2086. [Google Scholar] [CrossRef]
- Eliana, F.; Suwondo, P.; Makmun, L.H.; Harbuwono, D.S. ADMA as a marker of endothelial dysfunction in prediabetic women. Acta Medica Indones. 2011, 43, 92–98. [Google Scholar]
- Du, M.-R.; Yan, L.; Li, N.-S.; Wang, Y.-J.; Zhou, T.; Jiang, J.-L. Asymmetric dimethylarginine contributes to retinal neovascularization of diabetic retinopathy through EphrinB2 pathway. Vasc. Pharmacol. 2018, 108, 46–56. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, J.-M.; Jeon, H.J.; Oh, T.; Choi, H.J.; Kim, B.-J. Metabolomics profiles associated with diabetic retinopathy in type 2 diabetes patients. PLoS ONE 2020, 15, e241365. [Google Scholar] [CrossRef]
- Malecki, M.T.; Undas, A.; Cyganek, K.; Mirkiewicz-Sieradzka, B.; Wolkow, P.; Osmenda, G.; Walus-Miarka, M.; Guzik, T.J.; Sieradzki, J. Plasma asymmetric dimethylarginine (ADMA) is associated with retinopathy in type 2 diabetes. Diabetes Care 2007, 30, 2899–2901. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; Garcia-Arumi, J.; Gibson, J.; et al. The usefulness of serum biomarkers in the early stages of diabetic retinopathy: Results of the EUROCONDOR clinical trial. J. Clin. Med. 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, S.; Dilli, D.; Kabatas, E.U.; Akduman, H.; Sah Ipek, M.; Oguz, B.; Atlas, N.; Zenciroglu, A. The serum levels of asymmetric dimethylarginine, vascular endothelial growth factor, and insulin-like growth factor-1 in preterms with retinopathy of prematurity. Fetal Pediatr. Pathol. 2022, 41, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Wieczor, R.; Wieczor, A.M.; Kulwas, A.; Rosc, D. ADMA (asymmetric dimethylarginine) and angiogenic potential in patients with type 2 diabetes and prediabetes. Exp. Biol. Med. 2021, 246, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef]
- Jaroszynski, A.J.; Jaroszynska, A.; Wysokinski, A. Asymmetric dimethylarginine—The link between hart and kidney diseases. Chor. Serca I Naczyń 2012, 9, 225–229. [Google Scholar]
- Celik, M.; Cerrah, S.; Arabul, M.; Akalin, A. Relation of asymmetric dimethylarginine levels to macrovascular disease and inflammation markers in type 2 diabetic patients. J. Diabetes Res. 2014, 2014, 139215. [Google Scholar] [CrossRef]
- Kawata, T.; Daimon, M.; Hasegawa, R.; Teramoto, K.; Toyoda, T.; Sekine, T.; Yamamoto, K.; Uchida, D.; Himi, T.; Yoshida, K.; et al. Effect of angiotensin-converting enzyme inhibitor on serum asymmetric dimethylarginine and coronary circulation in patients with type 2 diabetes mellitus. Int. J. Cardiol. 2009, 132, 286–288. [Google Scholar] [CrossRef]
- Guo, X.; Xing, Y.; Jin, W. Role of ADMA in the pathogenesis of microvascular complications in type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1183586. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kaab, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Karimi, G. The role of SIRT1 in diabetic retinopathy. Biomed. Pharmacother. 2018, 97, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhu, G.; Cai, Z.; Wang, Y.; Lian, R.; Tang, X.; Ma, C.; Fu, S. miRNA, lncRNA and circRNA: Targeted Molecules Full of Therapeutic Prospects in the Development of Diabetic Retinopathy. Front. Endocrinol. 2021, 12, 771552. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Han, J.; Wang, L.; Liu, J.; Dong, Y.; Zhu, K.; Shi, L. MicroRNA-34a promotes apoptosis of retinal vascular endothelial cells by targeting SIRT1 in rats with diabetic retinopathy. Cell Cycle 2020, 19, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Zhang, H.; Han, Y.; Kuang, R. Expression and mechanism of microRNA 195 in diabetic retinopathy. Endocr. J. 2022, 69, 529–537. [Google Scholar] [CrossRef]
- Yin, C.; Lin, X.; Sun, Y.; Ji, X. Dysregulation of miR210 is involved in the development of diabetic retinopathy and serves a regulatory role in retinal vascular endothelial cell proliferation. Eur. J. Med. Res. 2020, 25, 20. [Google Scholar] [CrossRef]
- Pan, Q.; Gao, Z.; Zhu, C.; Peng, Z.; Song, M.; Li, L. Overexpression of histone deacetylase SIRT1 exerts an antiangiogenic role in diabetic retinopathy via miR-20a elevation and YAP/HIF1α/VEGFA depletion. Am. J. Physiol. Endocrinol. Metab. 2020, 319, 932–943. [Google Scholar] [CrossRef]
- Qin, B.; Liu, J.; Liu, S.; Li, B.; Ren, J. MiR-20b targets AKT3 and modulates vascular endothelial growth factor-mediated changes in diabetic retinopathy. Acta Biochim. Biophys. Sin. 2016, 48, 732–740. [Google Scholar] [CrossRef]
- Maisto, R.; Trotta, M.C.; Petrillo, F.; Izzo, S.; Cuomo, G.; Alfano, R.; Hermenean, A.; Barcia, J.M.; Galdiero, M.; Platania, C.B.M.; et al. Resolvin D1 Modulates the Intracellular VEGF-Related miRNAs of Retinal Photoreceptors Challenged With High Glucose. Front. Pharmacol. 2020, 11, 235. [Google Scholar] [CrossRef]
- Duan, P.; Chen, S.; Zeng, Y.; Xu, H.; Liu, Y. Osteopontin Upregulates Col IV Expression by Repressing miR-29a in Human Retinal Capillary Endothelial Cells. Mol. Ther. Nucleic Acids 2020, 20, 242–251. [Google Scholar] [CrossRef]
- Someya, H.; Ito, M.; Nishio, Y.; Sato, T.; Harimoto, K.; Takeuchi, M. Osteopontin-induced vascular hyperpermeability through tight junction disruption in diabetic retina. Exp. Eye Res. 2022, 220, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, Y.; Wang, K.; Chu, T. MicroRNA-148a-3p alleviates high glucose-induced diabetic retinopathy by targeting TGFB2 and FGF2. Acta Diabetol. 2020, 57, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Cao, N.J.; Li, X.; Qian, W.; Chen, X.L. Serum microRNA-211 as a biomarker for diabetic retinopathy via modulating Sirtuin 1. Biochem. Biophys. Res. Commun. 2018, 505, 1236–1243. [Google Scholar] [CrossRef]
- Miao, C.; Chang, J.; Zhang, G.; Fang, Y. MicroRNAs in type 1 diabetes: New research progress and potential directions. Biochem. Cell Biol. 2018, 96, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, K.; Margioula-Siarkou, G.; Giza, S.; Kotanidou, E.P.; Tsinopoulou, V.R.; Christoforidis, A.; Galli-Tsinopoulou, A. Micro-RNA Implications in Type-1 Diabetes Mellitus: A Review of Literature. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef]
- Li, E.H.; Huang, Q.Z.; Li, G.C.; Xiang, Z.Y.; Zhang, X. Effects of miRNA-200b on the development of diabetic retinopathy by targeting VEGFA gene. Biosci. Rep. 2017, 37, 2016572. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, K.P.; Wang, Y.X.; Liu, Z.C.; Tian, L.; Yang, X.Z.; Ding, J.Y.; Wu, W.T.; Yang, W.H.; Li, Y.L.; et al. RNA sequencing identified specific circulating miRNA biomarkers for early detection of diabetes retinopathy. Am. J. Physiol. Endocrinol. Metab. 2018, 315, 374–385. [Google Scholar] [CrossRef]
- Santovito, D.; Toto, L.; De Nardis, V.; Ces, D. Plasma microRNA signature associated with retinopathy in patients with type 2 diabetes. Sci Rep. 2021, 11, 4136. [Google Scholar] [CrossRef]
- McArthur, K.; Feng, B.; Wu, Y.; Chen, S.; Chakrabarti, S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 2011, 60, 1314–1323. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, W.; Wang, N.; Wang, Z.; Li, B.; Zeng, J.; Yoshida, S.; Ding, C.; Zhou, Y. Altered Expressions of Transfer RNA-Derived Small RNAs and microRNAs in the Vitreous Humor of Proliferative Diabetic Retinopathy. Front. Endocrinol. 2022, 13, 913370. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Kaczmarek, R. Exosomal miRNA Profiling in Vitreous Humor in Proliferative Diabetic Retinopathy. Cells 2022, 12, 123. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, P.; Liu, Z.; Dai, F.; Pan, M.; An, G.; Han, J.; Du, L.; Jin, X. The Aflibercept-Induced MicroRNA Profile in the Vitreous of Proliferative Diabetic Retinopathy Patients Detected by Next-Generation Sequencing. Front. Pharmacol. 2021, 12, 781276. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; El-Hefnawy, S.M.; Kasemy, Z.A.; Alhagaa, A.A.; Nooh, M.Z.; Arafat, E.S. Mi-RNA-93 and Mi-RNA-152 in the Diagnosis of Type 2 Diabetes and Diabetic Retinopathy. Br. J. Biomed. Sci. 2022, 79, 10192. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef]
- Ko, G.Y.; Yu, F.; Bayless, K.J.; Ko, M.L. MicroRNA-150 (miR-150) and Diabetic Retinopathy: Is miR-150 Only a Biomarker or Does It Contribute to Disease Progression? Int. J. Mol. Sci. 2022, 23, 99. [Google Scholar] [CrossRef]
- Zhou, H.; Peng, C.; Huang, D.S.; Liu, L.; Guan, P. microRNA Expression Profiling Based on Microarray Approach in Human Diabetic Retinopathy: A Systematic Review and Meta-Analysis. DNA Cell Biol. 2020, 39, 441–450. [Google Scholar] [CrossRef]
- Ma, L.; Wen, Y.; Li, Z.; Wu, N.; Wang, Q. Circulating MicroRNAs as Potential Diagnostic Biomarkers for Diabetic Retinopathy: A Meta-Analysis. Front. Endocrinol. 2022, 13, 929924. [Google Scholar] [CrossRef]
- Jenkins, H.N.; Rivera-Gonzalez, O.; Gibert, Y.; Speed, J.S. Endothelin-1 in the pathophysiology of obesity and insulin resistance. Obes. Rev. 2020, 21, e13086. [Google Scholar] [CrossRef]
- Ergul, A. Endothelin-1 and diabetic complications: Focus on the vasculature. Pharmacol. Res. 2011, 63, 477–482. [Google Scholar] [CrossRef]
- Stow, L.R.; Jacobs, M.E.; Wingo, C.S.; Cain, B.D. Endothelin-1 gene regulation. FASEB J. 2010, 25, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K. The causal relationship between endothelin-1 and hypertension: Focusing on endothelial dysfunction, arterial stiffness, vascular remodeling, and Blood Pressure Regulation. Life 2021, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Abman, S.H. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annu. Rev. Med. 2009, 60, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.S.; Leung, J.W.; Lam, A.K.; Acy, L. Selective over-expression of endothelin-1 in endothelial cells exacerbates inner retinal edema and neuronal death in ischemic retina. PLoS ONE 2011, 6, e26184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Rosa, R.H.; Kuo, L.; Hein, T.W. Hyperglycemia augments endothelin-1–induced constriction of human retinal venules. Transl. Vis. Sci. Technol. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Lajko, M.; Fawzi, A.A. Endothelin-1 is associated with fibrosis in proliferative diabetic retinopathy membranes. PLoS ONE 2018, 13, e0191285. [Google Scholar] [CrossRef]
- Kang, H.M.; Hasanuzzaman, M.; Kim, S.W.; Koh, H.J.; Lee, S.C. Elevated aqueous endothelin-1 concentrations in advanced diabetic retinopathy. PLoS ONE 2022, 17, e0268353. [Google Scholar] [CrossRef]
- Khuu, L.-A.; Tayyari, F.; Sivak, J.M.; Singer, S. Aqueous humor endothelin-1 and total retinal blood flow in patients with non-proliferative diabetic retinopathy. Eye 2017, 31, 1443–1450. [Google Scholar] [CrossRef]
- Ottosson-Seeberger, A.; Lundberg, J.M.; Alvestrand, A.; Ahlborg, G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol. Scand. 1997, 161, 211–220. [Google Scholar] [CrossRef]
- Anfossi, G.; Russo, I.; Doronzo, G.; Trovati, M. Relevance of the vascular effects of insulin in the rationale of its therapeutical use. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 228–249. [Google Scholar] [CrossRef]
- Wang, Z.; Yadav, A.S.; Leskova, W.; Harris, N.R. Attenuation of streptozotocin-induced microvascular changes in the mouse retina with the endothelin receptor A antagonist atrasentan. Exp. Eye Res. 2010, 91, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.C.; Rollins, S.D.; Ye, M.; Batlle, D.; Fawzi, A.A. Endothelin receptor-a antagonist attenuates retinal vascular and neuroretinal pathology in diabetic mice. Investig. Opthalmology Vis. Sci. 2014, 55, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Alrashdi, S.F.; Deliyanti, D.; Wilkinson-Berka, J.L. Intravitreal administration of endothelin type A receptor or endothelin type B receptor antagonists attenuates hypertensive and diabetic retinopathy in rats. Exp. Eye Res. 2018, 176, 1–9. [Google Scholar] [CrossRef]
- Bogdanov, P.; Simo-Servat, O.; Sampedro, J.; Garcia, M. Topical administration of Bosentan prevents retinal neurodegeneration in experimental diabetes. Int. J. Mol. Sci. 2018, 19, 3578. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Lu, C.H.; Wu, C.H.; Li, K.J. The Development of Maillard Reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) Signaling Inhibitors as Novel Therapeutic Strategies for Patients with AGE-Related Diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrinology 2020, 161, bqz006. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced Glycation End Products in Health and Disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Mao, L.; Yin, R.; Yang, L.; Zhao, D. Role of advanced glycation end products on vascular smooth muscle cells under diabetic atherosclerosis. Front. Endocrinol. 2022, 13, 983723. [Google Scholar] [CrossRef]
- Vlassara, H.; Bucala, R.; Striker, L. Pathogenic effects of advanced glycosylation: Biochemical, biologic, and clinical implications for diabetes and aging. Lab. Investig. 1988, 58, 317–326. [Google Scholar]
- Zhang, Q.; Wang, Y.; Fu, L. Dietary advanced glycation end-products: Perspectives linking food processing with health implications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2559–2587. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced Glycation End Products (AGEs) May Be a Striking Link Between Modern Diet and Health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.; Machowiak, A.; Rychter, A.M.; Rata, A.E. Accumulation of Advanced Glycation End-Products in the Body and Dietary Habits. Nutrients 2022, 14, 3982. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Oshitari, T. Advanced Glycation End-Products and Diabetic Neuropathy of the Retina. Int. J. Mol. Sci. 2023, 24, 2927. [Google Scholar] [CrossRef]
- Stitt, A.W. AGEs and diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4867–4874. [Google Scholar] [CrossRef]
- Mokini, Z.; Marcovecchio, M.L.; Chiarelli, F. Molecular pathology of oxidative stress in diabetic angiopathy: Role of mitochondrial and cellular pathways. Diabetes Res. Clin. Pract. 2010, 87, 313–321. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed. Res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.R.; Choi, J.A.; Koh, J.Y.; Yoon, Y.H. Ursodeoxycholic Acid Attenuates Endoplasmic Reticulum Stress-Related Retinal Pericyte Loss in Streptozotocin-Induced Diabetic Mice. J. Diabetes. Res. 2017, 2017, 1763292. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.; Heinemann, L. Skin Autofluorescence—A Non-invasive Measurement for Assessing Cardiovascular Risk and Risk of Diabetes. Eur. Endocrinol. 2014, 10, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diab. Rep. 2014, 14, 453. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.; Zeebregts, C.; Tio, R.; Smit, O. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc. Diabetol. 2008, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Jagger, J.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, E.G.; Lutgers, H.L.; Kleefstra, N.; Gano, N.O. Skin autofluorescence: A tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008, 31, 517–521. [Google Scholar] [CrossRef]
- Osawa, S.; Katakami, N.; Sato, I.; Sakamoto, F. Skin autofluorescence is associated with vascular complications in patients with type 2 diabetes. J. Diabetes Complicat. 2018, 32, 839–844. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |


| Receptor | VEGFR1 | VEGFR2 | VEGFR3 |
| VEGF variant | VEGF-A, -B, and PGF | VEGF-A, -C, and -D | VEGF-C and -D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszkiewicz, M.; Walulik, A.; Florek, K.; Górecki, I.; Sławatyniec, O.; Gomułka, K. Advances and Perspectives in Relation to the Molecular Basis of Diabetic Retinopathy—A Review. Biomedicines 2023, 11, 2951. https://doi.org/10.3390/biomedicines11112951
Błaszkiewicz M, Walulik A, Florek K, Górecki I, Sławatyniec O, Gomułka K. Advances and Perspectives in Relation to the Molecular Basis of Diabetic Retinopathy—A Review. Biomedicines. 2023; 11(11):2951. https://doi.org/10.3390/biomedicines11112951
Chicago/Turabian StyleBłaszkiewicz, Michał, Agata Walulik, Kamila Florek, Ignacy Górecki, Olga Sławatyniec, and Krzysztof Gomułka. 2023. "Advances and Perspectives in Relation to the Molecular Basis of Diabetic Retinopathy—A Review" Biomedicines 11, no. 11: 2951. https://doi.org/10.3390/biomedicines11112951





