Assessing the Layer-by-Layer Assembly of Cellulose Nanofibrils and Polyelectrolytes in Pancreatic Tumor Spheroid Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
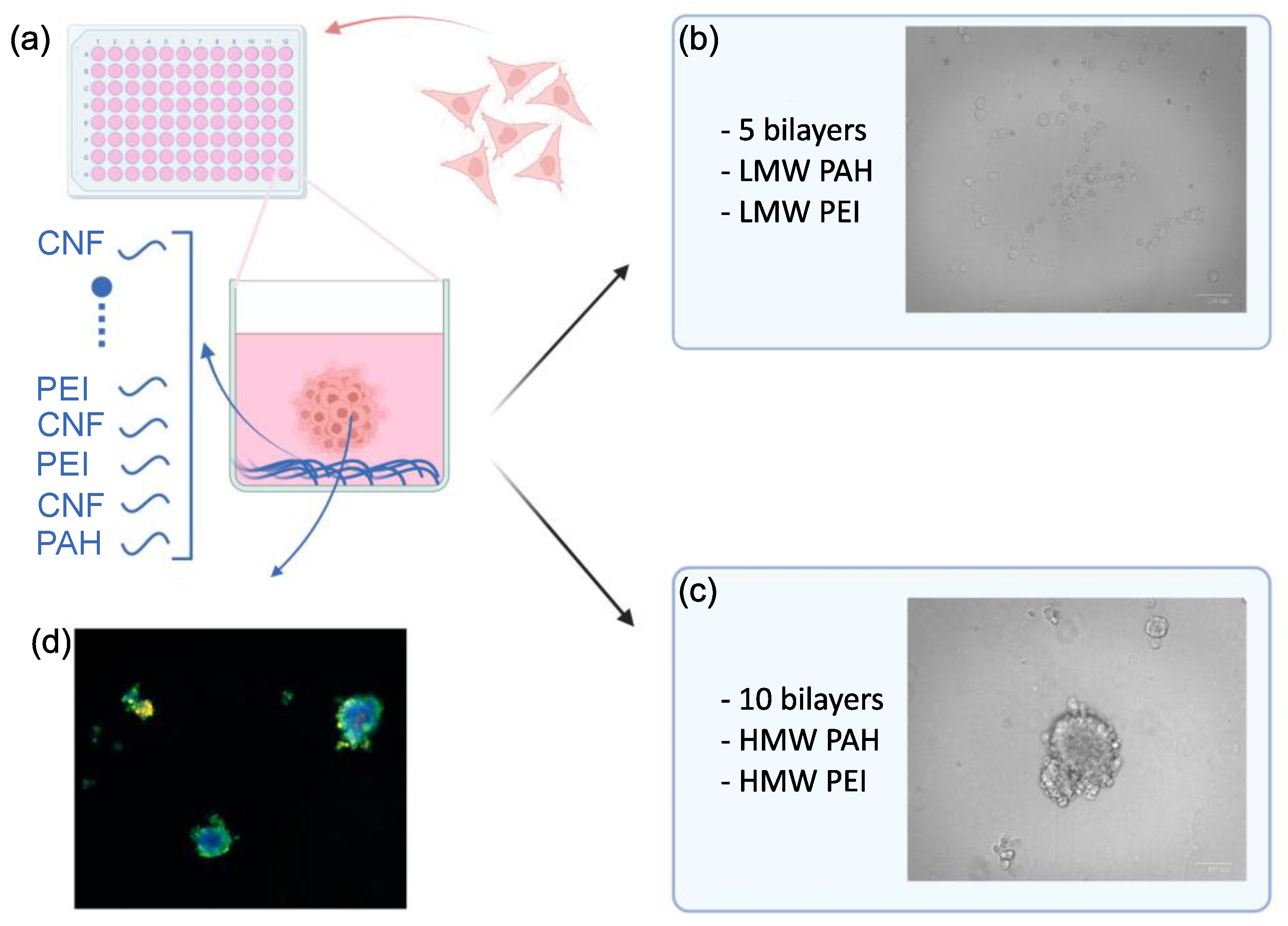
2.2. Layer-by-Layer Coating in 96-Well Plates
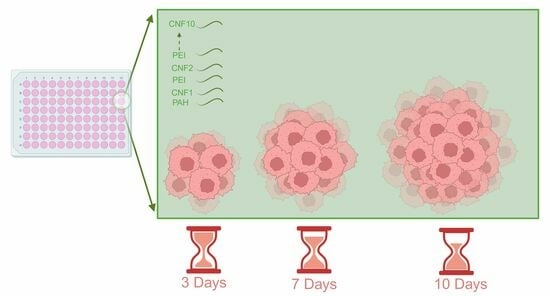
2.3. Spheroid Formation
2.4. Live–Dead Assay
2.5. Statistical Analysis
3. Results and Discussion
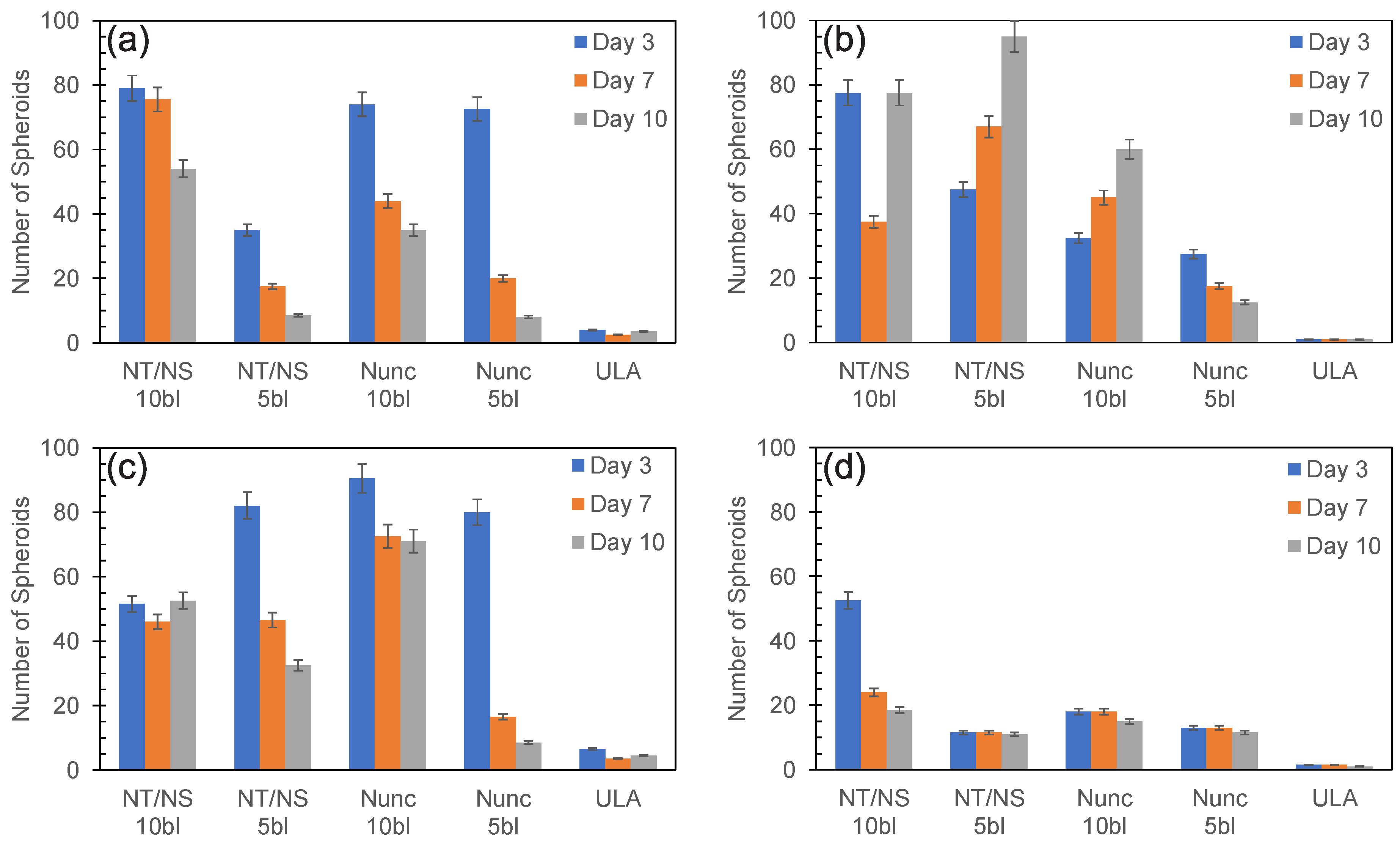
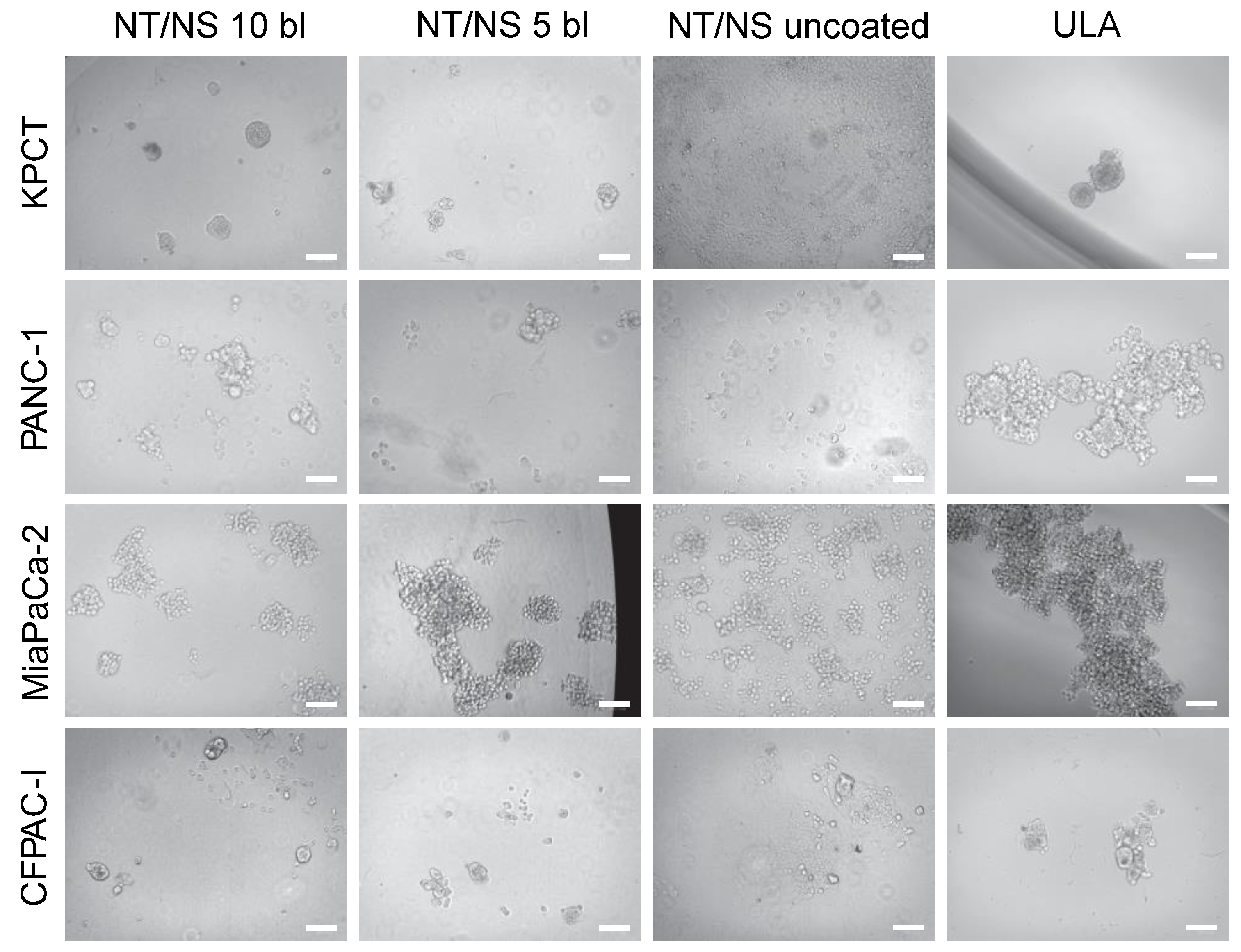
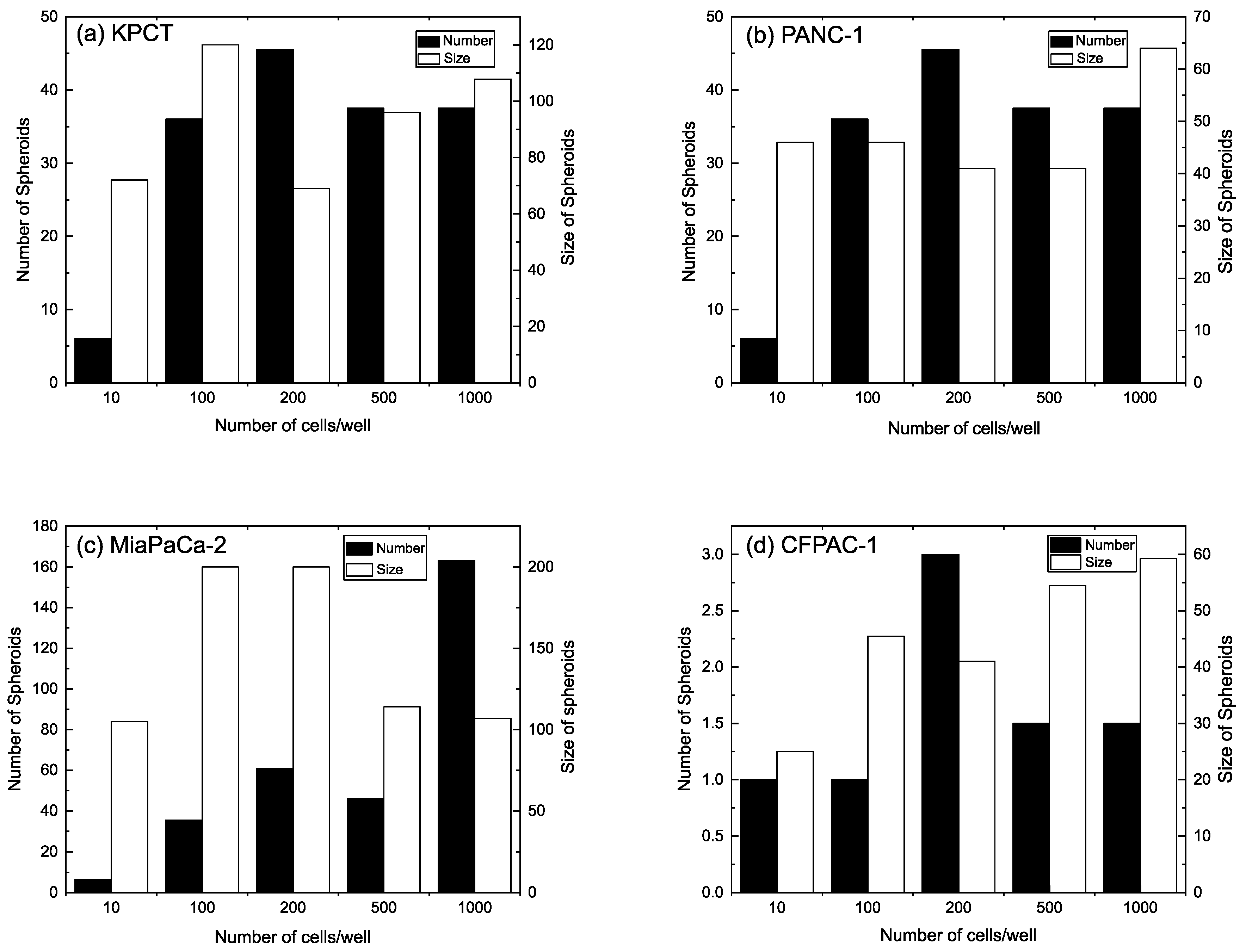
3.1. PDAC Spheroid Formation on Different Plates
3.2. Spheroid Morphology and Size
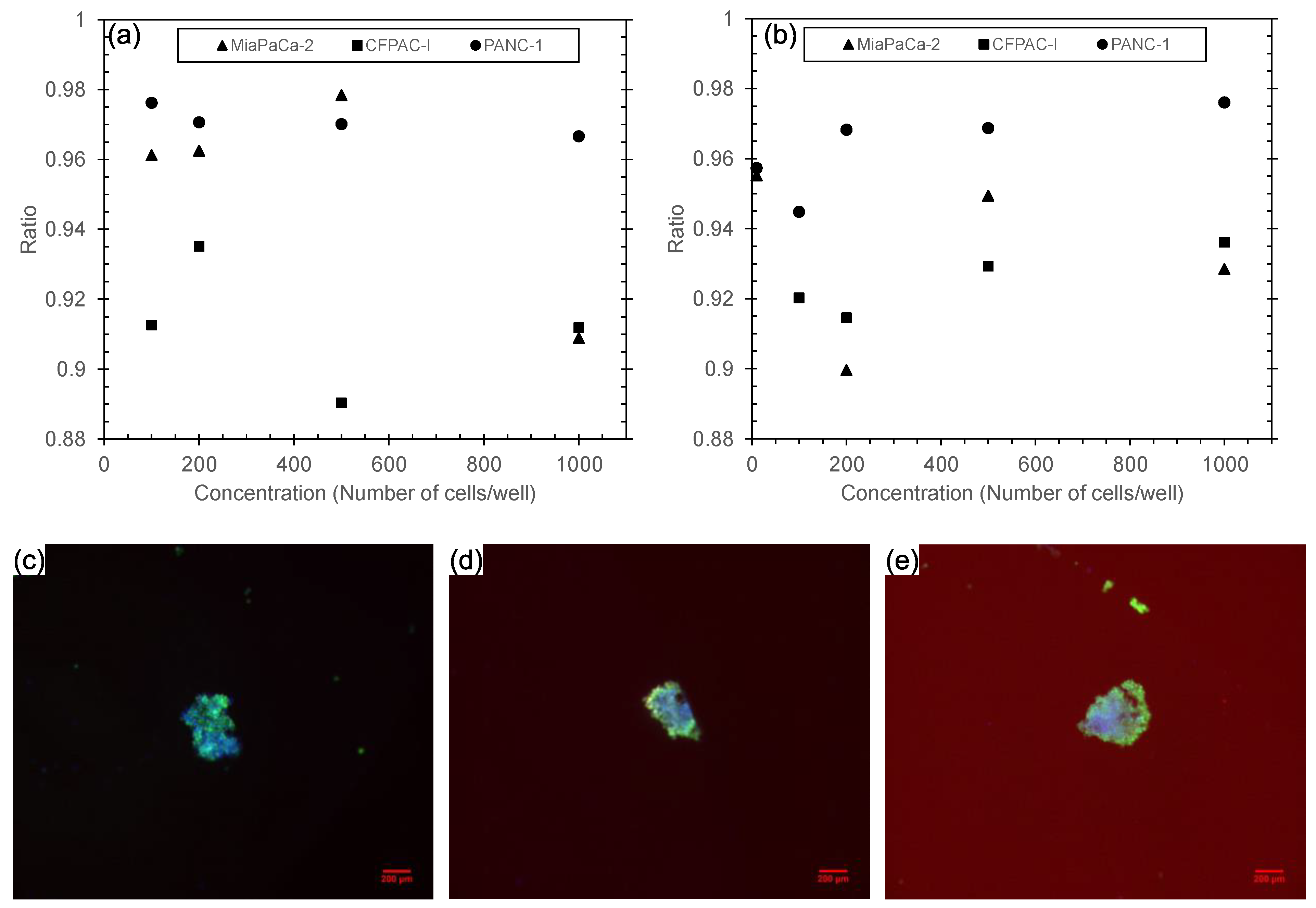
3.3. Analysis of Spheroids’ Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener 2018, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Gündel, B.; Liu, X.; Löhr, M.; Heuchel, R. Pancreatic Ductal Adenocarcinoma: Preclinical in vitro and ex vivo Models. Front. Cell Dev. Biol. 2021, 9, 741162. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Moshksayan, K.; Kashaninejad, N.; Ebrahimi Warkiani, M.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Said Saidi, M.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Di Caprio, N.; Burdick, J.A. Engineered biomaterials to guide spheroid formation, function, and fabrication into 3D tissue constructs. Acta Biomater. 2023, 165, 4–18. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 1620. [Google Scholar] [CrossRef]
- Türker, E.; Demirçak, N.; Arslan-Yildiz, A. Scaffold-free three-dimensional cell culturing using magnetic levitation. Biomater. Sci. 2018, 6, 1745–1753. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J. Mixed hydrogel bead-based tumor spheroid formation and anticancer drug testing. Analyst 2014, 139, 2449–2458. [Google Scholar] [CrossRef]
- Kamatar, A.; Gunay, G.; Acar, H. Natural and synthetic biomaterials for engineering multicellular tumor spheroids. Polymers 2020, 12, 2506. [Google Scholar] [CrossRef]
- Metzger, W.; Sossong, D.; Bächle, A.; Pütz, N.; Wennemuth, G.; Pohlemann, T.; Oberringer, M. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 2011, 13, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.P.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Tumor spheroid assembly on hyaluronic acid-based structures: A review. Carbohydr. Polym. 2016, 150, 139–148. [Google Scholar] [CrossRef]
- Abdolahinia, E.D.; Jafari, B.; Parvizpour, S.; Barar, J.; Nadri, S.; Omidi, Y. Role of cellulose family in fibril organization of collagen for forming 3D cancer spheroids: In vitro and in silico approach. BioImpacts 2021, 11, 111–117. [Google Scholar] [CrossRef]
- Dashtarzheneh, A.K.; Afrashtehpour, A.; Ramesh, B.S.; Loizidou, M. Harvestable tumor spheroids initiated in a gelatin-carboxymethyl cellulose hydrogel for cancer targeting and imaging with fluorescent gold nanoclusters. Vitr. Model. 2022, 1, 437–446. [Google Scholar] [CrossRef]
- Aljadi, Z.; Abbasi Aval, N.; Kumar, T.; Qin, T.; Ramachandraiah, H.; Pettersson, T.; Russom, A. Layer-by-Layer Cellulose Nanofibrils: A New Coating Strategy for Development and Characterization of Tumor Spheroids as a Model for In Vitro Anticancer Drug Screening. Macromol. Biosci. 2022, 22, e2200137. [Google Scholar] [CrossRef]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic ductal adenocarcinoma: Current and evolving therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Tanaka, H.; Ödberg, L.; Wågberg, L.; Lindström, T. Exchange of cationic polyacrylamides adsorbed on monodisperse polystyrene latex and cellulose fibers: Effect of molecular weight. J. Colloid Interface Sci. 1990, 134, 229–234. [Google Scholar] [CrossRef]
- Mirab, F.; Kang, Y.J.; Majd, S. Preparation and characterization of size-controlled glioma spheroids using agarose hydrogel microwells. PLoS ONE 2019, 14, e0211078. [Google Scholar] [CrossRef]
- Deer, E.L.; González-Hernández, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and genotype of pancreatic cancer cell lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef]
- Wang, C.; Huang, B.; Sun, L.; Wang, X.; Zhou, B.; Tang, H.; Geng, W. MK8722, an AMPK activator, inhibiting carcinoma proliferation, invasion and migration in human pancreatic cancer cells. Biomed. Pharmacother. 2021, 144, 112325. [Google Scholar] [CrossRef]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Ruppen, J.; Wildhaber, F.D.; Strub, C.; Hall, S.R.R.; Schmid, R.A.; Geiser, T.; Guenat, O.T. Towards personalized medicine: Chemosensitivity assays of patient lung cancer cell spheroids in a perfused microfluidic platform. Lab Chip 2015, 15, 3076–3085. [Google Scholar] [CrossRef] [PubMed]
- Norberg, K.J.; Liu, X.; Fernández Moro, C.; Strell, C.; Nania, S.; Blümel, M.; Balboni, A.; Bozóky, B.; Heuchel, R.L.; Löhr, J.M. A novel pancreatic tumour and stellate cell 3D co-culture spheroid model. BMC Cancer 2020, 20, 475. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi Aval, N.; Lahchaichi, E.; Tudoran, O.; Fayazbakhsh, F.; Heuchel, R.; Löhr, M.; Pettersson, T.; Russom, A. Assessing the Layer-by-Layer Assembly of Cellulose Nanofibrils and Polyelectrolytes in Pancreatic Tumor Spheroid Formation. Biomedicines 2023, 11, 3061. https://doi.org/10.3390/biomedicines11113061
Abbasi Aval N, Lahchaichi E, Tudoran O, Fayazbakhsh F, Heuchel R, Löhr M, Pettersson T, Russom A. Assessing the Layer-by-Layer Assembly of Cellulose Nanofibrils and Polyelectrolytes in Pancreatic Tumor Spheroid Formation. Biomedicines. 2023; 11(11):3061. https://doi.org/10.3390/biomedicines11113061
Chicago/Turabian StyleAbbasi Aval, Negar, Ekeram Lahchaichi, Oana Tudoran, Farzaneh Fayazbakhsh, Rainer Heuchel, Matthias Löhr, Torbjörn Pettersson, and Aman Russom. 2023. "Assessing the Layer-by-Layer Assembly of Cellulose Nanofibrils and Polyelectrolytes in Pancreatic Tumor Spheroid Formation" Biomedicines 11, no. 11: 3061. https://doi.org/10.3390/biomedicines11113061