Overexpression of MicroRNA-138 Affects the Proliferation and Invasion of Urothelial Carcinoma Cells by Suppressing SOX9 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Tissue Samples and Immunohistochemistry
2.3. miRNA Precursor and siRNA Transfection of UC Cell Lines
2.4. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analyses of miRNA and mRNA
2.5. Cell Proliferation and Viability Assays
2.6. Invasion Assay
2.7. Statistical Analyses
3. Results
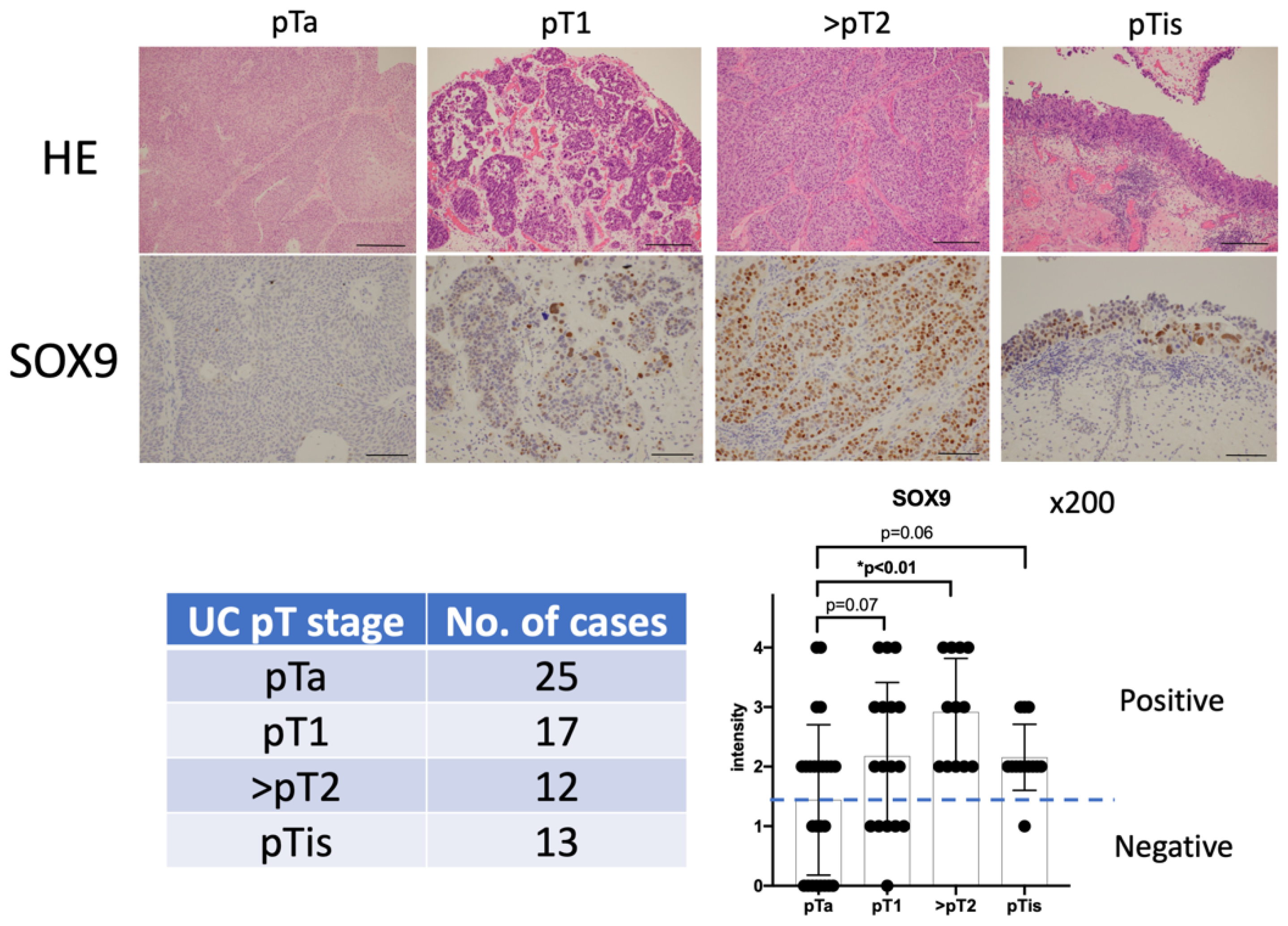
3.1. Evaluation of SOX9 Expression Levels in Non-Invasive and Invasive Urothelial Carcinoma via Immunohistochemical Staining
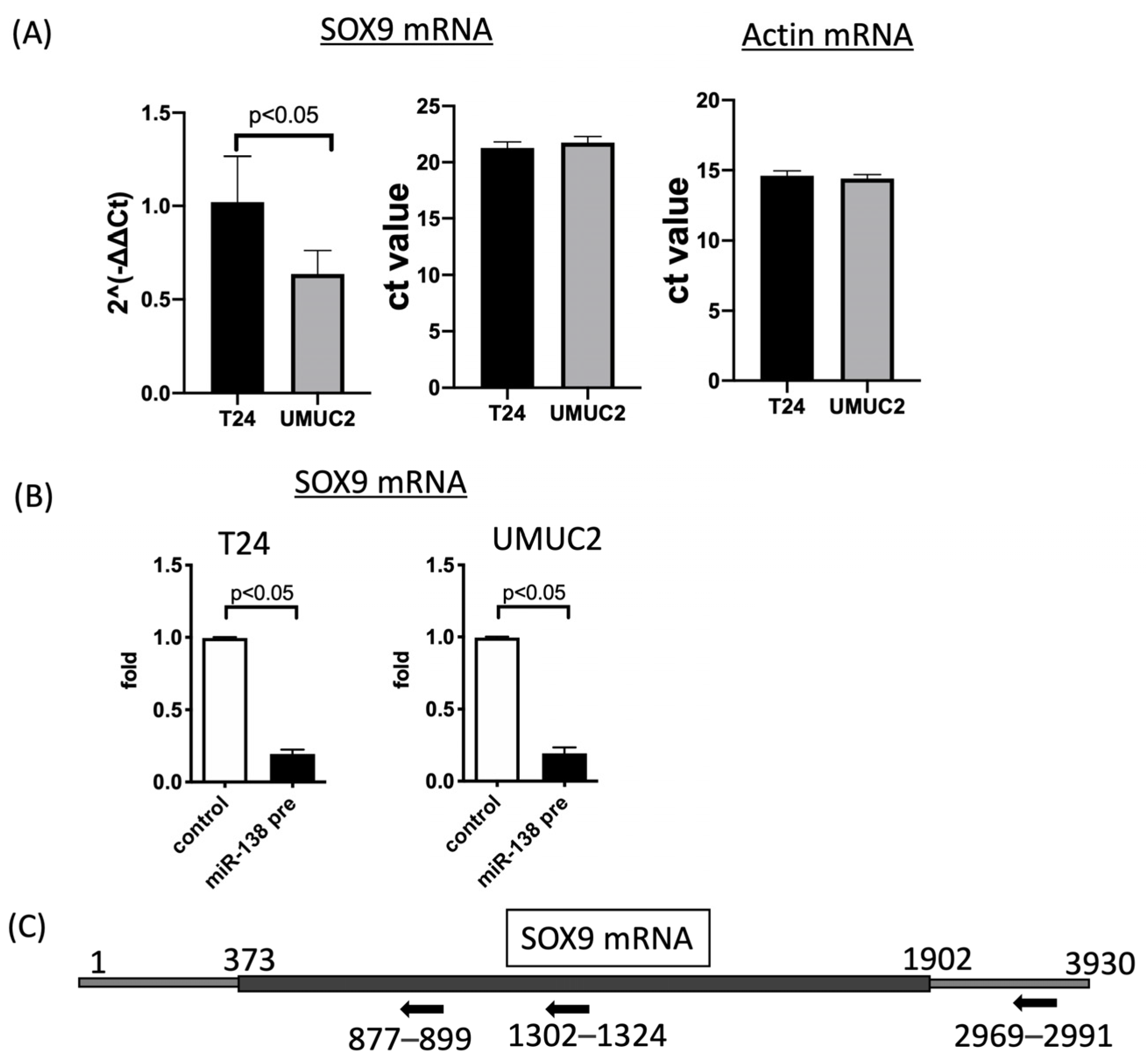
3.2. SOX9 Expression Is Regulated by MiR-138 in Urothelial Carcinoma Cells
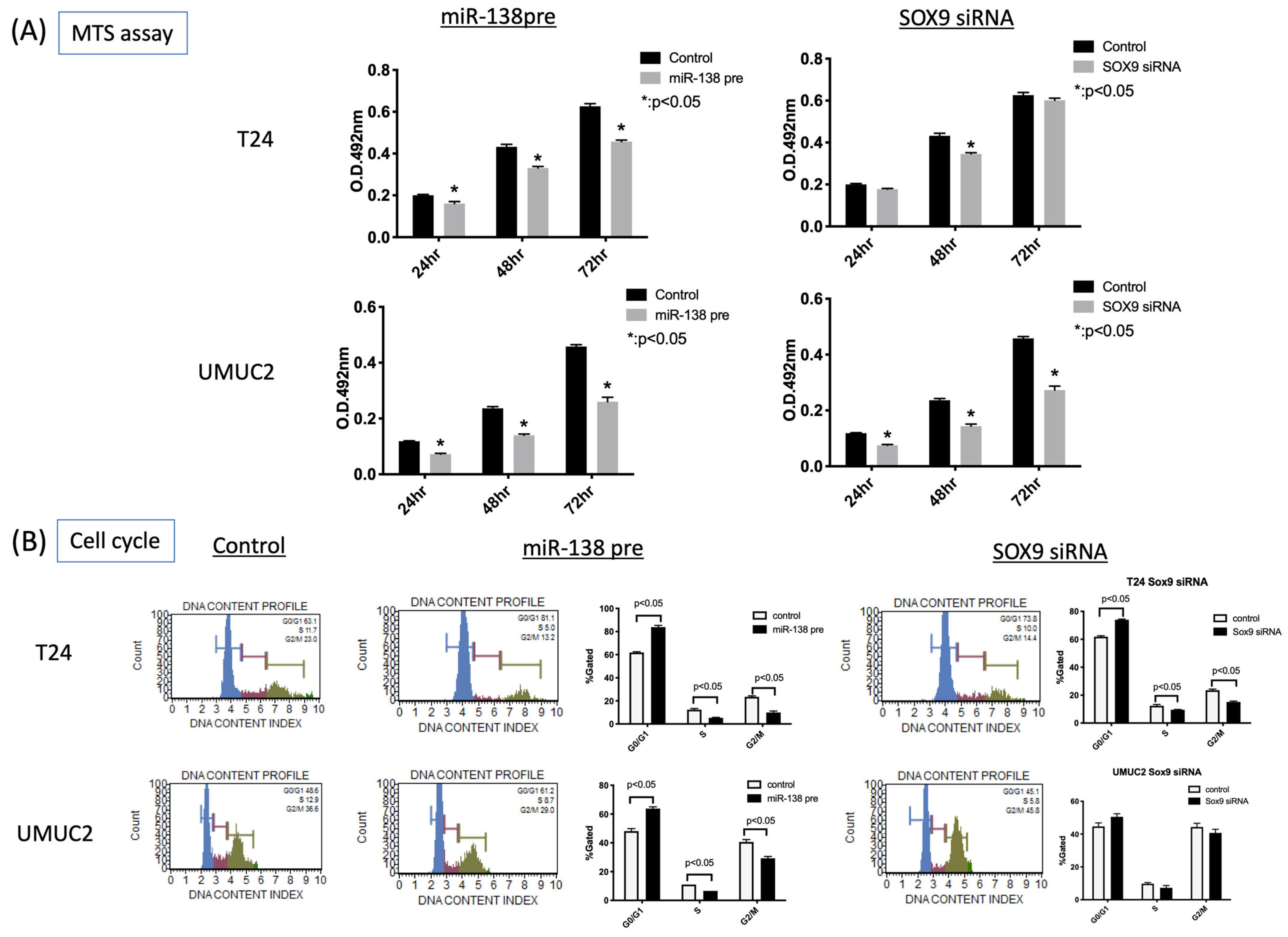
3.3. MiR-138 and SOX9 Are Involved in the Proliferation of T24, UMUC2, and UMUC3 Cell Lines
3.4. MiR-138 and SOX9 Affect the Invasiveness of T24 and UMUC2 Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Shimada, K.; Nakai, T.; Ohbayashi, C. MicroRNAs in smoking-related carcinogenesis: Biomarkers, functions, and therapy. J. Clin. Med. 2018, 7, 98. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Smith, A.B.; Meyer, A.M.; Kuo, T.M.; Tyree, S.; Kim, W.Y.; Milowsky, M.I.; Pruthi, R.S.; Millikan, R.C. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer 2014, 120, 86–95. [Google Scholar] [CrossRef]
- Parkin, D.M. The global burden of urinary bladder cancer. Scand. J. Urol. Nephrol. Suppl. 2008, 42 (Suppl. S218), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005, 66 (Suppl. S1), 4–34. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Martin, M.; Domingo-Domenech, J.; Karni-Schmidt, O.; Matos, T.; Cordon-Cardo, C. Molecular pathways of urothelial development and bladder tumorigenesis. Urol. Oncol. 2010, 28, 401–408. [Google Scholar] [CrossRef]
- Cordon-Cardo, C. Molecular alterations associated with bladder cancer initiation and progression. Scand. J. Urol. Nephrol. Suppl. 2008, 42 (Suppl. S218), 154–165. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory mechanism of microRNA expression in cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Garzon, R.; Fabbri, M.; Cimmino, A.; Calin, G.A.; Croce, C.M. MicroRNA expression and function in cancer. Trends Mol. Med. 2006, 12, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Garg, M. Urothelial cancer stem cells and epithelial plasticity: Current concepts and therapeutic implications in bladder cancer. Cancer Metastasis Rev. 2015, 34, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Hatina, J.; Parmar, H.S.; Kripnerova, M.; Hepburn, A.; Heer, R. Urothelial carcinoma stem cells: Current concepts, controversies, and methods. Methods Mol. Biol. 2018, 1655, 121–136. [Google Scholar] [PubMed]
- Fujii, T.; Shimada, K.; Tatsumi, Y.; Hatakeyama, K.; Obayashi, C.; Fujimoto, K.; Konishi, N. MicroRNA-145 promotes differentiation in human urothelial carcinoma through down-regulation of syndecan-1. BMC Cancer 2015, 15, 818. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Dumitriu, B.; Penzo-Mendez, A.; Han, Y.; Pallavi, B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int. J. Biochem. Cell Biol. 2007, 39, 2195–2214. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.K.; Francis, J.C.; Swain, A. The role of Sox9 in prostate development. Differentiation 2008, 76, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Leav, I.; Ibaragi, S.; Wegner, M.; Hu, G.F.; Lu, M.L.; Balk, S.P.; Yuan, X. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 2008, 68, 1625–1630. [Google Scholar] [CrossRef]
- Murakami, S.; Kan, M.; McKeehan, W.L.; de Crombrugghe, B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 1113–1118. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Yao, X.; Jin, J.; Scott, A.; Liu, B.; Wang, S.; Huo, L.; Wang, Y.; Wang, R.; et al. Epithelial SOX9 drives progression and metastases of gastric adenocarcinoma by promoting immunosuppressive tumour microenvironment. Gut 2023, 72, 624–637. [Google Scholar] [CrossRef]
- Huang, J.Q.; Wei, F.K.; Xu, X.L.; Ye, S.X.; Song, J.W.; Ding, P.K.; Zhu, J.; Li, H.F.; Luo, X.P.; Gong, H.; et al. SOX9 drives the epithelial-mesenchymal transition in non-small-cell lung cancer through the Wnt/beta-catenin pathway. J. Transl. Med. 2019, 17, 143. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Piao, J.; Ou, J.; Zhu, X. Circ_0109046 promotes the malignancy of endometrial carcinoma cells through the microRNA-105/SOX9/Wnt/beta-catenin axis. IUBMB Life 2021, 73, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, X.; Xiong, L.; Kong, X.; Xu, Y.; Liu, C.; Zou, L.; Li, Z.; Zhao, J.; Lin, N. MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 2012, 586, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, J.; Jin, X. MicroRNA-138 suppresses cell proliferation and invasion of renal cell carcinoma by directly targeting SOX9. Oncol. Lett. 2017, 14, 7583–7588. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Chen, Y.; Xie, B.F.; Li, Y.L.; Wei, Y.T.; Wang, F. MicroRNA-215-3p suppresses the growth and metastasis of cervical cancer cell via targeting SOX9. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5628–5639. [Google Scholar] [PubMed]
- Ling, S.; Chang, X.; Schultz, L.; Lee, T.K.; Chaux, A.; Marchionni, L.; Netto, G.J.; Sidransky, D.; Berman, D.M. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res. 2011, 71, 3812–3821. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.J.; Tan, Y.X.; Ren, H.; Qi, Z.T. MiR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis 2012, 33, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Pan, Z.G.; Shu, L.; Li, Q.J. Podocalyxin-like, targeted by miR-138, promotes colorectal cancer cell proliferation, migration, invasion and EMT. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8664–8674. [Google Scholar]
- Blanca, A.; Sanchez-Gonzalez, A.; Requena, M.J.; Carrasco-Valiente, J.; Gomez-Gomez, E.; Cheng, L.; Cimadamore, A.; Montironi, R.; Lopez-Beltran, A. Expression of miR-100 and miR-138 as prognostic biomarkers in non-muscle-invasive bladder cancer. APMIS 2019, 127, 545–553. [Google Scholar] [CrossRef]
- Wang, N.; Hao, F.; Ren, J.; Fei, X.; Chen, Y.; Xu, W.; Wang, J. Positive feedback loop of AKR1B10P1/miR-138/SOX4 promotes cell growth in hepatocellular carcinoma cells. Am. J. Transl. Res. 2020, 12, 5465–5480. [Google Scholar]
- Ma, F.; Zhang, M.; Gong, W.; Weng, M.; Quan, Z. MiR-138 suppresses cell proliferation by targeting Bag-1 in gallbladder carcinoma. PLoS ONE 2015, 10, e0126499. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.B.; Wang, X.P.; Sang, Y.; Xu, S.; Hu, K.; Wu, M.; Liang, Y.; Liu, P.; Tang, J.; et al. MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle 2012, 11, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Banik, N.L.; Ray, S.K. MiR-138 overexpression is more powerful than hTERT knockdown to potentiate apigenin for apoptosis in neuroblastoma in vitro and in vivo. Exp. Cell Res. 2013, 319, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Mitomo, S.; Maesawa, C.; Ogasawara, S.; Iwaya, T.; Shibazaki, M.; Yashima-Abo, A.; Kotani, K.; Oikawa, H.; Sakurai, E.; Izutsu, N.; et al. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008, 99, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, L.; Hu, J.; Ruan, J. miR-138 might reverse multidrug resistance of leukemia cells. Leuk. Res. 2010, 34, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, L.; Wang, A.; Xu, Y.; Luo, X.; Liu, X.; Hua, Y.; Zhang, D.; Wu, S.; Lin, T.; et al. MicroRNA-138 attenuates epithelial-to-mesenchymal transition by targeting SOX4 in clear cell renal cell carcinoma. Am. J. Transl. Res. 2017, 9, 3611–3622. [Google Scholar] [PubMed]
- Erdmann, K.; Kaulke, K.; Rieger, C.; Salomo, K.; Wirth, M.P.; Fuessel, S. MiR-26a and miR-138 block the G1/S transition by targeting the cell cycle regulating network in prostate cancer cells. J. Cancer Res. Clin. Oncol. 2016, 142, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, M.; Liang, H.; Guo, S.; Guo, X.; Yuan, M.; Lian, H.; Yan, X.; Zhang, S.; Chen, X.; et al. MiR-138-5p contributes to cell proliferation and invasion by targeting Survivin in bladder cancer cells. Mol. Cancer 2016, 15, 82. [Google Scholar] [CrossRef]



| Number | 67 |
|---|---|
| Male | 61 |
| Female | 5 |
| Mean age (y.o.) | 71.6 (37–95) |
| pT stage of Urothelial carcinomas | |
| pTa | 25 |
| pT1 | 17 |
| ≧pT2 | 12 |
| pTis | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitta, Y.; Fujii, T.; Uchiyama, T.; Sugimoto, A.; Nishikawa, T.; Takeda, M.; Miyake, M.; Shimada, K.; Fujimoto, K. Overexpression of MicroRNA-138 Affects the Proliferation and Invasion of Urothelial Carcinoma Cells by Suppressing SOX9 Expression. Biomedicines 2023, 11, 3064. https://doi.org/10.3390/biomedicines11113064
Nitta Y, Fujii T, Uchiyama T, Sugimoto A, Nishikawa T, Takeda M, Miyake M, Shimada K, Fujimoto K. Overexpression of MicroRNA-138 Affects the Proliferation and Invasion of Urothelial Carcinoma Cells by Suppressing SOX9 Expression. Biomedicines. 2023; 11(11):3064. https://doi.org/10.3390/biomedicines11113064
Chicago/Turabian StyleNitta, Yuji, Tomomi Fujii, Tomoko Uchiyama, Aya Sugimoto, Takeshi Nishikawa, Maiko Takeda, Makito Miyake, Keiji Shimada, and Kiyohide Fujimoto. 2023. "Overexpression of MicroRNA-138 Affects the Proliferation and Invasion of Urothelial Carcinoma Cells by Suppressing SOX9 Expression" Biomedicines 11, no. 11: 3064. https://doi.org/10.3390/biomedicines11113064






