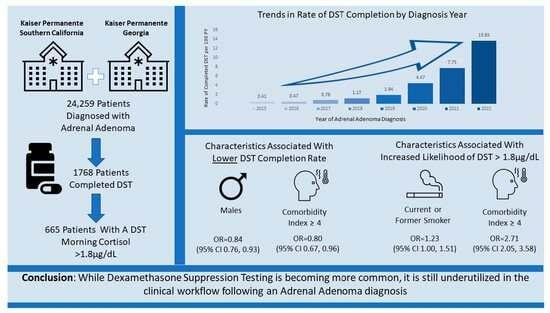
Dexamethasone Suppression Testing in a Contemporary Cohort with Adrenal Incidentalomas in Two U.S. Integrated Healthcare Systems
Abstract
:1. Introduction
2. Methods
2.1. Population
2.2. Outcomes of Interest
2.3. Covariates
2.4. Descriptive Analysis
2.5. Time to DST Completion
2.6. Elevated DST Result
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bancos, I.; Prete, A. Approach to the patient with adrenal incidentaloma. J. Clin. Endocrinol. Metab. 2021, 106, 3331–3353. [Google Scholar] [CrossRef]
- Ebbehoj, A.; Li, D.; Kaur, R.J.; Zhang, C.; Singh, S.; Li, T.; Atkinson, E.; Achenbach, S.; Khosla, S.; Arlt, W.; et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 894–902. [Google Scholar] [CrossRef]
- Reimondo, G.; Castellano, E.; Grosso, M.; Priotto, R.; Puglisi, S.; Pia, A.; Pellegrino, M.; Borretta, G.; Terzolo, M. Adrenal incidentalomas are tied to increased risk of diabetes: Findings from a prospective study. J. Clin. Endocrinol. Metab. 2020, 105, e973–e981. [Google Scholar] [CrossRef]
- Geelhoed, G.; Druy, E. Management of the adrenal “incidentaloma”. Surgery 1982, 92, 866–874. [Google Scholar] [PubMed]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Nassiri, N.; Bhanvadia, S.; Carmichael, J.D.; Duddalwar, V.; Daneshmand, S. Discrepancies in the recommended management of adrenal incidentalomas by various guidelines. J. Urol. 2021, 205, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2023, 189, G1–G42. [Google Scholar] [CrossRef] [PubMed]
- Deutschbein, T.; Reimondo, G.; Di Dalmazi, G.; Bancos, I.; Patrova, J.; Vassiliadi, D.A.; Nekić, A.B.; Debono, M.; Lardo, P.; Ceccato, F.; et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: An international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022, 10, 499–508. [Google Scholar] [CrossRef]
- Taya, M.; Paroder, V.; Bellin, E.; Haramati, L.B. The relationship between adrenal incidentalomas and mortality risk. Eur. Radiol. 2019, 29, 6245–6255. [Google Scholar] [CrossRef]
- Tsagarakis, S.; Kokkoris, P.; Roboti, C.; Malagari, C.; Kaskarelis, J.; Vlassopoulou, V.; Thalassinos, N. The low-dose dexamethasone suppression test in patients with adrenal incidentalomas: Comparisons with clinically euadrenal subjects and patients with Cushing’s syndrome. Clin. Endocrinol. 1998, 48, 627–633. [Google Scholar] [CrossRef]
- Diez Roux, A.V.; Mair, C. Neighborhoods and health. Ann. N. Y. Acad. Sci. 2010, 1186, 125–145. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Bradley, E.H.; McGraw, S.A.; Curry, L.; Buckser, A.; King, K.L.; Kasl, S.V.; Andersen, R. Expanding the Andersen model: The role of psychosocial factors in long-term care use. Health Serv. Res. 2002, 37, 1221–1242. [Google Scholar] [CrossRef]
- Andersen, R.M. Revisiting the behavioral model and access to medical care: Does it matter? J. Health Soc. Behav. 1995, 36, 1–10. [Google Scholar] [CrossRef]
- Hanna, F.W.F.; Hancock, S.; George, C.; Clark, A.; Sim, J.; Issa, B.G.; Powner, G.; Waldron, J.; Duff, C.J.; Lea, S.C.; et al. Adrenal incidentaloma: Prevalence and referral patterns from routine practice in a large UK university teaching hospital. J. Endocr. Soc. 2022, 6, bvab180. [Google Scholar] [CrossRef]
- Remde, H.; Kranz, S.; Morell, S.M.; Altieri, B.; Kroiss, M.; Detomas, M.; Fassnacht, M.; Deutschbein, T. Clinical course of patients with adrenal incidentalomas and cortisol autonomy: A German retrospective single center cohort study. Front. Endocrinol. 2023, 14, 1123132. [Google Scholar] [CrossRef]
- Kapoor, A.; Morris, T.; Rebello, R. Guidelines for the management of the incidentally discovered adrenal mass. Can. Urol. Assoc. J. 2011, 5, 241. [Google Scholar] [CrossRef]
- Atkins, J.S.; Hawley, J.M.; Owen, L.J.; Clayton, J.; Scargill, J.; Keevil, B.G. Serum cortisol assay performance following the 1 mg overnight dexamethasone suppression test. Ann. Clin. Biochem. 2023, 60, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Vijayashankar, N.P. Dexamethasone suppression test. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wood, P.J.; Barth, J.H.; Freedman, D.B.; Perry, L.; Sheridan, B. Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—Recommendations for a protocol for biochemistry laboratories. Ann. Clin. Biochem. 1997, 34, 222–229. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.J.; Sperl-Hillen, J.M.; Johnson, P.E.; Rush, W.A.; Biltz, G. Clinical inertia and outpatient medical errors. J. Med. Regul. 2008, 94, 19–29. [Google Scholar] [CrossRef]
- Debono, M.; Bradburn, M.; Bull, M.; Harrison, B.; Ross, R.J.; Newell-Price, J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J. Clin. Endocrinol. Metab. 2014, 99, 4462–4470. [Google Scholar] [CrossRef]
- Kjellbom, A.; Lindgren, O.; Puvaneswaralingam, S.; Löndahl, M.; Olsen, H. Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas: A cohort study. Ann. Intern. Med. 2021, 174, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, E.; Minnetti, M.; D’Aluisio, D.; Rizza, L.; Di Giorgio, M.R.; Vinci, F.; Isidori, A.M. Cardiovascular features of possible autonomous cortisol secretion in patients with adrenal incidentalomas. Eur. J. Endocrinol. 2018, 178, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.; Alahdab, F.; Prete, A.; Delivanis, D.; Khanna, A.; Murad, M.; O’Reilly, M.; Arlt, W.; Bancos, I. Natural history of adrenal incidentalomas with and without mild autonomous cortisol excess: A systematic review and meta-analysis. Ann. Intern. Med. 2019, 171, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Lázaro, C.R.; Ramírez, P.P.; Hernández, M.C.; Núñez, M.A.S.; Marazuela, M. Cardiometabolic profile of non-functioning and autonomous cortisol-secreting adrenal incidentalomas. Is the cardiometabolic risk similar or are there differences? Endocrine 2019, 66, 650–659. [Google Scholar] [CrossRef]
- Di Dalmazi, G.; Vicennati, V.; Garelli, S.; Casadio, E.; Rinaldi, E.; Giampalma, E.; Pasquali, R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: A 15-year retrospective study. Lancet Diabetes Endocrinol. 2014, 2, 396–405. [Google Scholar] [CrossRef]
- Berlińska, A.; Świątkowska-Stodulska, R.; Sworczak, K. Factors affecting dexamethasone suppression test results. Exp. Clin. Endocrinol. Diabetes 2020, 128, 667–671. [Google Scholar] [CrossRef]
- Vastbinder, M.; Kuindersma, M.; Mulder, A.H.; Schuijt, M.P.; Mudde, A.H. The influence of oral contraceptives on overnight 1 mg dexamethasone suppression test. Neth. J. Med. 2016, 74, 158–161. [Google Scholar]
- Araujo-Castro, M.; Cano, A.G.; Mendiguchía, L.J.; Escobar-Morreale, H.F.; Valderrábano, P. Diagnostic accuracy of the different hormonal tests used for the diagnosis of autonomous cortisol secretion. Sci. Rep. 2021, 11, 20539. [Google Scholar] [CrossRef]


| Total Population N (%) | KP Georgia N (%) | KP Southern California N (%) | Comparison Test p-Value | |
|---|---|---|---|---|
| Patient Characteristics at the Time of Adenoma Diagnosis | N = 24,259 | N = 2310 (9.52) | N = 21,949 (90.48) | |
| Age, mean (SD) | 63.1 (13.9) | 60.9 (12.6) | 63.4 (14.0) | <0.0001 |
| Age group, n (%) | <0.0001 | |||
| 18–39 | 1443 (5.9) | 106 (4.6) | 1337 (6.1) | |
| 40–49 | 2533 (10.4) | 317 (13.7) | 2215 (10.1) | |
| 50–59 | 4893 (20.2) | 597 (25.8) | 4293 (19.6) | |
| 60–69 | 7134 (29.4) | 722 (31.3) | 6408 (29.2) | |
| 70+ | 8274 (34.1) | 568 (24.6) | 7696 (35.1) | |
| Sex, n (%) | 0.29 | |||
| Male | 10,151 (41.8) | 942 (40.8) | 9198 (41.9) | |
| Female | 14,126 (58.2) | 1368 (59.2) | 12,751 (58.1) | |
| Patient Race/Ethnicity, n (%) | <0.0001 | |||
| Asian Pacific Islander | 1890 (7.8) | 73 (3.2) | 1821 (8.3) | |
| Black or African–American | 3727 (15.4) | 1097 (47.5) | 2631 (12.0) | |
| Non-Hispanic White | 10,653 (43.9) | 980 (42.4) | 9663 (44.0) | |
| Hispanic | 7179 (29.6) | 69 (3.0) | 7105 (32.4) | |
| Multiracial | 316 (1.3) | 10 (0.4) | 306 (1.4) | |
| Other | 48 (0.2) | 5 (0.2) | 43 (0.2) | |
| Unknown | 464 (1.9) | 76 (3.3) | 380 (1.7) | |
| Neighborhood Deprivation Index (NDI), Mean (SD) | <0.0001 | |||
| Q1 | −0.92 (0.2) | −1.08 (0.3) | −0.90 (0.2) | |
| Q2 | −0.39 (0.1) | −0.49 (0.1) | −0.38 (0.1) | |
| Q3 | 0.06 (0.1) | −0.07 (0.1) | 0.08 (0.1) | |
| Q4 | 0.60 (0.2) | 0.37 (0.2) | 0.63 (0.2) | |
| Q5 | 1.57 (0.6) | 1.24 (0.6) | 1.60 (0.5) | |
| Smoking status, n (%) | 0.0031 | |||
| Current or Past | 11,764 (48.5) | 1187 (51.4) | 10,568 (48.2) | |
| Never smoked | 12,513 (51.5) | 1123 (48.6) | 11,381 (51.9) | |
| Lab Values, mean ± SD (median) | ||||
| HbA1c | 6.4 ± 1.3 (6.0) | 6.3 ± 1.4 (5.9) | 6.4 ± 1.3 (6.0) | 0.0070 |
| Creatinine | 1.0 ± 0.9 (0.9) | 1.1 ± 1.2 (0.9) | 1.0 ± 0.7 (0.9) | 0.0002 |
| Total cholesterol | 172.3 ± 46.7 (169.0) | 181.3 ± 45.5 (178.0) | 171.4 ± 46.7 (168.0) | <0.0001 |
| LDL | 100.1 ± 39.2 (95.0) | 109.2 ± 40.1 (107.0) | 99.0 ± 38.9 (94.0) | <0.0001 |
| HDL | 49.4 ± 14.7 (47.0) | 51.3 ± 15.3 (49.5) | 49.2 ± 14.6 (47.0) | <0.0001 |
| Triglycerides | 168.7 ± 200.8 (137.0) | 130.8 ± 100.4 (109.0) | 173.7 ± 210.2 (141.0) | <0.0001 |
| Potassium | 4.0 ± 0.5 (4.0) | 4.0 ± 0.5 (4.0) | 4.0 ±0.5 (4.0) | <0.0001 |
| Comorbidities, n (%) | ||||
| Obesity | 11,680 (48.1) | 1205 (52.2) | 10,466 (47.7) | <0.0001 |
| Pre-diabetes | 6859 (28.3) | 617 (26.7) | 6239 (28.4) | 0.08 |
| Diabetes | 9036 (37.2) | 811 (35.1) | 8214 (37.4) | 0.03 |
| Hypertension | 16,317 (67.2) | 1671 (72.3) | 14,630 (66.7) | <0.0001 |
| Chronic Kidney Disease, Stage 3–5 | 4135 (17.0) | 311 (13.5) | 3812 (17.4) | <0.0001 |
| Dyslipidemia | 15,321 (63.1) | 1022 (44.2) | 14,284 (65.1) | <0.0001 |
| Cardiovascular Disease | 17,522 (72.2) | 1741 (75.4) | 15,765 (71.8) | 0.0003 |
| Coronary Artery Disease | 3380 (13.9) | 321 (13.9) | 3056 (13.9) | 0.97 |
| Myocardial Infarction | 1669 (6.9) | 154 (‘6.7) | 1514 (6.9) | 0.73 |
| Peripheral Vascular Disease | 6862 (28.3) | 464 (20.1) | 6391 (29.1) | <0.0001 |
| Cerebral Vascular Disease | 2457 (10.1) | 255 (11.0) | 2202 (10.0) | 0.13 |
| Deep Vein Thrombosis | 244 (1.0) | 28 (1.2) | 215 (1.0) | 0.27 |
| Transient Ischemic Attack | 760 (3.1) | 80 (3.5) | 680 (3.1) | 0.35 |
| Stroke | 1005 (4.1) | 118 (5.1) | 887 (4.0) | 0.02 |
| Osteoporosis | 2958 (12.2) | 150 6.5) | 2807 (12.8) | <0.0001 |
| Osteopenia | 3428 (14.1) | 206 (8.9) | 3222 (14.7) | <0.0001 |
| Charlson Comorbidity Index | <0.0001 | |||
| 0–1 | 11,388 (46.9) | 1192 (51.6) | 10,194 (46.4) | |
| 2 | 3581 (14.8) | 320 (13.9) | 3259 (14.8) | |
| 3 | 2339 (9.6) | 224 (9.7) | 2112 (9.6) | |
| 4+ | 6969 (28.7) | 574 (24.9) | 6384 (29.1) | |
| Year of incident adrenal adenoma diagnosis n (%) | <0.0001 | |||
| 2015 | 2513 (10.4) | 212 (9.2) | 2299 (10.5) | |
| 2016 | 2662 (11.0) | 269 (11.7) | 2391 (10.9) | |
| 2017 | 2921 (12.0) | 301 (13.0) | 2617 (11.9) | |
| 2018 | 3019 (12.4) | 288 (12.5) | 2730 (12.4) | |
| 2019 | 3195 (13.2) | 278 (12.0) | 2913 (13.3) | |
| 2020 | 2867 (11.8) | 211 (9.1) | 2656 (12.1) | |
| 2021 | 3569 (14.3) | 320 (13.9) | 3149 (14.3) | |
| 2022 | 3631 (15.0) | 431 (18.7) | 3194 (14.6) |
| Patient Characteristics at the Time of Adenoma Diagnosis | Mean Follow Up in Years (SD) | Number with Completed DST | Rate of Completed DST (95% CI) per 100 PY |
|---|---|---|---|
| Overall | 3.12 (2.43) | 1768 | 2.36 (2.35, 2.37) |
| Age group, n (%) | |||
| 18–39 | 2.78 (2.29) | 119 | 2.97 (2.93, 3.06) |
| 40–49 | 3.01 (2.37) | 238 | 3.12 (3.10, 3.16) |
| 50–59 | 3.28 (2.45) | 411 | 2.56 (2.55, 2.58) |
| 60–69 | 3.29 (2.46) | 557 | 2.38 (2.37, 2.39) |
| 70+ | 2.97 (2.40) | 443 | 1.80 (1.80, 1.81) |
| Sex, n (%) | |||
| Male | 3.14 (2.42) | 638 | 2.04 (2.03, 2.05) |
| Female | 3.09 (2.44) | 1130 | 2.55 (2.54, 2.55) |
| Patient Race/Ethnicity, n (%) | |||
| Asian Pacific Islander | 3.31 (2.47) | 139 | 2.21 (2.19, 2.26) |
| Black or African–American | 3.09 (2.49) | 324 | 2.81 (2.80, 2.84) |
| Non-Hispanic White | 3.13 (2.43) | 640 | 1.92 (1.92, 1.93) |
| Hispanic | 3.10 (2.38) | 603 | 2.71 (2.70, 2.72) |
| Multiracial | 3.45 (2.57) | 21 | 1.93 (1.80, 2.18) |
| Other | 3.35 (2.57) | 2 | 1.24 (0.55, 2.60) |
| Unknown | 2.32 (1.97) | 39 | 3.69 (3.51, 4.05) |
| Neighborhood Deprivation Index (NDI), n (%) | |||
| Q1 | 3.27 (2.47) | 280 | 1.76 (1.76, 1.78) |
| Q2 | 3.21 (2.45) | 335 | 2.14 (2.13, 2.16) |
| Q3 | 3.02 (2.37) | 369 | 2.51 (2.49, 2.53) |
| Q4 | 2.95 (2.34) | 394 | 2.76 (2.75, 2.79) |
| Q5 | 3.14 (2.49) | 390 | 2.57 (2.56, 2.59) |
| Smoking status, n (%) | |||
| Current or Past | 3.07 (2.44) | 989 | 2.50 (2.49, 2.50) |
| Never smoked | 3.17 (2.41) | 779 | 2.16 (2.16, 2.17) |
| Comorbidities, n (%) | |||
| Obesity | 3.16 (2.39) | 960 | 2.60 (2.60, 2.61) |
| Pre-diabetes | 3.16 (2.40) | 567 | 2.62 (2.61, 2.64) |
| Diabetes | 3.04 (2.38) | 648 | 2.36 (2.35, 2.37) |
| Hypertension | 3.12 (2.43) | 1106 | 2.18 (2.17, 2.18) |
| Chronic Kidney Disease, Stage 3–5 | 2.83 (2.34) | 222 | 1.90 (1.89, 1.93) |
| Dyslipidemia | 3.15 (2.42) | 1085 | 2.25 (2.25, 2.26) |
| Cardiovascular Disease | 3.12 (2.43) | 1196 | 2.19 (2.19, 2.20) |
| Coronary Artery Disease | 2.72 (2.30) | 161 | 1.75 (1.74, 1.78) |
| Myocardial Infarction | 2.68 (2.33) | 67 | 1.50 (1.47, 1.55) |
| Peripheral Vascular Disease | 2.78 (2.23) | 383 | 2.01 (2.00, 2.02) |
| Cerebral Vascular Disease | 2.73 (2.34) | 115 | 1.71 (1.69, 1.75) |
| Transient Ischemic Attack | 2.86 (2.39) | 36 | 1.65 (1.59, 1.77) |
| Charlson Comorbidity Index | |||
| 0–1 | 3.25 (2.45) | 953 | 2.57 (2.57, 2.58) |
| 2 | 3.46 (2.48) | 284 | 2.29 (2.28, 2.32) |
| 3 | 3.20 (2.39) | 178 | 2.38 (2.36, 2.43) |
| 4+ | 2.70 (2.30) | 353 | 1.88 (1.87, 1.89) |
| Year of incident adrenal adenoma diagnosis | |||
| 2015 | 5.28 (3.20) | 55 | 0.41 (0.41, 0.42) |
| 2016 | 4.87 (2.79) | 61 | 0.47 (0.47, 0.48) |
| 2017 | 4.30 (2.38) | 98 | 0.78 (0.77, 0.79) |
| 2018 | 3.67 (2.02) | 130 | 1.17 (1.16, 1.19) |
| 2019 | 2.07 (1.63) | 190 | 1.94 (1.93, 1.97) |
| 2020 | 2.34 (1.29) | 300 | 4.47 (4.44, 4.54) |
| 2021 | 1.67 (0.91) | 448 | 7.75 (7.71, 7.85) |
| 2022 | 0.98 (0.53) | 486 | 13.65 (13.54, 13.85) |
| Patient Characteristics | HR (95% CI) | |
|---|---|---|
| Patient Demographics and Behavioral Characteristics | Crude, Bivariate Model | Fully Adjusted Model * |
| Age group | ||
| 18–39 | Reference Group | Reference Group |
| 40–49 | 1.10 (0.89, 1.38) | 1.15 (0.92, 1.44) |
| 50–59 | 0.95 (0.78, 1.17) | 1.10 (0.89, 1.35) |
| 60–69 | 0.89 (0.73, 1.08) | 1.08 (0.87, 1.33) |
| 70+ | 0.64 (0.52, 0.78) | 0.84 (0.67, 1.05) |
| Sex | ||
| Male | 0.79 (0.72, 0.87) | 0.84 (0.76, 0.93) |
| Female | Reference Group | Reference Group |
| Patient Race/Ethnicity | ||
| Asian Pacific Islander | 1.20 (1.00, 1.44) | 1.11 (0.92, 1.34) |
| Black or African–American | 1.46 (1.28, 1.67) | 1.27 (1.10, 1.46) |
| Non-Hispanic White | Reference Group | Reference Group |
| Hispanic | 1.40 (1.26, 1.57) | 1.07 (0.95, 1.20) |
| Multiracial/Other/Unknown | 1.31 (1.01, 1.71) | 1.03 (0.79, 1.34) |
| Neighborhood Deprivation Index (NDI) | ||
| Q1 | Reference Group | Reference Group |
| Q2 | 1.21 (1.03, 1.42) | 1.19 (1.01, 1.39) |
| Q3 | 1.36 (1.16, 1.58) | 1.21 (1.03, 1.41) |
| Q4 | 1.47 (1.26, 1.71) | 1.23 (1.05, 1.45) |
| Q5 | 1.43 (1.23, 1.67) | 1.33 (1.13, 1.57) |
| Smoking status | ||
| Current or former | 0.85 (0.77, 0.93) | |
| Never | Reference | |
| Comorbidities | ||
| Obesity | 1.26 (1.15, 1.39) | 1.08 (0.98, 1.20) |
| Prediabetes | 1.19 (1.08, 1.32) | |
| Diabetes | 0.99 (0.90, 1.09) | 1.14 (1.02, 1.29) |
| Hypertension | 0.82 (0.74, 0.90) | 0.91 (0.82, 1.02) |
| Chronic kidney disease, stage 3–5 | 0.74 (0.64, 0.85) | |
| Dyslipidemia | 0.92 (0.84, 1.01) | |
| Cardiovascular disease | 0.81 (0.73, 0.90) | |
| Coronary artery disease | 0.66 (0.56, 0.77) | |
| Myocardial Infarction | 0.57 (0.45, 0.73) | |
| Peripheral Vascular Disease | 0.75 (0.67, 0.84) | |
| Cerebral Vascular Disease | 0.65 (0.54, 0.79) | |
| Stroke | 0.60 (0.44, 0.82) | |
| Transient ischemic attack | 0.67 (0.48, 0.93) | |
| Intracerebral hemorrhage | ||
| Deep Vein Thrombosis | 0.32 (0.15, 0.72) | |
| Osteoporosis | 0.66 (0.56, 0.78) | |
| Osteopenia | 0.85 (0.74, 0.98) | |
| Charlson Comorbidity Index | ||
| 0–1 | Reference Group | Reference Group |
| 2 | 0.92 (0.81, 1.06) | 1.01 (0.87, 1.16) |
| 3 | 0.92 (0.78, 1.08) | 1.01 (0.85, 1.21) |
| 4+ | 0.66 (0.58, 0.74) | 0.80 (0.67, 0.96) |
| Year of incident adrenal adenoma diagnosis | ||
| 2015 | Reference Group | Reference Group |
| 2016 | 1.24 (0.85, 1.81) | 1.53 (1.04, 2.25) |
| 2017 | 2.14 (1.50, 3.04) | 3.13 (2.12, 4.60) |
| 2018 | 3.39 (2.40, 4.80) | 5.62 (3.78, 8.63) |
| 2019 | 5.63 (4.02, 7.88) | 10.86 (7.01, 16.81) |
| 2020 | 12.40 (8.93, 17.23) | 26.71 (16.86, 42.34) |
| 2021 | 18.23 (13.18, 25.02) | 41.45 (25.65, 66.97) |
| 2022 | 22.44 (16.23, 31.03) | 54.03 (33.01, 88.43) |
| Total Population N (%) | KP Georgia N (%) | KP Southern California N (%) | ||||
|---|---|---|---|---|---|---|
| Patient Characteristics at Time of Dexamethasone Suppression Test (DST) | DST ≤ 1.8 μg/dL N = 1103 (62.4) | DST > 1.8 μg/dL N = 665 (37.6) | DST ≤ 1.8 μg/dL N = 138 (60.8) | DST > 1.8 μg/dL N = 89 (39.2) | DST ≤ 1.8 μg/dL N = 965 (62.6) | DST > 1.8 μg/dL N = 576 (37.4) |
| Age, mean (SD) | 59.2 (13.0) | 62.4 (12.2) | 57.4 (10.8) | 60.1 (10.8) | 59.5 (13.2) | 62.7 (12.3) |
| Age group, n (%) | ||||||
| 18–39 | 92 (8.5) | 27 (4.1) | 7 (5.1) | 4 (4.5) | 85 (8.8) | 23 (4.0) |
| 40–49 | 159 (14.4) | 79 (11.9) | 22 (15.9) | 11 (12.4) | 137 (14.2) | 68 (11.8) |
| 50–59 | 262 (23.8) | 149 (22.4) | 50 (36.2) | 27 (30.3) | 212 (22.0) | 122 (21.2) |
| 60–69 | 347 (31.5) | 210 (31.6) | 38 (27.5) | 30 (33.7) | 309 (32.2) | 180 (31.3) |
| 70+ | 243 (22.0) | 200 (30.1) | 21 (15.2) | 17 (19.1) | 222 (23.0) | 183 (31.8) |
| Sex, n (%) | ||||||
| Male | 407 (36.9) | 231 (34.7) | 55 (39.9) | 35 (39.3) | 352 (36.5) | 196 (34.0) |
| Female | 696 (63.1) | 434 (65.3) | 83 (60.1) | 54 (60.7) | 613 (63.5) | 380 (66.0) |
| Patient Race/Ethnicity, n (%) | ||||||
| Asian Pacific Islander | 90 (8.2) | 49 (7.4) | 3 (2.2) | 4 (4.5) | 87 (9.0) | 45 (7.8) |
| Black or African–American | 175 (15.9) | 149 (22.4) | 67 (48.6) | 45 (50.6) | 108 (11.2) | 104 (18.1) |
| Non-Hispanic white | 386 (35.0) | 254 (38.2) | 58 (42.0) | 35 (39.3) | 328 (34.0) | 219 (38.0) |
| Hispanic | 413 (37.4) | 190 (28.6) | 4 (2.9) | 4 (4.5) | 409 (42.4) | 186 (32.3) |
| Multiracial | 10 (0.9) | 11 (1.7) | 1 (0.8) | 1 (1.1) | 9 (0.9) | 10 (1.7) |
| Other | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0 (0) | 1 (0.1) | 1 (0.2) |
| Unknown | 28 (2.5) | 11 (1.7) | 5 (3.6) | 0 (0) | 23 (2.4) | 11 (1.9) |
| Neighborhood Deprivation Index (NDI), mean (SD) | ||||||
| Q1 | −0.93 (0.22) | −0.89 (0.24) | −1.04 (0.27) | −1.13 (0.29) | −0.92 (0.21) | −0.86 (0.22) |
| Q2 | −0.39 (0.13) | −0.40 (0.14) | −0.46 (0.14) | −0.51 (0.12) | −0.39 (0.13) | −0.39 (0.13) |
| Q3 | 0.06 (0.15) | 0.09 (0.14) | −0.06 (0.11) | −0.02 (0.10) | 0.07 (0.15) | 0.10 (0.14) |
| Q4 | 0.59 (0.20) | 0.60 (0.22) | 0.39 (0.15) | 0.34 (0.17) | 0.62 (0.19) | 0.66 (0.19) |
| Q5 | 1.58 (0.55) | 1.54 (0.52) | 1.28 (0.84) | 1.20 (0.62) | 1.61 (0.49) | 1.59 (0.49) |
| Smoking status, n (%) | ||||||
| Current or Past | 452 (41.0) | 327 (49.2) | 67 (48.5) | 48 (53.9) | 385 (39.9) | 297 (51.6) |
| Never smoked | 651 (59.0) | 338 (50.8) | 71 (51.5) | 41 (46.1) | 580 (60.1) | 279 (48.4) |
| Lab Values, mean ± SD (median) | ||||||
| HbA1c | 6.3 ± 1.2 (5.9) | 6.5 ± 1.4 (6.0) | 6.3 ± 1.3 (5.8) | 6.2 ± 1.3 (5.8) | 6.3 ± 1.2 (6.0) | 6.5 ± 1.4 (6.0) |
| Creatinine | 0.9 ± 0.5 (0.9) | 1.1 ± 0.9 (0.9) | 0.9 ± 0.3 (0.9) | 1.2 ± 0.7 (0.9) | 0.9 ± 0.5 (0.8) | 1.1 ± 1.0 (0.9) |
| Total cholesterol | 177.2 ± 43.0 (177.0) | 175.2 ± 45.9 (172) | 187.9 ± 41.8 (186.0) | 173.9 ± 41.3 (174) | 175.8 ± 42.9 (176.0) | 175.4 ± 46.6 (172.0) |
| LDL | 106.5 ± 36.0 (105.0) | 102.8 ± 39.5 (98.5) | 120.1 ± 37.0 (117.0) | 101.6 ± 32.3 (99.0) | 103.9 ± 35.3 (102.0) | 103.0 ± 40.8 (98.0) |
| HDL | 50.1 ± 13.2 (49.0) | 50.9 ± 15.3 (48.0) | 51.1 ± 13.3 (50.0) | 46.3 ± 13.1 (45.3) | 50.0 ± 13.2 (48.0) | 51.5 ± 15.5 (49.0) |
| Triglycerides | 171.3 ± 110.5 (141.0) | 178.8 ± 137.5 (142.0) | 122.2 ± 78.4 (104.6) | 148.8 ± 78.5 (134.0) | 184.2 ± 114.2 (148.0) | 183.6 ± 145.6 (151.0) |
| Potassium | 4.0 ± 0.4 (4.0) | 4.0 ± 0.5 (4.0) | 4.0 ± 0.4 (4.0) | 4.0 ± 0.4 (4.0) | 4.0 ± 0.4 (4.0) | 4.0 ± 0.5 (4.0) |
| Comorbidities, n (%) | ||||||
| Obesity | 626 (56.8) | 334 (50.2) | 84 (60.9) | 53 (59.6) | 542 (56.2) | 281 (48.8) |
| Pre-diabetes | 365 (33.1) | 202 (30.4) | 44 (31.9) | 29 (32.6) | 321 (33.3) | 173 (30.0) |
| Diabetes | 343 (31.1) | 305 (45.9) | 38 (27.5) | 37 (41.6) | 305 (31.6) | 268 (46.5) |
| Hypertension | 637 (57.0) | 469 (70.5) | 86 (62.3) | 69 (77.5) | 601 (62.3) | 392 (68.1) |
| Chronic Kidney Disease, Stage 3–5 | 111 (10.1) | 111 (16.7) | 11 (8.0) | 12 (13.5) | 100 (10.4) | 99 (17.2) |
| Dyslipidemia | 656 (59.5) | 429 (64.5) | 55 (39.9) | 37 (41.6) | 582 (62.2) | 388 (67.7) |
| Cardiovascular Disease | 696 (63.1) | 500 (75.2) | 93 (67.4) | 72 (80.9) | 603 (62.5) | 428 (74.3) |
| Coronary Artery Disease | 91 (8.3) | 70 (10.5) | 7 (5.1) | 10 (11.2) | 84 (8.7) | 60 (10.4) |
| Myocardial Infarction | 37 (3.4) | 30 (4.5) | 3 (2.2) | 3 (3.4) | 34 (3.5) | 27 (4.7) |
| Patient Characteristics | Crude OR (95% CI) |
|---|---|
| DST > 1.8 μg/dL | |
| Patient demographics and behavioral characteristics | |
| Age group | |
| 18–39 | Reference Group |
| 40–49 | 1.69 (1.02, 2.81) |
| 50–59 | 1.94 (1.21, 3.11) |
| 60–69 | 2.06 (1.30, 3.27) |
| 70+ | 2.80 (1.76, 4.48) |
| Sex | |
| Male | 0.91 (0.74, 1.11) |
| Female | Reference Group |
| Patient race/Ethnicity | |
| Asian Pacific Islander | 0.83 (0.57, 1.54) |
| Black or African–American | 1.29 (0.99, 1.70) |
| Non-Hispanic White | Reference |
| Hispanic | 0.69 (0.55, 0.88) |
| Other | 0.90 (0.52, 1.54) |
| Neighborhood Deprivation Index (NDI) | |
| Q1 | Reference Group |
| Q2 | 1.23 (0.89, 1.71) |
| Q3 | 1.19 (0.86, 1.64) |
| Q4 | 1.14 (0.83, 1.57) |
| Q5 | 1.20 (0.87, 1.65) |
| Smoking status, Current or Former | 1.39 (1.15, 1.69) |
| Comorbidities | |
| Obesity | 0.77 (0.63, 0.93) |
| Pre-diabetes | 0.89 (0.72,1.09) |
| Diabetes | 1.88 (1.54, 2.29) |
| Hypertension | 1.75 (1.43, 2.15) |
| Chronic Kidney Disease, Stage 3–5 | 1.79 (1.35, 2.38) |
| Dyslipidemia | 1.24 (1.02, 1.51) |
| Cardiovascular Disease | 1.77 (1.43, 2.20) |
| Coronary Artery Disease | 1.31 (0.94, 1.82) |
| Myocardial Infarction | 1.36 (0.83, 2.23) |
| Peripheral Vascular Disease | 1.30 (1.04, 1.64) |
| Cerebral Vascular Disease | 1.63 (1.11, 2.38) |
| Stroke | 1.77 (0.95, 3.28) |
| Transient Ischemic Attack | 2.37 (1.21, 4.62) |
| Deep Vein Thrombosis | 8.35 (0.97, 71.61) |
| Osteoporosis | 1.24 (0.88, 1.75) |
| Osteopenia | 1.26 (0.95, 1.68) |
| Charlson Comorbidity Index | |
| 0–1 | Reference Group |
| 2 | 1.43 (1.09, 1.90) |
| 3 | 1.64 (1.18, 2.27) |
| 4+ | 2.77 (2.16, 3.56) |
| Year of incident adrenal adenoma diagnosis | |
| 2015 | Reference Group |
| 2016 | 1.66 (0.77, 3.57) |
| 2017 | 2.06 (1.03, 4.13) |
| 2018 | 1.86 (0.95, 3.62) |
| 2019 | 1.46 (0.77, 2.77) |
| 2020 | 1.64 (0.89, 3.04) |
| 2021 | 1.21 (0.66, 2.21) |
| 2022 | 1.07 (0.58, 1.95) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, M.; McDonald, B.; Chen, W.; Chowdhry, H.; Contreras, R.; Reyes, I.A.C.; Dhakal, E.; Villanueva, T.; Barzilay, J.I.; Vaughn, C.F.; et al. Dexamethasone Suppression Testing in a Contemporary Cohort with Adrenal Incidentalomas in Two U.S. Integrated Healthcare Systems. Biomedicines 2023, 11, 3167. https://doi.org/10.3390/biomedicines11123167
Crawford M, McDonald B, Chen W, Chowdhry H, Contreras R, Reyes IAC, Dhakal E, Villanueva T, Barzilay JI, Vaughn CF, et al. Dexamethasone Suppression Testing in a Contemporary Cohort with Adrenal Incidentalomas in Two U.S. Integrated Healthcare Systems. Biomedicines. 2023; 11(12):3167. https://doi.org/10.3390/biomedicines11123167
Chicago/Turabian StyleCrawford, Mackenzie, Bennett McDonald, Wansu Chen, Hina Chowdhry, Richard Contreras, Iris Anne C. Reyes, Eleena Dhakal, Tish Villanueva, Joshua I. Barzilay, Candace F. Vaughn, and et al. 2023. "Dexamethasone Suppression Testing in a Contemporary Cohort with Adrenal Incidentalomas in Two U.S. Integrated Healthcare Systems" Biomedicines 11, no. 12: 3167. https://doi.org/10.3390/biomedicines11123167