New Enhancing MRI Lesions Associate with IL-17, Neutrophil Degranulation and Integrin Microparticles: Multi-Omics Combined with Frequent MRI in Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Sample Material (MRI, Plasma)
2.2. MRI Analysis of Five Patients
2.3. Proteomics—Sample Preparation and UPLC-Tandem MassSpec Analysis
2.4. Proteomics—Mass Spectrometry Analysis
2.5. Proteomics—Data Analysis
2.6. EV Array Analysis—Preparation of EV Stress Optimized EV Array
2.7. EV Array Analysis—Interpretation and Statistical Analysis of Data
2.8. Multiplex Analysis for Neuroinflammatory Profiling
2.9. Statistical Analysis
3. Results
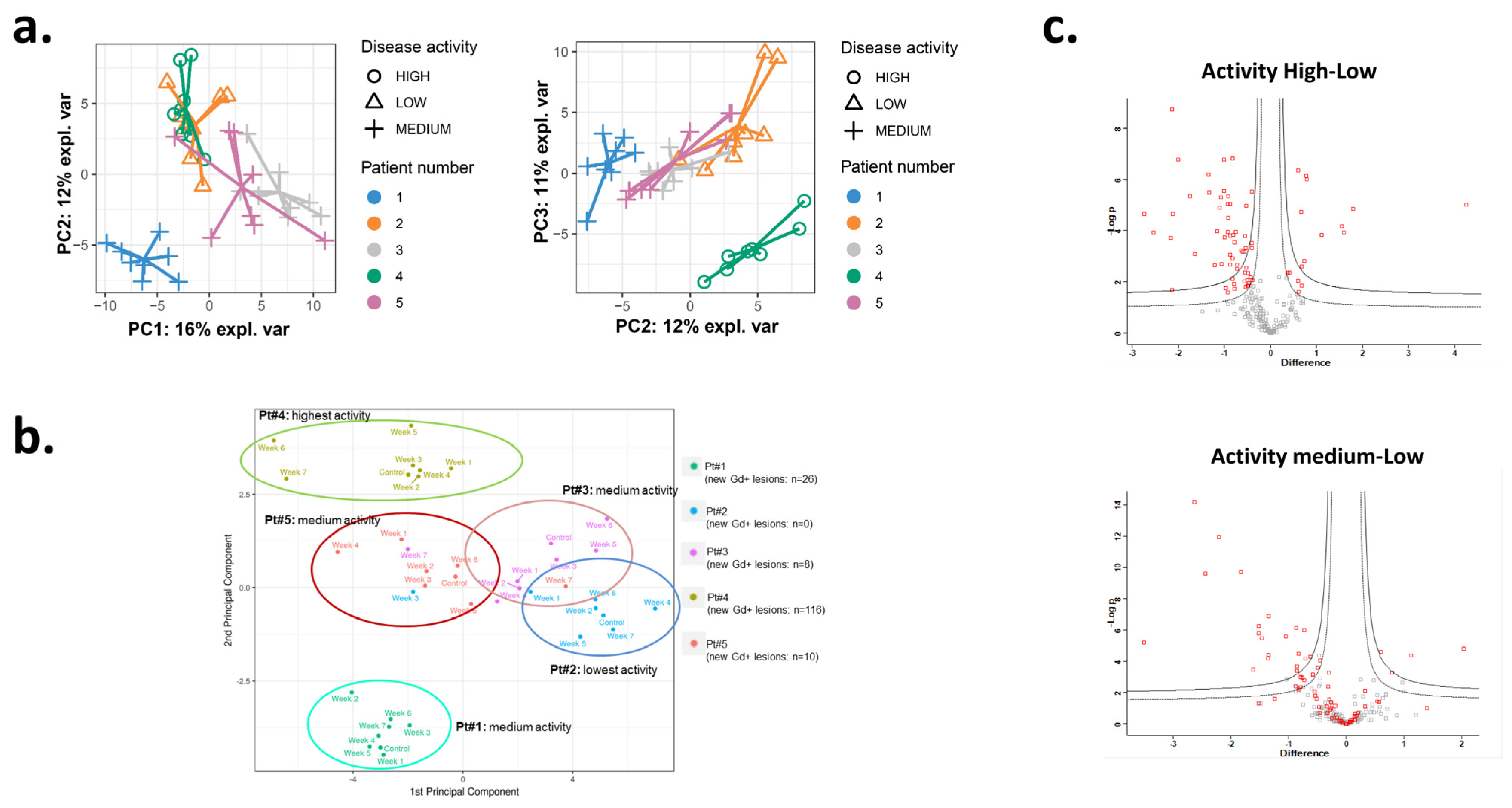
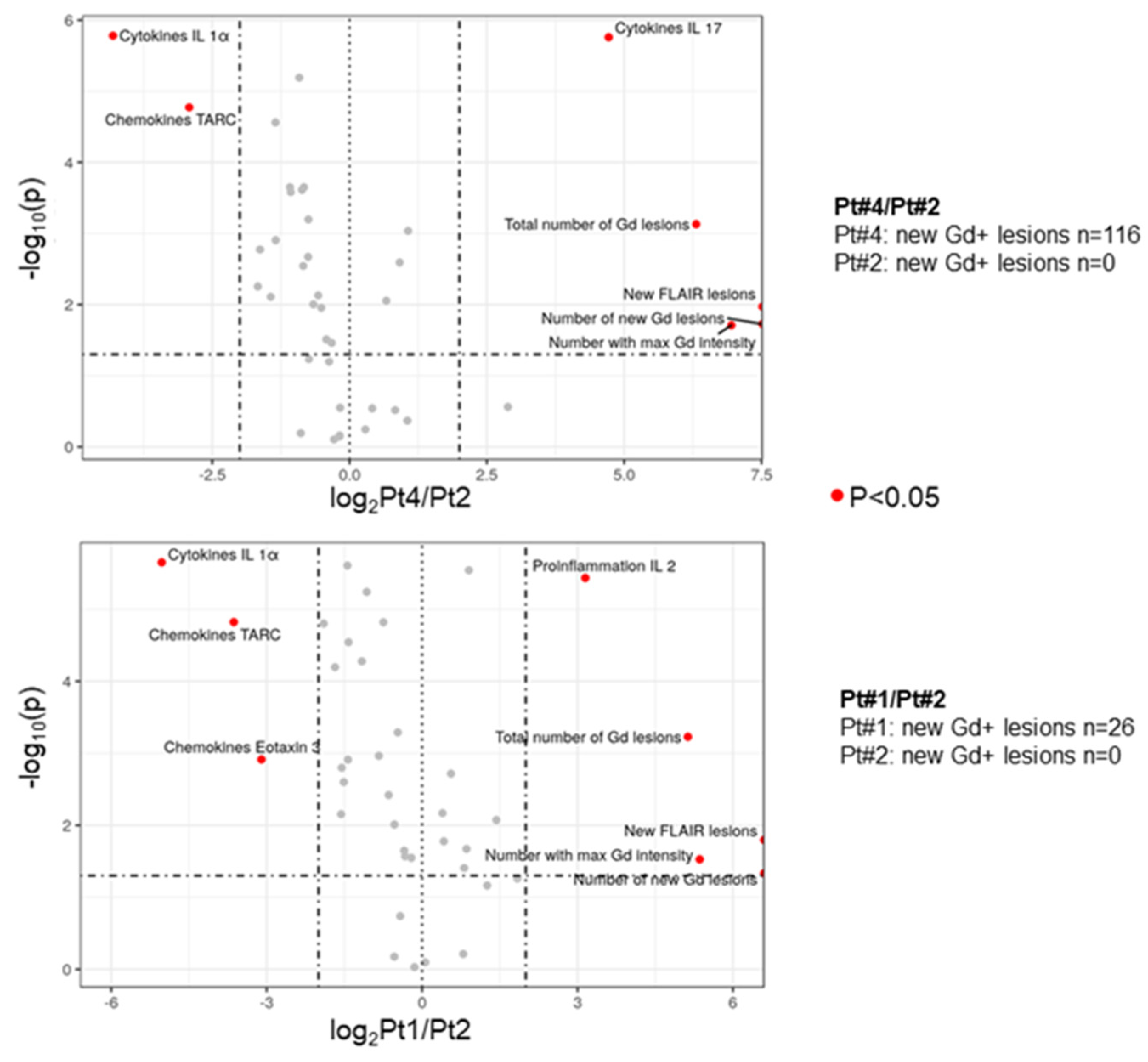
3.1. The Inflammatory Profile in Patients Reflects the Number of New Gd-Enhancing and FLAIR Lesions
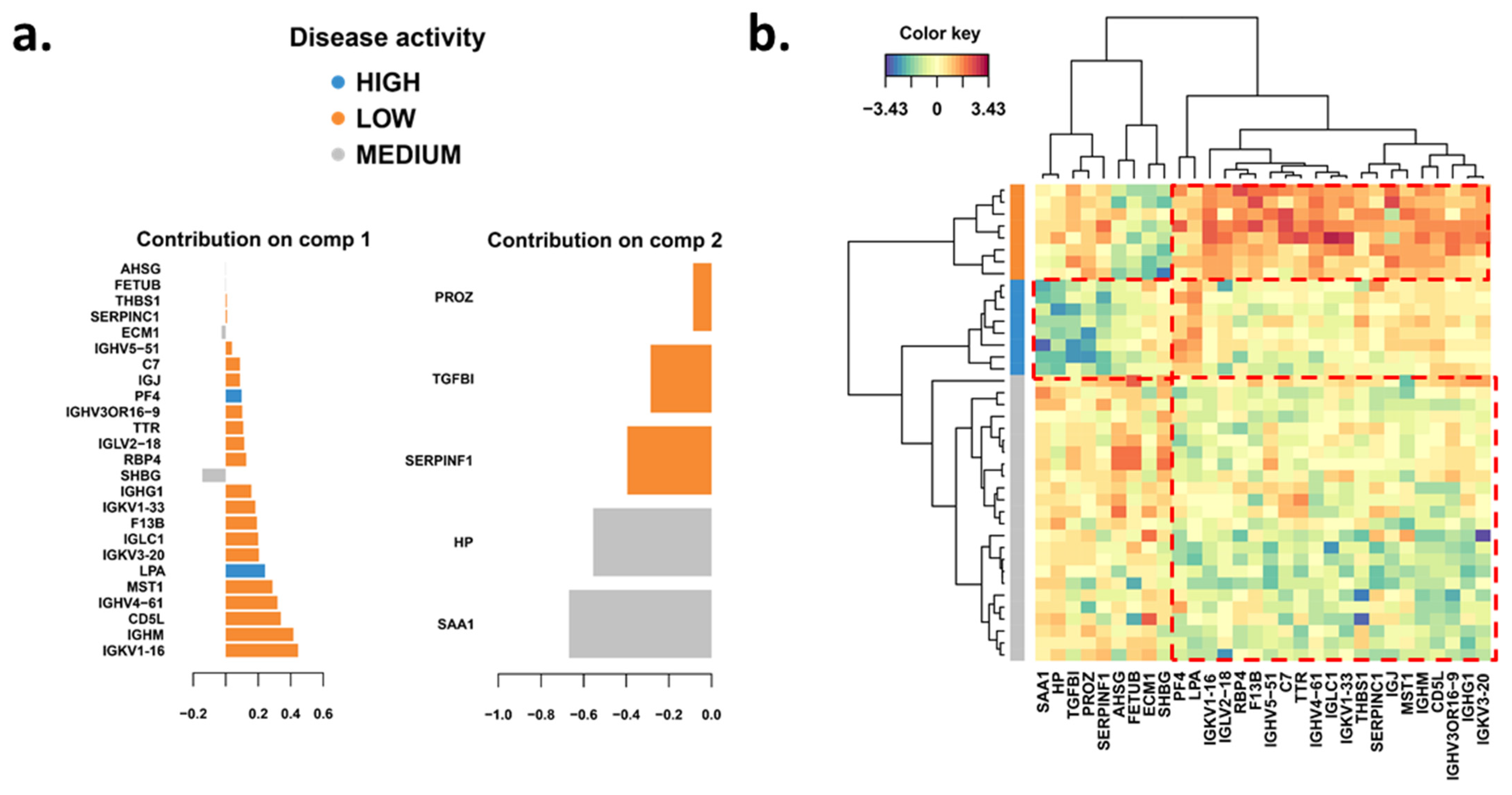
3.2. Significantly Changed Inflammatory and Vascular Markers in Patients with High, Low and Medium MRI Activity
3.3. Weekly Level of Inflammatory and Vascular Markers Related to MRI Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guttmann, C.R.; Rousset, M.; Roch, J.A.; Hannoun, S.; Durand-Dubief, F.; Belaroussi, B.; Cavallari, M.; Rabilloud, M.; Sappey-Marinier, D.; Vukusic, S.; et al. Multiple Sclerosis Lesion Formation and Early Evolution Revisited: A Weekly High-Resolution Magnetic Resonance Imaging Study. Mult. Scler. J. 2016, 22, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Eisele, P.; Alonso, A.; Griebe, M.; Szabo, K.; Hennerici, M.G.; Gass, A. Investigation of Cerebral Microbleeds in Multiple Sclerosis as a Potential Marker of Blood-Brain Barrier Dysfunction. Mult. Scler. Relat. Disord. 2016, 7, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Touyz, R.M. Cellular Biomarkers of Endothelial Health: Microparticles, Endothelial Progenitor Cells, and Circulating Endothelial Cells. J. Am. Soc. Hypertens. JASH 2012, 6, 85–99. [Google Scholar] [CrossRef]
- Greenwood, J.; Heasman, S.J.; Alvarez, J.I.; Prat, A.; Lyck, R.; Engelhardt, B. Review: Leucocyte-Endothelial Cell Crosstalk at the Blood-Brain Barrier: A Prerequisite for Successful Immune Cell Entry to the Brain. Neuropathol. Appl. Neurobiol. 2011, 37, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Welton, J.L.; Loveless, S.; Stone, T.; von Ruhland, C.; Robertson, N.P.; Clayton, A. Cerebrospinal Fluid Extracellular Vesicle Enrichment for Protein Biomarker Discovery in Neurological Disease; Multiple Sclerosis. J. Extracell. Vesicles 2017, 6, 1369805. [Google Scholar] [CrossRef] [PubMed]
- Croese, T.; Furlan, R. Extracellular Vesicles in Neurodegenerative Diseases. Mol. Asp. Med. 2018, 60, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, A.; Hamilton, T.; Carter, N.; Brown, M.; McPheat, W.; Dobrian, A. Endothelial Extracellular Vesicles: From Keepers of Health to Messengers of Disease. Int. J. Mol. Sci. 2021, 22, 4640. [Google Scholar] [CrossRef]
- Baylis, C. Arginine, Arginine Analogs and Nitric Oxide Production in Chronic Kidney Disease. Nat. Clin. Pract. Nephrol. 2006, 2, 209–220. [Google Scholar] [CrossRef]
- Sabatier, F.; Camoin-Jau, L.; Anfosso, F.; Sampol, J.; Dignat-George, F. Circulating Endothelial Cells, Microparticles and Progenitors: Key Players towards the Definition of Vascular Competence. J. Cell. Mol. Med. 2009, 13, 454–471. [Google Scholar] [CrossRef]
- Rautou, P.-E.; Leroyer, A.S.; Ramkhelawon, B.; Devue, C.; Duflaut, D.; Vion, A.-C.; Nalbone, G.; Castier, Y.; Leseche, G.; Lehoux, S.; et al. Microparticles From Human Atherosclerotic Plaques Promote Endothelial ICAM-1–Dependent Monocyte Adhesion and Transendothelial MigrationNovelty and Significance. Circ. Res. 2011, 108, 335–343. [Google Scholar] [CrossRef]
- Jy, W.; Minagar, A.; Jimenez, J.J.; Sheremata, W.A.; Mauro, L.M.; Horstman, L.L.; Bidot, C.; Ahn, Y.S. Endothelial Microparticles (EMP) Bind and Activate Monocytes: Elevated EMP-Monocyte Conjugates in Multiple Sclerosis. Front. Biosci. J. Virtual Libr. 2004, 9, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, K.R.; Paulsen, B.S.; Bæk, R.; Varming, K.; Sorensen, B.S.; Jørgensen, M.M. Exosomal Proteins as Potential Diagnostic Markers in Advanced Non-Small Cell Lung Carcinoma. J. Extracell. Vesicles 2015, 4, 26659. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple Sclerosis Progression: Time for a New Mechanism-Driven Framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- Rocca, M.A.; Colombo, B.; Falini, A.; Ghezzi, A.; Martinelli, V.; Scotti, G.; Comi, G.; Filippi, M. Cortical Adaptation in Patients with MS: A Cross-Sectional Functional MRI Study of Disease Phenotypes. Lancet Neurol. 2005, 4, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Vermersch, P.; Berger, T.; Gold, R.; Lukas, C.; Rovira, A.; Meesen, B.; Chard, D.; Comabella, M.; Palace, J.; Trojano, M. The Clinical Perspective: How to Personalise Treatment in MS and How May Biomarkers Including Imaging Contribute to This? Mult. Scler. 2016, 22, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, M.P.; Rovira, À.; Miller, D.; Yousry, T.A.; Sormani, M.P.; De Stefano, N.; Tintoré, M.; Auger, C.; Tur, C.; Filippi, M.; et al. MAGNIMS Consensus Guidelines on the Use of MRI in Multiple Sclerosis—Establishing Disease Prognosis and Monitoring Patients. Nat. Rev. Neurol. 2015, 11, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.; Hayden, Z.; Cseh, T.; Berki, T.; Illes, Z. Osteopontin Levels Are Associated with Late-Time Lower Regional Brain Volumes in Multiple Sclerosis. Sci. Rep. 2021, 11, 23604. [Google Scholar] [CrossRef]
- Orsi, G.; Cseh, T.; Hayden, Z.; Perlaki, G.; Nagy, S.A.; Giyab, O.; Olsen, D.A.; Madsen, J.S.; Berki, T.; Illes, Z. Microstructural and Functional Brain Abnormalities in Multiple Sclerosis Predicted by Osteopontin and Neurofilament Light. Mult. Scler. Relat. Disord. 2021, 51, 102923. [Google Scholar] [CrossRef]
- Krämer, J.; Brück, W.; Zipp, F.; Cerina, M.; Groppa, S.; Meuth, S.G. Imaging in Mice and Men: Pathophysiological Insights into Multiple Sclerosis from Conventional and Advanced MRI Techniques. Prog. Neurobiol. 2019, 182, 161663. [Google Scholar] [CrossRef]
- Katz, D.; Taubenberger, J.K.; Cannella, B.; McFarlin, D.E.; Raine, C.S.; McFarland, H.F. Correlation between Magnetic Resonance Imaging Findings and Lesion Development in Chronic, Active Multiple Sclerosis. Ann. Neurol. 1993, 34, 661–669. [Google Scholar] [CrossRef]
- Willoughby, E.W.; Grochowski, E.; Li, D.K.B.; Oger, J.; Kastrukoff, L.F.; Paty, D.W. Serial Magnetic Resonance Scanning in Multiple Sclerosis: A Second Prospective Study in Relapsing Patients. Ann. Neurol. 1989, 25, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.S.; Weiner, H.L.; Guttmann, C.R.G. Time-Series Modeling of Multiple Sclerosis Disease Activity: A Promising Window on Disease Progression and Repair Potential? Neurother. J. Am. Soc. Exp. NeuroTher. 2007, 4, 485–498. [Google Scholar] [CrossRef]
- Cotton, F.; Weiner, H.L.; Jolesz, F.A.; Guttmann, C.R.G. MRI Contrast Uptake in New Lesions in Relapsing-Remitting MS Followed at Weekly Intervals. Neurology 2003, 60, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E.W. Bovine Lactoferrin Regulates Cell Survival, Apoptosis and Inflammation in Intestinal Epithelial Cells and Preterm Pig Intestine. J. Proteom. 2016, 139, 95–102. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Côté, R.G.; Csordas, A.; Dianes, J.A.; Fabregat, A.; Foster, J.M.; Griss, J.; Alpi, E.; Birim, M.; Contell, J.; et al. The Proteomics Identifications (PRIDE) Database and Associated Tools: Status in 2013. Nucleic Acids Res. 2013, 41, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- de Jong, O.G.; Verhaar, M.C.; Chen, Y.; Vader, P.; Gremmels, H.; Posthuma, G.; Schiffelers, R.M.; Gucek, M.; Van Balkom, B.W. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 2012, 1, 18396. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.M.; Bæk, R.; Varming, K. Potentials and Capabilities of the Extracellular Vesicle (EV) Array. J. Extracell. Vesicles 2015, 4, 26048. [Google Scholar] [CrossRef]
- Revenfeld, A.L.S.; Steffensen, R.; Pugholm, L.H.; Jørgensen, M.M.; Stensballe, A.; Varming, K. Presence of HLA-DR Molecules and HLA-DRB1 MRNA in Circulating CD4 + T Cells. Scand. J. Immunol. 2016, 84, 211–221. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote)Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. MixOmics: An R Package for ‘omics Feature Selection and Multiple Data Integration. PLOS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Liquet, B.; Cao, K.A.L.; Hocini, H.; Thiébaut, R. A Novel Approach for Biomarker Selection and the Integration of Repeated Measures Experiments from Two Assays. BMC Bioinform. 2012, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Anderson, D.E.; Baecher-Allan, C.; Hastings, W.D.; Bettelli, E.; Oukka, M.; Kuchroo, V.K.; Hafler, D.A. IL-21 and TGF-Beta Are Required for Differentiation of Human T(H)17 Cells. Nature 2008, 454, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Swallow, C.J.; Partridge, E.A.; Macmillan, J.C.; Tajirian, T.; DiGuglielmo, G.M.; Hay, K.; Szweras, M.; Jahnen-Dechent, W.; Wrana, J.L.; Redston, M.; et al. Alpha2HS-Glycoprotein, an Antagonist of Transforming Growth Factor Beta in Vivo, Inhibits Intestinal Tumor Progression. Cancer Res. 2004, 64, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
- Bhakuni, T.; Ali, M.F.; Ahmad, I.; Bano, S.; Ansari, S.; Jairajpuri, M.A. Role of Heparin and Non Heparin Binding Serpins in Coagulation and Angiogenesis: A Complex Interplay. Arch. Biochem. Biophys. 2016, 604, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Liu, R.; Tong, H.; Gao, S.; Yang, G.; Hossain, A.; Li, T.; He, W. Fetuin B Overexpression Suppresses Proliferation, Migration, and Invasion in Prostate Cancer by Inhibiting the PI3K/AKT Signaling Pathway. Biomed. Pharmacother. 2020, 131, 110689. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.A.; Rauova, L.; Poncz, M. Role of the Platelet Chemokine Platelet Factor 4 (PF4) in Hemostasis and Thrombosis. Thromb. Res. 2010, 125, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Vieira, P.M.; Yore, M.M.; Sontheimer-Phelps, A.; Castoldi, A.; Norseen, J.; Aryal, P.; Sjödin, K.S.; Kahn, B.B. Retinol Binding Protein 4 Primes the NLRP3 Inflammasome by Signaling through Toll-like Receptors 2 and 4. Proc. Natl. Acad. Sci. USA 2020, 117, 31309–31318. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, E.; Ożyhar, A. Transthyretin: From Structural Stability to Osteoarticular and Cardiovascular Diseases. Cells 2021, 10, 1768. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The Thrombospondins. Int. J. Biochem. Cell Biol. 2004, 36, 961–968. [Google Scholar] [CrossRef]
- Faherty, N.; Curran, S.P.; O’Donovan, H.; Martin, F.; Godson, C.; Brazil, D.P.; Crean, J.K. CCN2/CTGF Increases Expression of MiR-302 Micrornas, Which Target the TGFβ Type II Receptor with Implications for Nephropathic Cell Phenotypes. J. Cell Sci. 2012, 125, 5621–5629. [Google Scholar] [CrossRef]
- Sanchez-Moral, L.; Ràfols, N.; Martori, C.; Paul, T.; Téllez, É.; Sarrias, M.R. Multifaceted Roles of CD5L in Infectious and Sterile Inflammation. Int. J. Mol. Sci. 2021, 22, 4076. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Kern, R.; Thomas, K. Multiple Sclerosis: Clinical Profiling and Data Collection as Prerequisite for Personalized Medicine Approach. BMC Neurol. 2016, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- De Bondt, M.; Hellings, N.; Opdenakker, G.; Struyf, S. Neutrophils: Underestimated Players in the Pathogenesis of Multiple Sclerosis (MS). Int. J. Mol. Sci. 2020, 21, 4558. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zeng, H.; Gao, C. The Role of Neutrophil Extracellular Traps in Central Nervous System Diseases and Prospects for Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021, 9931742. [Google Scholar] [CrossRef] [PubMed]
- Bennike, T.B.; Carlsen, T.G.; Ellingsen, T.; Bonderup, O.K.; Glerup, H.; Bøgsted, M.; Christiansen, G.; Birkelund, S.; Stensballe, A.; Andersen, V. Neutrophil Extracellular Traps in Ulcerative Colitis: A Proteome Analysis of Intestinal Biopsies. Inflamm. Bowel Dis. 2015, 21, 2052–2067. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.N.; Muk, T.; Stensballe, A.; Nguyen, D.N.; Sangild, P.T.; Jiang, P.P. Early Protein Markers of Necrotizing Enterocolitis in Plasma of Preterm Pigs Exposed to Antibiotics. Front. Immunol. 2020, 11, 565862. [Google Scholar] [CrossRef]
- Naegele, M.; Tillack, K.; Reinhardt, S.; Schippling, S.; Martin, R.; Sospedra, M. Neutrophils in Multiple Sclerosis Are Characterized by a Primed Phenotype. J. Neuroimmunol. 2012, 242, 60–71. [Google Scholar] [CrossRef]
- Bisgaard, A.K.; Pihl-Jensen, G.; Frederiksen, J.L. The Neutrophil-to-Lymphocyte Ratio as Disease Actvity Marker in Multiple Sclerosis and Optic Neuritis. Mult. Scler. Relat. Disord. 2017, 18, 213–217. [Google Scholar] [CrossRef]
- Orian, J.M.; D’Souza, C.S.; Kocovski, P.; Krippner, G.; Hale, M.W.; Wang, X.; Peter, K. Platelets in Multiple Sclerosis: Early and Central Mediators of Inflammation and Neurodegeneration and Attractive Targets for Molecular Imaging and Site-Directed Therapy. Front. Immunol. 2021, 12, 620963. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-Producing CD4+ Effector T Cells Develop via a Lineage Distinct from the T Helper Type 1 and 2 Lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 Lymphocytes Promote Blood-Brain Barrier Disruption and Central Nervous System Inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-Microarray Analysis of Multiple Sclerosis Lesions Yields New Targets Validated in Autoimmune Encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Anderson, A.C.; Bettelli, E.; Oukka, M. The Dynamics of Effector T Cells and Foxp3+ Regulatory T Cells in the Promotion and Regulation of Autoimmune Encephalomyelitis. J. Neuroimmunol. 2007, 191, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 Production in Central Nervous System-Infiltrating T Cells and Glial Cells Is Associated with Active Disease in Multiple Sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.C.; Hygino, J.; Ferreira, T.B.; Kasahara, T.M.; Barros, P.O.; Sacramento, P.M.; Andrade, R.M.; Camargo, S.; Rueda, F.; Alves-Leon, S.V.; et al. Interleukin-17- and Interleukin-22-Secreting Myelin-Specific CD4(+) T Cells Resistant to Corticoids Are Related with Active Brain Lesions in Multiple Sclerosis Patients. Immunology 2016, 147, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.K.; Wang, L.; Han, P.; Zhang, X.; Ekholm, S.; Srinivasan, A.; Irani, D.N.; Segal, B.M. Dysregulation of the IL-23/IL-17 Axis and Myeloid Factors in Secondary Progressive MS. Neurology 2014, 83, 1500. [Google Scholar] [CrossRef] [PubMed]
- Durelli, L.; Conti, L.; Clerico, M.; Boselli, D.; Contessa, G.; Ripellino, P.; Ferrero, B.; Eid, P.; Novelli, F. T-Helper 17 Cells Expand in Multiple Sclerosis and Are Inhibited by Interferon-Beta. Ann. Neurol. 2009, 65, 499–509. [Google Scholar] [CrossRef]
- Carnero Contentti, E.; Farez, M.F.; Correale, J. Mucosal-Associated Invariant T Cell Features and TCR Repertoire Characteristics During the Course of Multiple Sclerosis. Front. Immunol. 2019, 10, 2690. [Google Scholar] [CrossRef]
- Illés, Z.; Shimamura, M.; Newcombe, J.; Oka, N.; Yamamura, T. Accumulation of Vα7.2–Jα33 Invariant T Cells in Human Autoimmune Inflammatory Lesions in the Nervous System. Int. Immunol. 2004, 16, 223–230. [Google Scholar] [CrossRef]
- Haas, J.; Schneider, K.; Schwarz, A.; Korporal-Kuhnke, M.; Faller, S.; Von Glehn, F.; Jarius, S.; Wildemann, B. Th17 Cells: A Prognostic Marker for MS Rebound after Natalizumab Cessation? Mult. Scler. 2017, 23, 114–118. [Google Scholar] [CrossRef]
- Bühler, U.; Fleischer, V.; Luessi, F.; Rezk, A.; Belikan, P.; Graetz, C.; Gollan, R.; Wolf, C.; Lutz, J.; Bar-Or, A.; et al. Role of IL-17-Producing Lymphocytes in Severity of Multiple Sclerosis upon Natalizumab Treatment. Mult. Scler. 2017, 23, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Havrdová, E.; Belova, A.; Goloborodko, A.; Tisserant, A.; Wright, A.; Wallstroem, E.; Garren, H.; Maguire, R.P.; Johns, D.R. Activity of Secukinumab, an Anti-IL-17A Antibody, on Brain Lesions in RRMS: Results from a Randomized, Proof-of-Concept Study. J. Neurol. 2016, 263, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Brucklacher-Waldert, V.; Stuerner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and Functional Characterization of T Helper 17 Cells in Multiple Sclerosis. Brain J. Neurol. 2009, 132, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, A.F.; Abbas, A.R.; Jeet, S.; Wong, K.; Bischof, A.; Peng, I.; Lee, J.; Bremer, M.; Eggers, E.L.; DeVoss, J.; et al. IL-17A Is Associated with the Breakdown of the Blood-Brain Barrier in Relapsing-Remitting Multiple Sclerosis. J. Neuroimmunol. 2019, 332, 147–154. [Google Scholar] [CrossRef]
- Bai, Z.; Chen, D.; Wang, L.; Zhao, Y.; Liu, T.; Yu, Y.; Yan, T.; Cheng, Y. Cerebrospinal Fluid and Blood Cytokines as Biomarkers for Multiple Sclerosis: A Systematic Review and Meta-Analysis of 226 Studies With 13,526 Multiple Sclerosis Patients. Front. Neurosci. 2019, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector TH17 and Regulatory T Cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Bennett, J.L.; de Seze, J.; Haramura, M.; Kleiter, I.; Weinshenker, B.G.; Kang, D.; Mughal, T.; Yamamura, T. Interleukin-6 in Neuromyelitis Optica Spectrum Disorder Pathophysiology. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, 5. [Google Scholar] [CrossRef]
- Baranzini, S.E.; Elfstrom, C.; Chang, S.-Y.; Butunoi, C.; Murray, R.; Higuchi, R.; Oksenberg, J.R. Transcriptional Analysis of Multiple Sclerosis Brain Lesions Reveals a Complex Pattern of Cytokine Expression. J. Immunol. 2000, 165, 6576–6582. [Google Scholar] [CrossRef]
- De Flon, P.; Söderström, L.; Laurell, K.; Dring, A.; Sundström, P.; Gunnarsson, M.; Svenningsson, A. Immunological Profile in Cerebrospinal Fluid of Patients with Multiple Sclerosis after Treatment Switch to Rituximab and Compared with Healthy Controls. PLoS ONE 2018, 13, 192516. [Google Scholar] [CrossRef]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and Type 17 Helper T Cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef]
- Hauser, S.L.; Doolittle, T.H.; Lincoln, R.; Brown, R.H.; Dinarello, C.A. Cytokine Accumulations in CSF of Multiple Sclerosis Patients: Frequent Detection of Interleukin-1 and Tumor Necrosis Factor but Not Interleukin-6. Neurology 1990, 40, 1735–1739. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Depino, A.M.; Prada, F.; Muraro, N.; Campbell, S.; Podhajcer, O.; Perry, V.H.; Anthony, D.C.; Pitossi, F.J. Reversible Demyelination, Blood-Brain Barrier Breakdown, and Pronounced Neutrophil Recruitment Induced by Chronic IL-1 Expression in the Brain. Am. J. Pathol. 2004, 165, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Hofman, F.M.; Hinton, D.R.; Johnson, K.; Merrill, J.E. Tumor Necrosis Factor Identified in Multiple Sclerosis Brain. J. Exp. Med. 1989, 170, 607–612. [Google Scholar] [CrossRef]
- Cannella, B.; Raine, C.S. The Adhesion Molecule and Cytokine Profile of Multiple Sclerosis Lesions. Ann. Neurol. 1995, 37, 424–435. [Google Scholar] [CrossRef]
- Seppi, D.; Puthenparampil, M.; Federle, L.; Ruggero, S.; Toffanin, E.; Rinaldi, F.; Perini, P.; Gallo, P. Cerebrospinal Fluid IL-1β Correlates with Cortical Pathology Load in Multiple Sclerosis at Clinical Onset. J. Neuroimmunol. 2014, 270, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Studer, V.; Motta, C.; Germani, G.; Macchiarulo, G.; Buttari, F.; Mancino, R.; Castelli, M.; De Chiara, V.; Weiss, S.; et al. Cerebrospinal Fluid Detection of Interleukin-1β in Phase of Remission Predicts Disease Progression in Multiple Sclerosis. J. Neuroinflamm. 2014, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Ashbaugh, J.J.; Magliozzi, R.; Dellarole, A.; Karmally, S.; Szymkowski, D.E.; Bethea, J.R. Inhibition of Soluble Tumour Necrosis Factor Is Therapeutic in Experimental Autoimmune Encephalomyelitis and Promotes Axon Preservation and Remyelination. Brain J. Neurol. 2011, 134, 2736–2754. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.L.; Nawrocki, A.; Kacprowski, T.; Lassen, P.; Simonsen, A.H.; Marignier, R.; Sejbaek, T.; Nielsen, H.H.; Wermuth, L.; Rashid, A.Y.; et al. CSF Proteome in Multiple Sclerosis Subtypes Related to Brain Lesion Transcriptomes. Sci. Rep. 2021, 11, 4132. [Google Scholar] [CrossRef]
- Ramos, T.N.; Bullard, D.C.; Barnum, S.R. ICAM-1: Isoforms and Phenotypes. J. Immunol. 2014, 192, 4469–4474. [Google Scholar] [CrossRef]
- Selmaj, I.; Mycko, M.P.; Raine, C.S.; Selmaj, K.W. The Role of Exosomes in CNS Inflammation and Their Involvement in Multiple Sclerosis. J. Neuroimmunol. 2017, 306, 1–10. [Google Scholar] [CrossRef]
- Chu, A.J. Tissue Factor, Blood Coagulation, and Beyond: An Overview. Int. J. Inflamm. 2011, 2011, 367284. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A.; Jy, W.; Jimenez, J.J.; Sheremata, W.A.; Mauro, L.M.; Mao, W.W.; Horstman, L.L.; Ahn, Y.S. Elevated Plasma Endothelial Microparticles in Multiple Sclerosis. Neurology 2001, 56, 1319–1324. [Google Scholar] [CrossRef] [PubMed]



| Marker | Correlation with the number of | |||
|---|---|---|---|---|
| New Gd+ Lesions | New FLAIR Lesions | Total Gd+ Lesions | Lesions with Maximum Gd+ | |
| Tie 2 | −0.56079 *** | −0.59616 *** | −0.74088 *** | −0.63491 *** |
| VEGF-C | 0.35236 * | −0.36838 * | ||
| VEGF-D | −0.38390 * | −0.42184 * | −0.36100 * | −0.34755 * |
| sFLT-1 | −0.46322 ** | −0.42402 ** | ||
| CCL11, eotaxin | −0.41500 ** | −0.31424 * | ||
| CCL2, MCP-1 | −0.32893 * | |||
| CCL13, MCP-4 | −0.44751 ** | −0.36185 * | ||
| CCL22, MDC | −0.43846 ** | −0.33729 * | ||
| CCL3, MIP-1a | −0.39215 * | −0.42601 * | −0.54656 *** | −0.40649 ** |
| CCL4, MIP-1b | −0.34290 * | −0.37318 * | −0.48321 ** | −0.40504 ** |
| CCL17, TARC | −0.38653 * | −0.40675 * | −0.55210 ** | −0.39932 * |
| sICAM-1 | −0.36958 * | −0.40194 * | −0.49975 ** | −0.39018 * |
| sVCAM-1 | −0.35616 * | −0.36749 * | −0.35089 * | |
| IL-15 | −0.37681 * | −0.40334 * | −0.46042 ** | −0.46021 ** |
| IL-16 | −0.35487 * | −0.36831 * | −0.48427 ** | −0.39520 * |
| TNF-a | −0.36675 * | −0.38338 * | −0.59531 *** | −0.49169 ** |
| IL-17 | 0.64576 *** | 0.71139 *** | 0.68207 *** | 0.47769 ** |
| IL-1b | 0.47589 ** | 0.52346 *** | 0.31339 * | |
| IL-6 | 0.35851 * | |||
| EV-LAMP2 | −0.40376 ** | |||
| EV-TSG101 | 0.34669 * | 0.35821 * | ||
| EV-ICAM1 | 0.65587 *** | 0.57176 *** | 0.57701 *** | 0.73735 *** |
| Biomarker | Most important a | 2nd Most Important a | 3rd Most Important a |
|---|---|---|---|
| CCL17 (TARC) | 10 | 5 | 3 |
| CCL3 (MIP-1a) | 6 | 8 | 4 |
| IL-17 | 2 | 5 | 7 |
| TNF-a | 0 | 0 | 4 |
| Model (Number of Lesions Types) | R-Squared a | RMSE (MSE) |
|---|---|---|
| New Gd+ lesions | 0.015 | 3.585 |
| New FLAIR lesions | 0.245 | 2.868 |
| Total Gd+ lesions | 0.0197 | 21.478 |
| Lesions with max Gd intensity | 0.085 | 11.511 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illes, Z.; Jørgensen, M.M.; Bæk, R.; Bente, L.-M.; Lauridsen, J.T.; Hyrlov, K.H.; Aboo, C.; Baumbach, J.; Kacprowski, T.; Cotton, F.; et al. New Enhancing MRI Lesions Associate with IL-17, Neutrophil Degranulation and Integrin Microparticles: Multi-Omics Combined with Frequent MRI in Multiple Sclerosis. Biomedicines 2023, 11, 3170. https://doi.org/10.3390/biomedicines11123170
Illes Z, Jørgensen MM, Bæk R, Bente L-M, Lauridsen JT, Hyrlov KH, Aboo C, Baumbach J, Kacprowski T, Cotton F, et al. New Enhancing MRI Lesions Associate with IL-17, Neutrophil Degranulation and Integrin Microparticles: Multi-Omics Combined with Frequent MRI in Multiple Sclerosis. Biomedicines. 2023; 11(12):3170. https://doi.org/10.3390/biomedicines11123170
Chicago/Turabian StyleIlles, Zsolt, Malene Møller Jørgensen, Rikke Bæk, Lisa-Marie Bente, Jørgen T. Lauridsen, Kirsten H. Hyrlov, Christopher Aboo, Jan Baumbach, Tim Kacprowski, Francois Cotton, and et al. 2023. "New Enhancing MRI Lesions Associate with IL-17, Neutrophil Degranulation and Integrin Microparticles: Multi-Omics Combined with Frequent MRI in Multiple Sclerosis" Biomedicines 11, no. 12: 3170. https://doi.org/10.3390/biomedicines11123170









