Identification and Characterization of Retinitis Pigmentosa in a Novel Mouse Model Caused by PDE6B-T592I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fundus Examination and Histology
2.3. Electroretinography
2.4. DNA Sequencing
2.5. Western Blot Analysis
2.6. Immunohistochemistry
2.7. Generation of rAAV-hRho-PDE6B and Subretinal Injection
3. Results
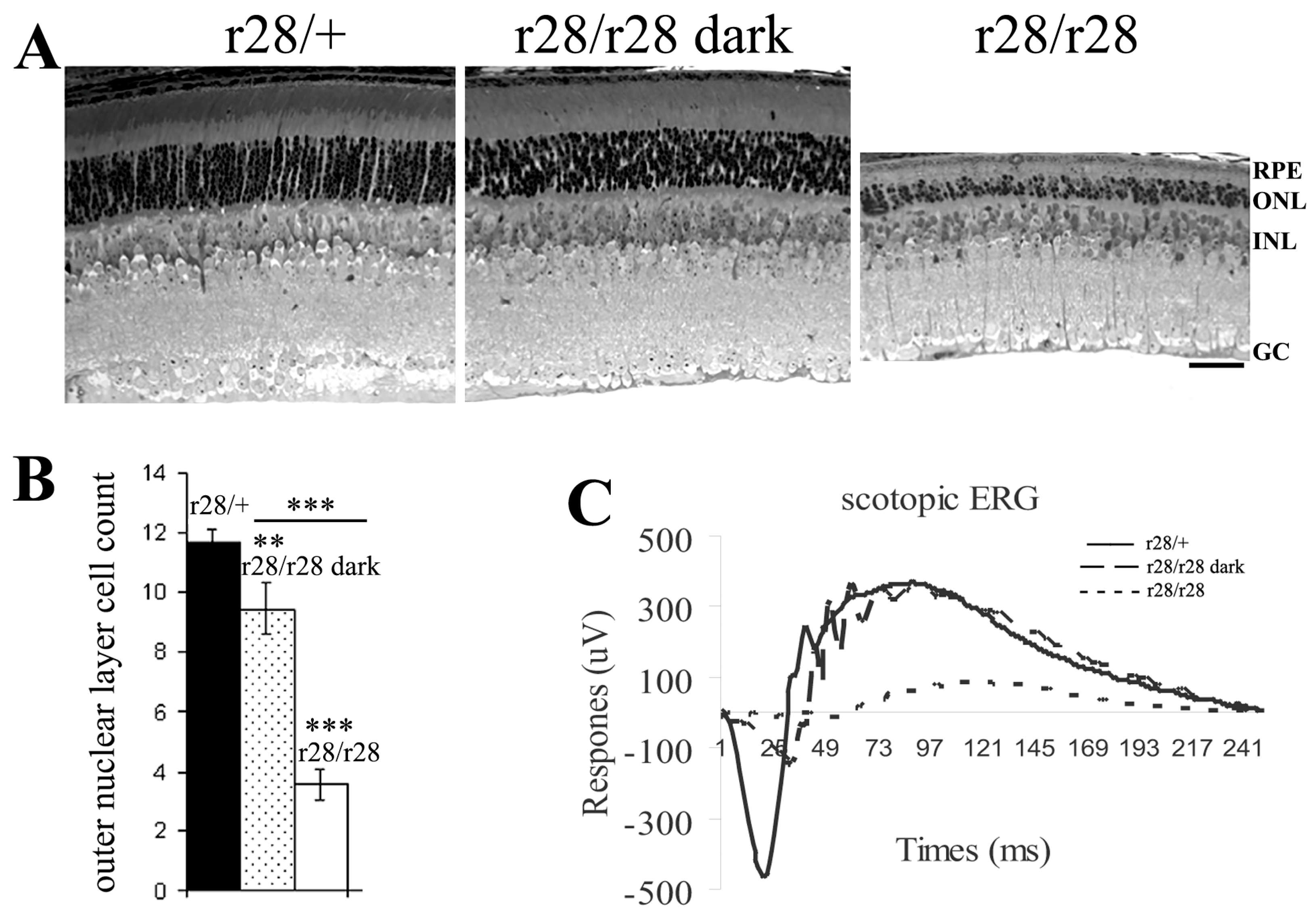
3.1. Loss of Photoreceptor Cells in the r28 Mutant Mice
3.2. Dark Rearing Rescues Photoreceptor Cell Loss in the r28 Mutant Mice
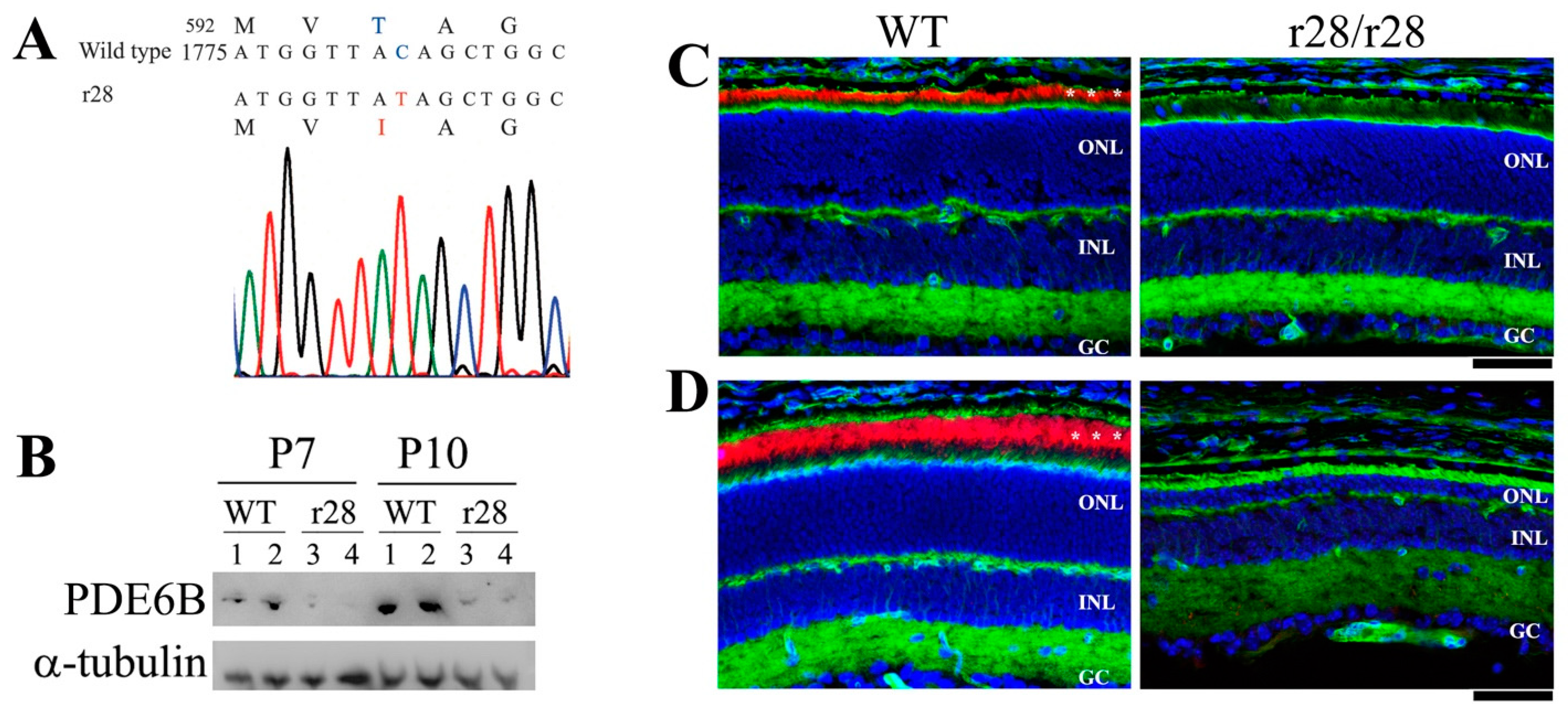
3.3. A Pde6b Point Mutation Leads to Mutant PDE6B-T592I Protein in the r28 Mutant Mice
3.4. Subretinal Injection of rAAV-hRho-wt-PDE6B Rescues Photoreceptor Cell Loss in the r28 Mutant Mice
3.5. The T592I Mutation in the r28 Mutant Mice Disrupts the Hydrogen Bond Formation between T592-Y524 and T592-Y645
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaumet-Riffaud, A.E.; Chaumet-Riffaud, P.; Cariou, A.; Devisme, C.; Audo, I.; Sahel, J.A.; Mohand-Said, S. Impact of Retinitis Pigmentosa on Quality of Life, Mental Health, and Employment Among Young Adults. Am. J. Ophthalmol. 2017, 177, 169–174. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- O’Neal, T.B.; Luther, E.E. Retinitis Pigmentosa. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Cross, N.; van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Retinitis Pigmentosa: Burden of Disease and Current Unmet Needs. Clin. Ophthalmol. 2022, 16, 1993–2010. [Google Scholar] [CrossRef]
- McLaughlin, M.E.; Sandberg, M.A.; Berson, E.L.; Dryja, T.P. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat. Genet. 1993, 4, 130–134. [Google Scholar] [CrossRef]
- McLaughlin, M.E.; Ehrhart, T.L.; Berson, E.L.; Dryja, T.P. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 1995, 92, 3249–3253. [Google Scholar] [CrossRef]
- Farber, D.B. From mice to men: The cyclic GMP phosphodiesterase gene in vision and disease. The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 1995, 36, 263–275. [Google Scholar]
- Bowes, C.; Li, T.; Danciger, M.; Baxter, L.C.; Applebury, M.L.; Farber, D.B. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature 1990, 347, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Pittler, S.J.; Baehr, W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc. Natl. Acad. Sci. USA 1991, 88, 8322–8326. [Google Scholar] [CrossRef] [PubMed]
- Keeler, C.E. On the Occurrence in the House Mouse of Mendelizing Structural Defect of the Retina Producing Blindness. Proc. Natl. Acad. Sci. USA 1926, 12, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Cote, R.H. Characteristics of photoreceptor PDE (PDE6): Similarities and differences to PDE5. Int. J. Impot. Res. 2004, 16, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Cote, R.H. The catalytic and GAF domains of the rod cGMP phosphodiesterase (PDE6) heterodimer are regulated by distinct regions of its inhibitory gamma subunit. J. Biol. Chem. 2001, 276, 27527–27534. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Palczewski, K.; Engel, A.; Stahlberg, H.; Kovacik, L. Cryo-EM structure of phosphodiesterase 6 reveals insights into the allosteric regulation of type I phosphodiesterases. Sci. Adv. 2019, 5, eaav4322. [Google Scholar] [CrossRef] [PubMed]
- Pugh, E.N., Jr.; Lamb, T.D. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1993, 1141, 111–149. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Rohrer, B. Sustained elevation of intracellular cGMP causes oxidative stress triggering calpain-mediated apoptosis in photoreceptor degeneration. Curr. Eye Res. 2007, 32, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.Y.; Lewis, A.; Barabas, P.; Stearns, G.; Suzuki, S.; Krizaj, D.; Brockerhoff, S.E. Loss of Pde6 reduces cell body Ca(2+) transients within photoreceptors. Cell Death Dis. 2013, 4, e797. [Google Scholar] [CrossRef]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Chang, B.; Hawes, N.L.; Pardue, M.T.; German, A.M.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Rengarajan, K.; Boyd, A.P.; Sidney, S.S.; et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vis. Res. 2007, 47, 624–633. [Google Scholar] [CrossRef]
- Wang, T.; Reingruber, J.; Woodruff, M.L.; Majumder, A.; Camarena, A.; Artemyev, N.O.; Fain, G.L.; Chen, J. The PDE6 mutation in the rd10 retinal degeneration mouse model causes protein mislocalization and instability and promotes cell death through increased ion influx. J. Biol. Chem. 2018, 293, 15332–15346. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.P.; Strauss, R.W.; Singh, M.S.; Dalkara, D.; Roska, B.; Picaud, S.; Sahel, J.A. Emerging therapies for inherited retinal degeneration. Sci. Transl. Med. 2016, 8, 368rv366. [Google Scholar] [CrossRef] [PubMed]
- Kalloniatis, M.; Nivison-Smith, L.; Chua, J.; Acosta, M.L.; Fletcher, E.L. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: A review. Exp. Eye Res. 2016, 150, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Federico, S.; Relitti, N.; Gemma, S.; Butini, S.; Campiani, G. Retinitis Pigmentosa and Retinal Degenerations: Deciphering Pathways and Targets for Drug Discovery and Development. ACS Chem. Neurosci. 2020, 11, 2173–2191. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Tabeta, K.; Hoebe, K.; Liu, H.; Mann, N.; Mudd, S.; Crozat, K.; Sovath, S.; Gong, X.; Beutler, B. Velvet, a dominant Egfr mutation that causes wavy hair and defective eyelid development in mice. Genetics 2004, 166, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.H.; Liu, H.; Cheung, D.; Wang, M.; Cheng, C.; Du, X.; Chang, B.; Beutler, B.; Gong, X. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum. Mol. Genet. 2008, 17, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, M.; Xia, C.H.; Du, X.; Flannery, J.G.; Ridge, K.D.; Beutler, B.; Gong, X. Severe retinal degeneration caused by a novel rhodopsin mutation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.H.; Lu, E.; Liu, H.; Du, X.; Beutler, B.; Gong, X. The role of Vldlr in intraretinal angiogenesis in mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6572–6579. [Google Scholar] [CrossRef]
- Xia, C.H.; Ferguson, I.; Li, M.; Kim, A.; Onishi, A.; Li, L.; Su, B.; Gong, X. Essential function of NHE8 in mouse retina demonstrated by AAV-mediated CRISPR/Cas9 knockdown. Exp. Eye Res. 2018, 176, 29–39. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Bateman, J.B.; Klisak, I.; Kojis, T.; Mohandas, T.; Sparkes, R.S.; Li, T.S.; Applebury, M.L.; Bowes, C.; Farber, D.B. Assignment of the beta-subunit of rod photoreceptor cGMP phosphodiesterase gene PDEB (homolog of the mouse rd gene) to human chromosome 4p16. Genomics 1992, 12, 601–603. [Google Scholar] [CrossRef]
- Han, J.; Dinculescu, A.; Dai, X.; Du, W.; Smith, W.C.; Pang, J. Review: The history and role of naturally occurring mouse models with Pde6b mutations. Mol. Vis. 2013, 19, 2579–2589. [Google Scholar]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Horn, G.; Hebert, E.J.; Bechert, J.; Shaw, K.; Urbanikova, L.; Scholtz, J.M.; Sevcik, J. Tyrosine hydrogen bonds make a large contribution to protein stability. J. Mol. Biol. 2001, 312, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Weh, E.; Scott, K.; Wubben, T.J.; Besirli, C.G. Dark-reared rd10 mice experience rapid photoreceptor degeneration with short exposure to room-light during in vivo retinal imaging. Exp. Eye Res. 2022, 215, 108913. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Manfredi, A.; Iodice, C.; Di Vicino, U.; Auricchio, A. AAV-mediated gene replacement, either alone or in combination with physical and pharmacological agents, results in partial and transient protection from photoreceptor degeneration associated with betaPDE deficiency. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, S.K.; Rana, P.; West, E.R.; Hong, C.M.; Feng, H.; Wu, D.M.; Cepko, C.L. AAV-Txnip prolongs cone survival and vision in mouse models of retinitis pigmentosa. eLife 2021, 10, e66240. [Google Scholar] [CrossRef]
- Lem, J.; Flannery, J.G.; Li, T.; Applebury, M.L.; Farber, D.B.; Simon, M.I. Retinal degeneration is rescued in transgenic rd mice by expression of the cGMP phosphodiesterase beta subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 4422–4426. [Google Scholar] [CrossRef]
- Flannery, J.G.; Visel, M. Adeno-associated viral vectors for gene therapy of inherited retinal degenerations. Methods Mol. Biol. 2013, 935, 351–369. [Google Scholar]
- Pellissier, L.P.; Quinn, P.M.; Alves, C.H.; Vos, R.M.; Klooster, J.; Flannery, J.G.; Heimel, J.A.; Wijnholds, J. Gene therapy into photoreceptors and Muller glial cells restores retinal structure and function in CRB1 retinitis pigmentosa mouse models. Hum. Mol. Genet. 2015, 24, 3104–3118. [Google Scholar] [CrossRef]
- Mowat, F.M.; Occelli, L.M.; Bartoe, J.T.; Gervais, K.J.; Bruewer, A.R.; Querubin, J.; Dinculescu, A.; Boye, S.L.; Hauswirth, W.W.; Petersen-Jones, S.M. Gene Therapy in a Large Animal Model of PDE6A-Retinitis Pigmentosa. Front. Neurosci. 2017, 11, 342. [Google Scholar] [CrossRef]
- Thompson, D.A.; Ali, R.R.; Banin, E.; Branham, K.E.; Flannery, J.G.; Gamm, D.M.; Hauswirth, W.W.; Heckenlively, J.R.; Iannaccone, A.; Jayasundera, K.T.; et al. Advancing therapeutic strategies for inherited retinal degeneration: Recommendations from the Monaciano Symposium. Investig. Ophthalmol. Vis. Sci. 2015, 56, 918–931. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, C.-H.; Liu, H.; Li, M.; Zhang, H.; Xing, X.; Gong, X. Identification and Characterization of Retinitis Pigmentosa in a Novel Mouse Model Caused by PDE6B-T592I. Biomedicines 2023, 11, 3173. https://doi.org/10.3390/biomedicines11123173
Xia C-H, Liu H, Li M, Zhang H, Xing X, Gong X. Identification and Characterization of Retinitis Pigmentosa in a Novel Mouse Model Caused by PDE6B-T592I. Biomedicines. 2023; 11(12):3173. https://doi.org/10.3390/biomedicines11123173
Chicago/Turabian StyleXia, Chun-Hong, Haiquan Liu, Mei Li, Haiwei Zhang, Xinfang Xing, and Xiaohua Gong. 2023. "Identification and Characterization of Retinitis Pigmentosa in a Novel Mouse Model Caused by PDE6B-T592I" Biomedicines 11, no. 12: 3173. https://doi.org/10.3390/biomedicines11123173



