Synergy of Muscle and Cortical Activation through Vojta Reflex Locomotion Therapy in Young Healthy Adults: A Pilot Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants Recruitment and Eligibility Criteria
2.3. Allocation and Randomization
2.4. Blinding
2.5. Procedure
- 10 min of absolute rest without recording.
- 1 min of rest prior to stimulation with EMG and fNIRS recording (R1).
- 4 min of right-sided styling stimulation with EMG and fNIRS recording (S1).
- 1 min of rest with EMG and fNIRS (R2).
- 4 min of stimulation of the left side with EMG recording and fNIRS (S2).
- 1 min of rest with EMG recording (R3).
- 10 min absolute rest without EMG or fNIRS recording.
2.6. Tactile Stimuli Location and Sham Stimuli Location
2.7. Electromyographic Recording (rEMG) and Cerebral Oxygenation Recording (rCO)
2.8. rEMG and rCO Data Analysis
2.9. Statistical Analysis
3. Results
3.1. Sociodemographic Data
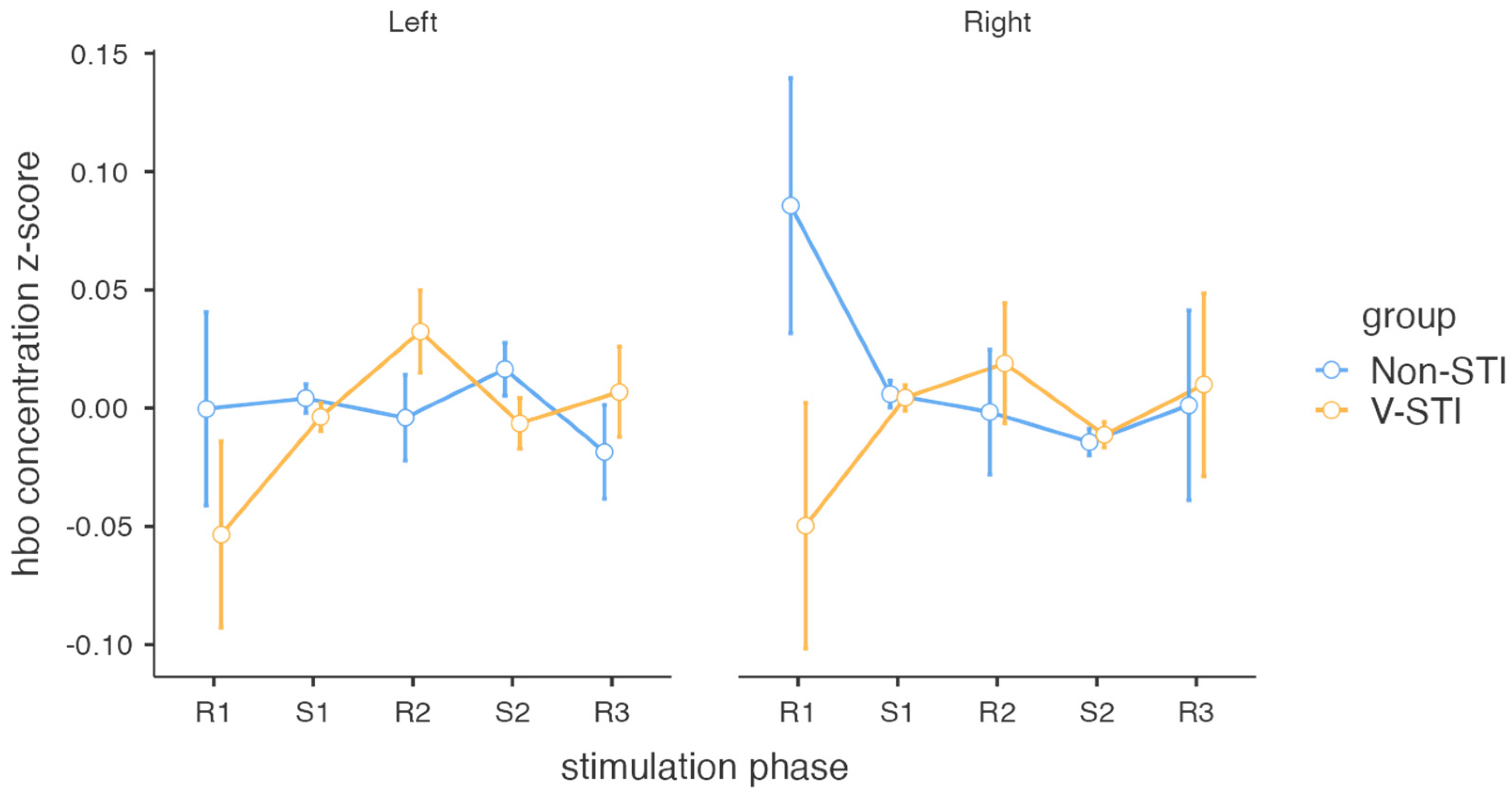
3.2. Intervention Effects on Cortical Activity
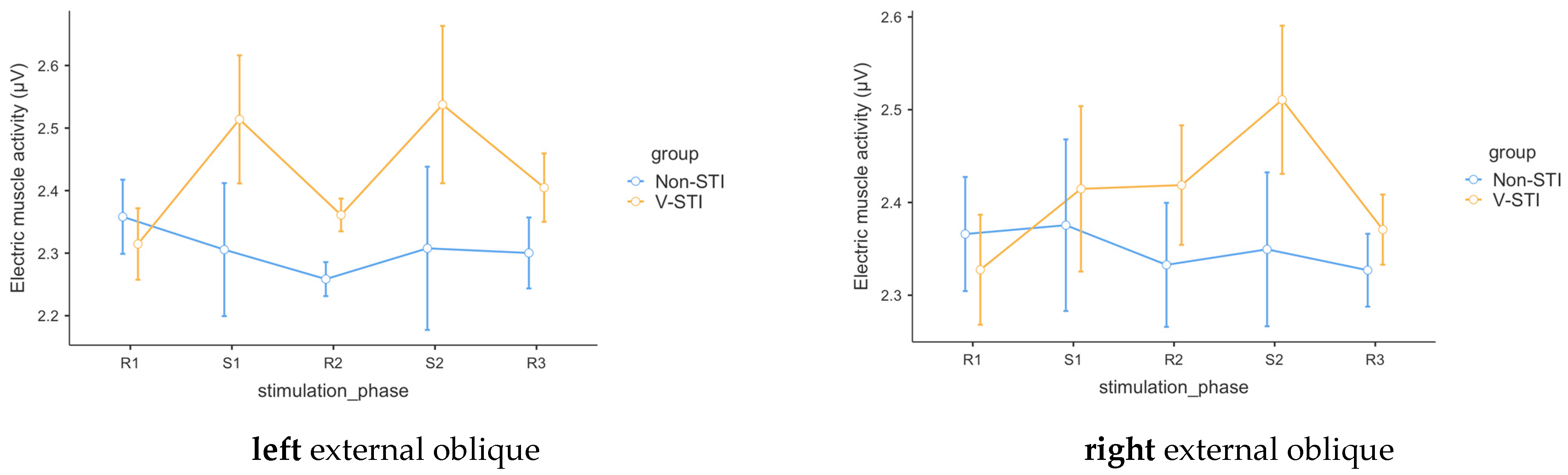
3.3. Intervention Effects on Muscle Activity
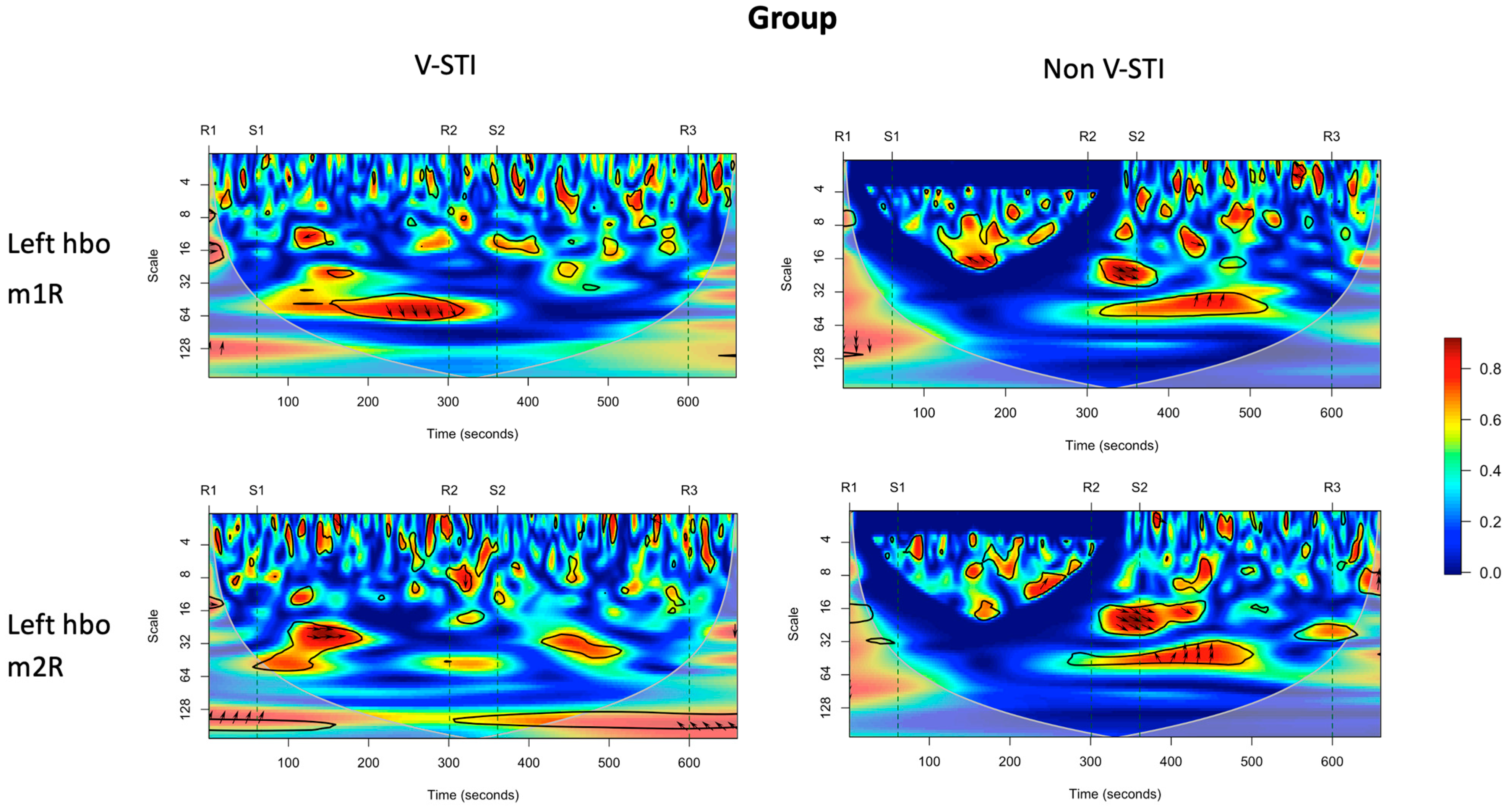
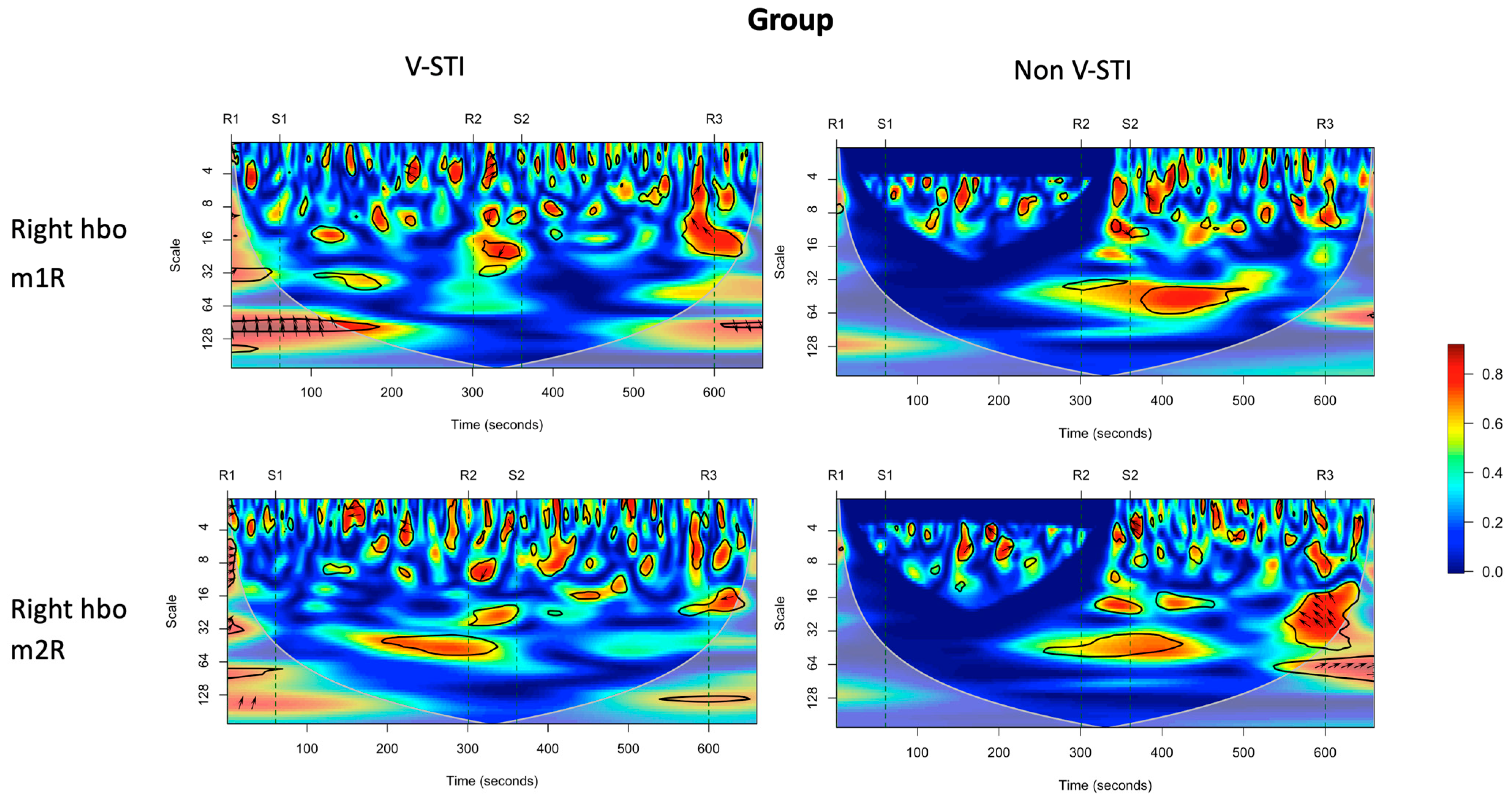
3.4. Intervention Effects on Coherence of Cortical Activity and Muscle Activity
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vojta, V. Reflex rotation as a pathway to human locomotion. Z. Orthop. Ihre Grenzgeb. 1970, 108, 446–452. [Google Scholar]
- Sanz-Mengibar, J.M.; Menendez-Pardiñas, M.; Santonja-Medina, F. Is the implementation of Vojta therapy associated with faster gross motor development in children with cerebral palsy? Clin. Neurosci. 2021, 74, 329–336. [Google Scholar] [CrossRef]
- Vojta, V.; Peters, A. The Vojta Principle; Springer: Berlin, Germany, 2007. [Google Scholar]
- Ha, S.-Y.; Sung, Y.-H. Effects of Vojta method on trunk stability in healthy individuals. J. Exerc. Rehabil. 2016, 12, 542–547. [Google Scholar] [CrossRef]
- Jung, M.W.; Landenberger, M.; Jung, T.; Lindenthal, T.; Philippi, H. Vojta therapy and neurodevelopmental treatment in children with infantile postural asymmetry: A randomised controlled trial. J. Phys. Ther. Sci. 2017, 29, 301–306. [Google Scholar] [CrossRef]
- Perales López, L.; Pérez Gorricho, A.M.; Atin, M.A.; Varela, E. Efecto de la terapia Vojta en la rehabilitación de la marcha en dos pacientes adultos con daño cerebral adquirido en fase tardía. Fisioterapia 2009, 31, 151–162. [Google Scholar] [CrossRef]
- Belda-Lois, J.-M.; Mena-del Horno, S.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; Iosa, M.; Molinari, M.; Tamburella, F.; Ramos, A.; et al. Rehabilitation of gait after stroke: A review towards a top-down approach. J. NeuroEng. Rehabil. 2011, 8, 66. [Google Scholar] [CrossRef]
- Epple, C.; Maurer-Burkhard, B.; Lichti, M.-C.; Steiner, T. Vojta therapy improves postural control in very early stroke rehabilitation: A randomised controlled pilot trial. Neurol. Res. Pract. 2020, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.P.; Palmero, N.V.; Ruano, L.G.; San Leon Pascual, C.; Orile, P.W.; Down, A.V.; Gor Garcia-Fogeda, M.D.; Toré, S. The implementation of a reflex locomotion program according to Vojta produces short-term automatic postural control changes in patients with multiple sclerosis. J. Bodyw. Mov. Ther. 2021, 26, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Pavlikova, M.; Cattaneo, D.; Jonsdottir, J.; Gervasoni, E.; Stetkarova, I.; Angelova, G.; Markova, M.; Prochazkova, M.; Prokopiusova, T.; Hruskova, N.; et al. The impact of balance specific physiotherapy, intensity of therapy and disability on static and dynamic balance in people with multiple sclerosis: A multi-center prospective study. Mult. Scler. Relat. Disord. 2020, 40, 101974. [Google Scholar] [CrossRef]
- Carratalá-Tejada, M.; Cuesta-Gómez, A.; Ortiz-Gutiérrez, R.; Molina-Rueda, F.; Luna-Oliva, L.; Miangolarra-Page, J.C. Reflex Locomotion Therapy for Balance, Gait, and Fatigue Rehabilitation in Subjects with Multiple Sclerosis. J. Clin. Med. 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Appaji, G.; Mundt, D. VOJTA neurophysiologic therapy. Indian J. Pediatr. 1992, 59, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, T. Effects of Vojta Therapy on Gait of Children with Spastic Diplegia. J. Phys. Ther. Sci. 2013, 25, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Huber, J.; Kulczyk, A.; Lipiec, J.; Sobieska, M. An attempt to explain the Vojta therapy mechanism of action using the surface polyelectromyography in healthy subjects: A pilot study. J. Bodyw. Mov. Ther. 2018, 22, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Esteban, I.; Calvo-Lobo, C.; Ríos-Lago, M.; Álvarez-Linera, J.; Muñoz-García, D.; Rodríguez-Sanz, D. Mapping the human brain during a specific Vojta’s tactile input. Medicine 2018, 97, e0253. [Google Scholar] [CrossRef]
- Sanz-Esteban, I.; Cano-de-la-Cuerda, R.; San-Martín-Gómez, A.; Jiménez-Antona, C.; Monge-Pereira, E.; Estrada-Barranco, C.; Serrano, J.I. Cortical activity during sensorial tactile stimulation in healthy adults through Vojta therapy. A randomized pilot controlled trial. J. NeuroEng. Rehabil. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Laufens, G.; Poltz, W.; Reimann, G.; Seitz, S. Physiologische Mechanismen bei der Vojta-Physiotherapie an MS-Patienten. Phys. Med. Rehabil. Kurortmed. 1994, 4, 1–4. [Google Scholar] [CrossRef]
- Laufens, G.; Poltz, W.; Prinz, E.; Reimann, G.; Schmiegelt, F. Alternierende Laufband-Vojta-Laufband-Therapie bei stark gehbehinderten Patienten mit multipler Sklerose. Phys. Med. Rehabil. Kurortmed. 2004, 14, 134–139. [Google Scholar] [CrossRef]
- Pérez-Robledo, F.; Sánchez-González, J.L.; Bermejo-Gil, B.M.; Llamas-Ramos, R.; Llamas-Ramos, I.; de la Fuente, A.; Martín-Nogueras, A.M. Electromyographic Response of the Abdominal Muscles and Stabilizers of the Trunk to Reflex Locomotion Therapy (RLT). A Preliminary Study. J. Clin. Med. 2022, 11, 3866. [Google Scholar] [CrossRef]
- Laufens, G.; Poltz, W.; Jügelt, E.; Prinz, E.; Reimann, G.; van Slobbe, T. Motorische Verbesserungen durch Vojta-Physiotherapie bei Patienten mit multipler Sklerose und der Einfluß von Behandlungspositionen. Phys. Med. Rehabil. Kurortmed. 1995, 5, 115–119. [Google Scholar] [CrossRef]
- Sanz-Esteban, I.; Cano-de-la-Cuerda, R.; San-Martin-Gomez, A.; Jimenez-Antona, C.; Monge-Pereira, E.; Estrada-Barranco, C.; Garcia-Sanchez, P.C.; Serrano, J.I. Innate Muscle Patterns Reproduction During Afferent Somatosensory Input with Vojta Therapy in Healthy Adults. A Randomized Controlled Trial. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2232–2241. [Google Scholar] [CrossRef]
- Perales-López, L.; Fernández-Aceñero, M.J. ¿Es transferible la terapia de locomoción refleja a una plataforma de teleneurorrehabilitación en el tratamiento del paciente adulto? Rehabilitación 2013, 47, 205–212. [Google Scholar] [CrossRef]
- Chen, W.-L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.-W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Abay, T.Y.; Kyriacou, P.A. Non-Invasive Techniques for Multimodal Monitoring in Traumatic Brain Injury: Systematic Review and Meta-Analysis. J. Neurotrauma 2020, 37, 2445–2453. [Google Scholar] [CrossRef]
- Torricelli, A.; Contini, D.; Pifferi, A.; Caffini, M.; Re, R.; Zucchelli, L.; Spinelli, L. Time domain functional NIRS imaging for human brain mapping. NeuroImage 2014, 85, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Weigl, W.; Milej, D.; Janusek, D.; Wojtkiewicz, S.; Sawosz, P.; Kacprzak, M.; Gerega, A.; Maniewski, R.; Liebert, A. Application of optical methods in the monitoring of traumatic brain injury: A review. J. Cereb. Blood Flow Metab. 2016, 36, 1825–1843. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, F.; Khellaf, A.; Ku, J.C.; Donnelly, J.; Thelin, E.P.; Zeiler, F.A. Continuous Near-infrared Spectroscopy Monitoring in Adult Traumatic Brain Injury: A Systematic Review. J. Neurosurg. Anesthesiol. 2020, 32, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Miyai, I.; Yagura, H.; Hatakenaka, M.; Oda, I.; Konishi, I.; Kubota, K. Longitudinal Optical Imaging Study for Locomotor Recovery After Stroke. Stroke 2003, 34, 2866–2870. [Google Scholar] [CrossRef]
- Arun, K.M.; Smitha, K.A.; Sylaja, P.N.; Kesavadas, C. Identifying Resting-State Functional Connectivity Changes in the Motor Cortex Using fNIRS During Recovery from Stroke. Brain Topogr. 2020, 33, 710–719. [Google Scholar] [CrossRef]
- Plenger, P.; Krishnan, K.; Cloud, M.; Bosworth, C.; Qualls, D.; Marquez de la Plata, C. fNIRS-based investigation of the Stroop task after TBI. Brain Imaging Behav. 2016, 10, 357–366. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Merletti, R.; Hermens, H. Introduction to the special issue on the SENIAM European Concerted Action. J. Electromyogr. Kinesiol. 2000, 10, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Hramov, A.E.; Koronovskii, A.A.; Makarov, V.A.; Pavlov, A.N.; Sitnikova, E. Wavelets in Neuroscience; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Gramfort, A. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.W.; Boudewyn, M.A.; Carter, C.S.; Phillips, M.L. Transcranial direct current stimulation: A roadmap for research, from mechanism of action to clinical implementation. Mol. Psychiatry 2020, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.Y.; Yau, S.S.Y.; Han, Y.M.Y. The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: A systematic review and meta-analyses of human and rodent studies. Neurosci. Biobehav. Rev. 2021, 125, 392–416. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.B.; Lerud, K.D.; Munsch, F.; Alsop, D.C.; Schlaug, G. Effects of tDCS dose and electrode montage on regional cerebral blood flow and motor behavior. NeuroImage 2021, 237, 118144. [Google Scholar] [CrossRef]
- Porro, C.A.; Francescato, M.P.; Cettolo, V.; Diamond, M.E.; Baraldi, P.; Zuiani, C.; Bazzocchi, M.; di Prampero, P.E. Primary Motor and Sensory Cortex Activation during Motor Performance and Motor Imagery: A Functional Magnetic Resonance Imaging Study. J. Neurosci. 1996, 16, 7688–7698. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. Neural Simulation of Action: A Unifying Mechanism for Motor Cognition. NeuroImage 2001, 14, S103–S109. [Google Scholar] [CrossRef]
- Opavsky, J.; Slachtova, M.; Kutin, M.; Hok, P.; Uhlir, P.; Opavska, H.; Hlustik, P. The effects of sustained manual pressure stimulation according to Vojta therapy on heart rate variability. Biomed. Pap. 2018, 162, 206–211. [Google Scholar] [CrossRef]
- Martínek, M.; Pánek, D.; Nováková, T.; Pavlů, D. Analysis of Intracerebral Activity during Reflex Locomotion Stimulation According to Vojta’s Principle. Appl. Sci. 2022, 12, 2225. [Google Scholar] [CrossRef]
- de Goede, A.A.; van Putten, M.J.A.M. Infraslow activity as a potential modulator of corticomotor excitability. J. Neurophysiol. 2019, 122, 325–335. [Google Scholar] [CrossRef]
- Neske, G.T. The Slow Oscillation in Cortical and Thalamic Networks: Mechanisms and Functions. Front. Neural Circuits 2016, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Malouin, F.; Richards, C.L.; Jackson, P.L.; Dumas, F.; Doyon, J. Brain activations during motor imagery of locomotor-related tasks: A PET study. Hum. Brain Mapp. 2003, 19, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-Y.; Sung, Y.-H. Effects of Vojta approach on diaphragm movement in children with spastic cerebral palsy. J. Exerc. Rehabil. 2018, 14, 1005–1009. [Google Scholar] [CrossRef] [PubMed]

| Groups (n) | Age (years) Mean (±Standard Deviation) | Weight (Kilograms) Mean (±Standard Deviation) | Height (Centimeters) Mean (±Standard Deviation) |
|---|---|---|---|
| All sample | 23.62 ± 1.54 | 72.77 ± 7.84 | 178.74 ± 3.93 |
| Non-STI group (13) | 24 ± 2.12 | 72.30 ± 9.49 | 179.76 ± 2.68 |
| V-STI group (14) | 23.28 ± 0.61 | 73.21 ± 6.27 | 177.78 ± 4.72 |
| Hemisphere | Group | Stimulation Phase | M | SE |
|---|---|---|---|---|
| Left | Non-STI | R1 | −3.19 × 10−4 | 0.04088 |
| S1 | 0.00414 | 0.00611 | ||
| R2 | −0.00405 | 0.01811 | ||
| S2 | 0.01642 | 0.01116 | ||
| R3 | −0.01852 | 0.01982 | ||
| V-STI | R1 | −0.05346 | 0.03939 | |
| S1 | −0.00373 | 0.00589 | ||
| R2 | 0.03239 | 0.01745 | ||
| S2 | −0.00642 | 0.01075 | ||
| R3 | 0.00686 | 0.01910 | ||
| Right | Non-STI | R1 | 0.08562 | 0.05390 |
| S1 | 0.00593 | 0.00566 | ||
| R2 | −0.00168 | 0.02638 | ||
| S2 | −0.01434 | 0.00560 | ||
| R3 | 0.00123 | 0.04013 | ||
| V-STI | R1 | −0.04970 | 0.05194 | |
| S1 | 0.00438 | 0.00546 | ||
| R2 | 0.01894 | 0.02542 | ||
| S2 | −0.01128 | 0.00539 | ||
| R3 | 0.00991 | 0.03867 |
| Group | Stim Phase | Left External Oblique | Right External Oblique | ||
|---|---|---|---|---|---|
| M | SE | M | SE | ||
| Non-STI | R1 | 2.36 | 0.0593 | 2.37 | 0.0615 |
| S1 | 2.31 | 0.1063 | 2.38 | 0.0925 | |
| R2 | 2.26 | 0.0272 | 2.33 | 0.0669 | |
| S2 | 2.31 | 0.1305 | 2.35 | 0.0829 | |
| R3 | 2.30 | 0.0567 | 2.33 | 0.0393 | |
| V-STI | R1 | 2.31 | 0.0571 | 2.33 | 0.0593 |
| S1 | 2.51 | 0.1025 | 2.41 | 0.0892 | |
| R2 | 2.36 | 0.0262 | 2.42 | 0.0644 | |
| S2 | 2.54 | 0.1258 | 2.51 | 0.0799 | |
| R3 | 2.40 | 0.0547 | 2.37 | 0.0378 | |
| VLFO | LFO | Respiration | Cardiac | ||
|---|---|---|---|---|---|
| 0.01 to 0.02 Hz (scale 45–65) | 0.02 to 0.04 Hz (scale 25–45) | 0.04–0.15 Hz (scale 6–25) | 0.16–0.6 Hz (scale 2.5–6.25) | 0.8–1.5 Hz (scale 2–2.5) | |
| Left HbO—m1R | ns | S1: Non-STI > V-STI * | ns | S2: Non-STI > V-STI ** | ns |
| Left HbO—m2R | R2: V-STI > Non-STI * | S1: Non-STI > V-STI * | S1: Non-STI > V-STI * | R1: Non-STI > V-STI * | R3: V-STI > Non-STI * |
| Right HbO—m1R | ns | R2: V-STI > Non-STI * | ns | S1: Non-STI > V-STI * | ns |
| Right HbO—m2R | S1: Non-STI > V-STI * | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-González, J.L.; Díez-Villoria, E.; Pérez-Robledo, F.; Sanz-Esteban, I.; Llamas-Ramos, I.; Llamas-Ramos, R.; de la Fuente, A.; Bermejo-Gil, B.M.; Canal-Bedia, R.; Martín-Nogueras, A.M. Synergy of Muscle and Cortical Activation through Vojta Reflex Locomotion Therapy in Young Healthy Adults: A Pilot Randomized Controlled Trial. Biomedicines 2023, 11, 3203. https://doi.org/10.3390/biomedicines11123203
Sánchez-González JL, Díez-Villoria E, Pérez-Robledo F, Sanz-Esteban I, Llamas-Ramos I, Llamas-Ramos R, de la Fuente A, Bermejo-Gil BM, Canal-Bedia R, Martín-Nogueras AM. Synergy of Muscle and Cortical Activation through Vojta Reflex Locomotion Therapy in Young Healthy Adults: A Pilot Randomized Controlled Trial. Biomedicines. 2023; 11(12):3203. https://doi.org/10.3390/biomedicines11123203
Chicago/Turabian StyleSánchez-González, Juan Luis, Emiliano Díez-Villoria, Fátima Pérez-Robledo, Ismael Sanz-Esteban, Inés Llamas-Ramos, Rocío Llamas-Ramos, Antonio de la Fuente, Beatriz María Bermejo-Gil, Ricardo Canal-Bedia, and Ana María Martín-Nogueras. 2023. "Synergy of Muscle and Cortical Activation through Vojta Reflex Locomotion Therapy in Young Healthy Adults: A Pilot Randomized Controlled Trial" Biomedicines 11, no. 12: 3203. https://doi.org/10.3390/biomedicines11123203







