Osteoprotegerin Gene as a Biomarker in the Development of Osteoporosis in Postmenopausal Women
Abstract
:1. Introduction
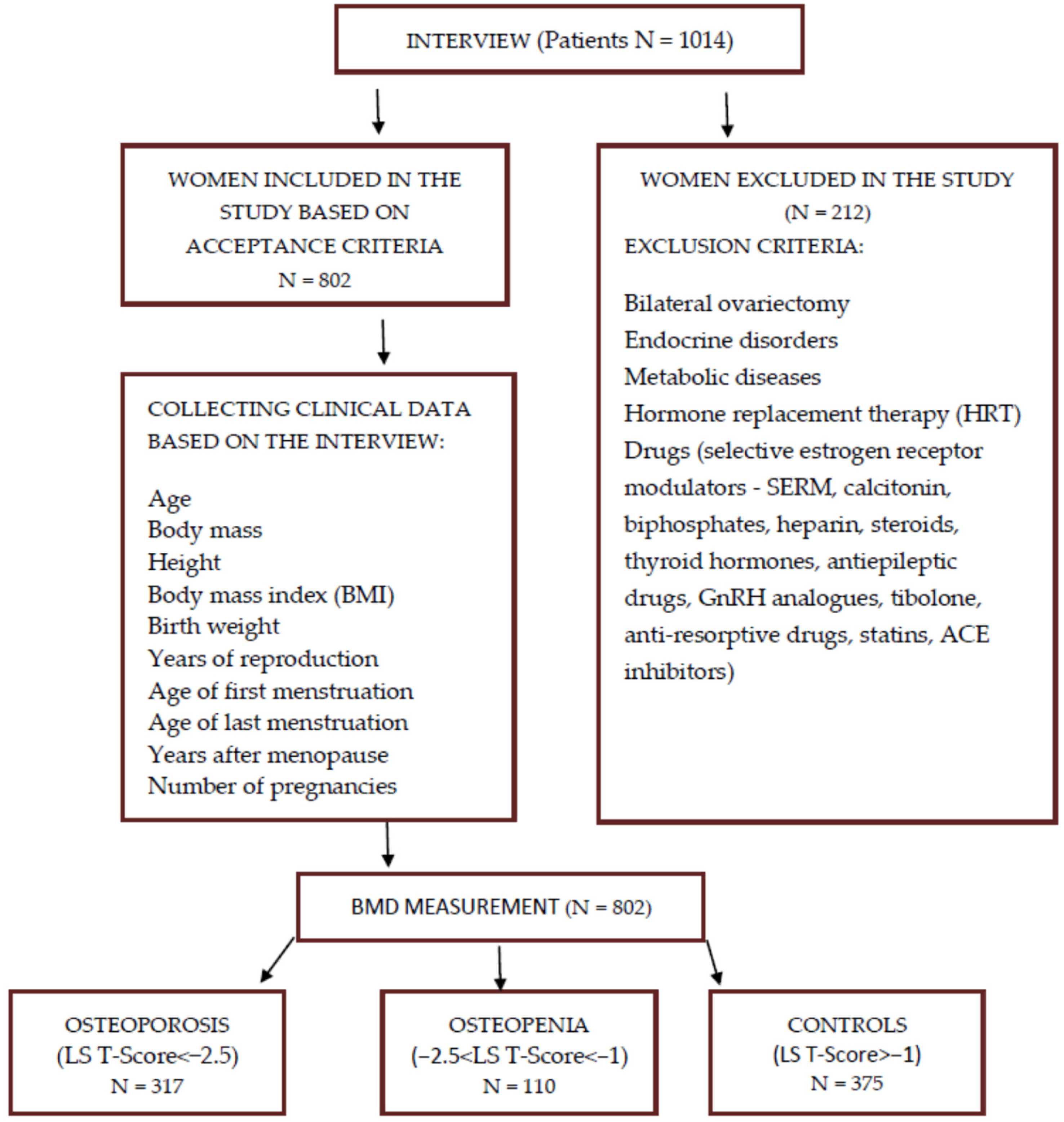
2. Materials and Methods
2.1. Study Group
2.2. Determination of OPG Polymorphisms
2.3. Statistical Analysis
3. Results
3.1. Evaluation of Clinical Data
3.2. Figures, Tables and Schemes
3.3. Evaluation of the Prevalence of the Polymorphisms Studied
3.4. Effect of the Prevalence of the Polymorphisms Studied on Osteopenia
3.5. Effect of the Prevalence of the Polymorphisms Studied on Osteoporosis
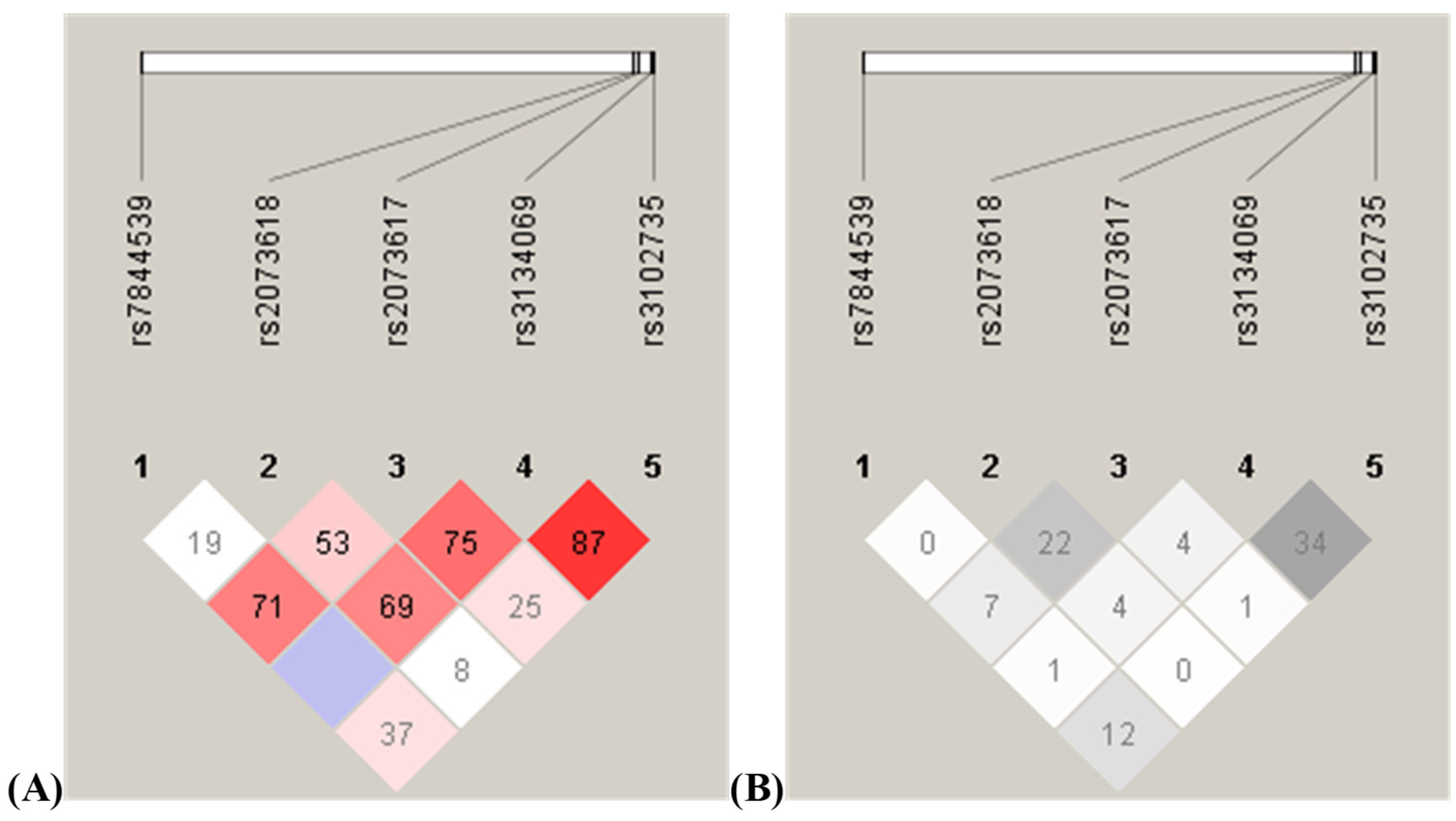
3.6. Linkage Disequilibrium Analysis and Haplotype Frequency Analysis of the Analyzed Polymorphisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Feng, X. RANKing Intracellular Signaling in Osteoclasts. IUBMB Life 2005, 57, 389–395. [Google Scholar] [CrossRef]
- Arko, B.; Preželj, J.; Kocijančič, A.; Komel, R.; Marc, J. Association of the osteoprotegerin gene polymorphisms with bone mineral density in postmenopausal women. Maturitas 2005, 51, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Pettit, A.R.; Ji, H.; von Stechow, D.; Müller, R.; Goldring, S.R.; Choi, Y.; Benoist, C.; Gravallese, E.M. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 2001, 159, 1689–1699. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Mochizuki, S.-I.; Yano, K.; Fujise, N.; Sato, Y.; Goto, M.; Yamaguchi, K.; Kuriyama, M.; et al. Identity of Osteoclastogenesis Inhibitory Factor (OCIF) and Osteoprotegerin (OPG): A Mechanism by which OPG/OCIF Inhibits Osteoclastogenesis in vitro. Endocrinology 1998, 139, 1329–1337. [Google Scholar] [CrossRef]
- Kostenuik, P.; Shalhoub, V. Osteoprotegerin A Physiological and Pharmacological Inhibitor of Bone Resorption. Curr. Pharm. Des. 2001, 7, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Théoleyre, S.; Tat, S.K.; Vusio, P.; Blanchard, F.; Gallagher, J.; Ricard-Blum, S.; Fortun, Y.; Padrines, M.; Rédini, F.; Heymann, D. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: Role in the interactions with receptor activator of nuclear factor κB ligand (RANKL) and RANK. Biochem. Biophys. Res. Commun. 2006, 347, 460–467. [Google Scholar] [CrossRef]
- Nair, S.; Bhadricha, H.; Patil, A.; Surve, S.; Joshi, B.; Balasinor, N.; Desai, M. Association of OPG and RANKL gene polymorphisms with bone mineral density in Indian women. Gene 2022, 840, 146746. [Google Scholar] [CrossRef]
- Han, X.; Zheng, L.; Mu, Y.-Y.; Li, H.-Z.; He, X.-F. Association between OPG polymorphisms and osteoporosis risk: An updated meta-analysis. Front. Genet. 2022, 13, 1032110. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Tariq, S.; Abualhamael, S.A.; Baig, M.; Malik, A.A.; Shahzad, M. Osteoprotegerin genetic polymorphisms and their influence on therapeutic response to ibandronate in postmenopausal osteoporotic females. PLoS ONE 2023, 18, e0291959. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Koh, J.M.; Chang, J.S.; Park, B.L.; Kim, L.H.; Park, E.K.; Kim, S.-Y.; Shin, H.D. Association of the OSCAR Promoter Polymorphism with BMD in Postmenopausal Women: Oscar polymorphism and BMD. J. Bone Miner. Res. 2005, 20, 1342–1348. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Liu, M. The effect of low-body weight combined with T149C and A163G polymorphism of osteoprotegerin promoter region on osteoporosis. Chin. J. Geriatr. 2006, 25, 645–650. [Google Scholar]
- García-Unzueta, M.T.; Riancho, J.A.; Zarrabeitia, M.T.; Sañudo, C.; Berja, A.; Valero, C.; Pesquera, C.; Paule, B.; González-Macías, J.; Amado, J.A. Association of the 163A/G and 1181G/C Osteoprotegerin Polymorphism with Bone Mineral Density. Horm. Metab. Res. 2008, 40, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Brambila-Tapia, A.J.L.; Durán-González, J.; Sandoval-Ramírez, L.; Mena, J.P.; Salazar-Páramo, M.; Gámez-Nava, J.I.; González-López, L.; Lazalde-Medina, B.B.; Dávalos, N.O.; Peralta-Leal, V.; et al. MTHFR C677T, MTHFR A1298C, and OPG A163G Polymorphisms in Mexican Patients with Rheumatoid Arthritis and Osteoporosis. Dis. Markers 2012, 32, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Boroń, D.; Kotrych, D.; Bartkowiak-Wieczorek, J.; Uzar, I.; Bogacz, A.; Kamiński, A. Polymorphisms of OPG and their relation to the mineral density of bones in pre- and postmenopausal women. Int. Immunopharmacol. 2015, 28, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bonfá, A.C.; Seguro, L.P.C.; Caparbo, V.; Bonfá, E.; Pereira, R.M.R. RANKL and OPG gene polymorphisms: Associations with vertebral fractures and bone mineral density in premenopausal systemic lupus erythematosus. Osteoporos. Int. 2015, 26, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Blaščáková, M.M.; Blaščáková, Ľ.; Poráčová, J.; Mydlár, J.; Vašková, J.; Bernasovská, J.; Boroňová, I.; Petrejčíková, E.; Bernasovský, I. Relationship between A163G osteoprotegerin gene polymorphism and other osteoporosis parameters in Roma and non-Roma postmenopausal women in eastern Slovakia. J. Clin. Lab. Anal. 2017, 31, 22093. [Google Scholar] [CrossRef] [PubMed]
- Abdi, S.; Binbaz, R.A.; Mohammed, A.K.; Ansari, M.G.; Wani, K.; Amer, O.E.; Alnaami, A.M.; Aljohani, N.; Al-Daghri, N.M. Association of RANKL and OPG Gene Polymorphism in Arab Women with and without Osteoporosis. Genes 2021, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L.; Carstens, M.; Stenkjaer, L.; Eriksen, E.F. Polymorphisms in the Osteoprotegerin Gene Are Associated With Osteoporotic Fractures. J. Bone Miner. Res. 2002, 17, 1245–1255. [Google Scholar] [CrossRef]
- Kusk, P.; Madsen, B.; Fenger, M.; Lauritzen, J.B.; Jørgensen, H.L. Serum osteoprotegerin (OPG) and the A163G polymorphism in the OPG promoter region are related to peripheral measures of bone mass and fracture odds ratios. J. Bone Miner. Metab. 2004, 22, 132–138. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Niu, T.; Terwedow, H.A.; Xu, X.; Feng, Y.; Li, Z.; Brain, J.D.; Rosen, C.J.; Laird, N.; Xu, X. Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Hum. Genet. 2006, 118, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Seremak-Mrozikiewicz, A.; Tatuśko, J.; Drews, K.; Barlik, M.; Krajewski, P.; Spaczyński, M.; Mrozikiewicz, P.M. Polymorphism of osteoprotegerin gene and osteoporosis in postmenopausal women. Ginekol. Pol. 2009, 80, 354–360. [Google Scholar] [PubMed]
- Hussien, Y.M.; Shehata, A.; Karam, R.A.; Alzahrani, S.S.; Magdy, H.; El-Shafey, A.M. Polymorphism in vitamin D receptor and osteoprotegerin genes in Egyptian rheumatoid arthritis patients with and without osteoporosis. Mol. Biol. Rep. 2013, 40, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Cvijetic, S.; Grazio, S.; Kosovic, P.; Uremovic, M.; Nemcic, T.; Bobic, J. Osteoporosis and polymorphisms of osteoprotegerin gene in postmenopausal women—A pilot study. Rheumatology 2016, 54, 10–13. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Feng, J.Q.; Liu, M.; Lin, W.; Huang, Y.; Shi, Q.W. The relationship between body fat ratio combined with G209A and T245G polymorphism of osteoprotegerin promoter region and osteoporosis. Chin. J. Geriatr. 2007, 12, 2409–2413. [Google Scholar]
- Kim, J.G.; Kim, J.H.; Kim, J.Y.; Ku, S.Y.; Jee, B.C.; Suh, C.S.; Kim, S.H.; Choi, Y.M. Association between osteoprotegerin (OPG), receptor activator of nuclear factor-kappaB (RANK), and RANK ligand (RANKL) gene polymorphisms and circulating OPG, soluble RANKL levels, and bone mineral density in Korean postmenopausal women. Menopause 2007, 14, 913–918. [Google Scholar] [CrossRef]
- Mencej-Bedrač, S.; Preželj, J.; Marc, J. TNFRSF11B gene polymorphisms 1181G>C and 245T>G as well as haplotype CT influence bone mineral density in postmenopausal women. Maturitas 2011, 69, 263–267. [Google Scholar] [CrossRef]
- Zavala-Cerna, M.G.; Moran-Moguel, M.C.; Cornejo-Toledo, J.A.; Gonzalez-Montoya, N.G.; Sanchez-Corona, J.; Salazar-Paramo, M.; Nava-Zavala, A.H.; Aguilar-Chavez, E.A.; Alcaraz-Lopez, M.F.; Gonzalez-Sanchez, A.G.; et al. Osteoprotegerin Polymorphisms in a Mexican Population with Rheumatoid Arthritis and Generalized Osteoporosis: A Preliminary Report. J. Immunol. Res. 2015, 2015, 376197. [Google Scholar] [CrossRef]
- Zajíčková, K.; Zemanová, A.; Hill, M.; Žofková, I. Is A163G polymorphism in the osteoprotegerin gene associated with heel velocity of sound in postmenopausal women? Physiol. Res. 2008, 57 (Suppl. S1), 153–157. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Liu, M.; Feng, J.Q. Relationships among single T~(950) -C mutation polymorphism in the promoter region of the osteoprotegerin gene, bone mineral density and metabolism. Chin. J. Geriatr. 2005, 3, 247–250. [Google Scholar]
- Vidal, N.; Brandstrom, H.; Jonsson, K.; Ohlsson, C. Osteoprotegerin mRNA is expressed in primary human osteoblast-like cells: Down-regulation by glucocorticoids. J. Endocrinol. 1998, 159, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Wynne, F.; Drummond, F.; O’Sullivan, K.; Daly, M.; Shanahan, F.; Molloy, M.; Quane, K. Investigation of the Genetic Influence of the OPG, VDR (Fok1), and COLIA1 Sp1 Polymorphisms on BMD in the Irish Population. Calcif. Tissue Int. 2002, 71, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Brincat, M.; Anastasi, A.X. TNFRSF11B gene variants and bone mineral density in postmenopausal women in Malta. Maturitas 2006, 53, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Bollerslev, J.; Wilson, S.; Dick, I.; Islam, F.; Mullin, B.; Devine, A.; Prince, R. No associations between OPG gene polymorphisms or serum levels and measures of osteoporosis in elderly Australian women. Bone 2007, 40, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Woo, J.-H.; Choi, S.J.; Ji, J.D.; Song, G.G. Associations between osteoprotegerin polymorphisms and bone mineral density: A meta-analysis. Mol. Biol. Rep. 2010, 37, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tang, K.; Quan, Z.; Zhao, Z.; Jiang, D. Association Between Seven Common OPG Genetic Polymorphisms and Osteoporosis Risk: A Meta-Analysis. DNA Cell Biol. 2014, 33, 29–39. [Google Scholar] [CrossRef]
- Li, S.; Jiang, H.; Du, N. Association between osteoprotegerin gene T950C polymorphism and osteoporosis risk in the Chinese population: Evidence via meta-analysis. PLoS ONE 2017, 12, e0189825. [Google Scholar] [CrossRef]
- Nava-Valdivia, C.A.; Saldaña-Cruz, A.M.; Corona-Sanchez, E.G.; Murillo-Vazquez, J.D.; Moran-Moguel, M.C.; Salazar-Paramo, M.; Perez-Guerrero, E.E.; Vazquez-Villegas, M.L.; Bonilla-Lara, D.; Rocha-Muñoz, A.D.; et al. Polymorphism rs2073618 of the TNFRSF11B (OPG) Gene and Bone Mineral Density in Mexican Women with Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 7680434. [Google Scholar] [CrossRef]
- González-Mercado, A.; Sánchez-López, J.Y.; Perea-Díaz, F.J.; Magaña-Torres, M.T.; Salazar-Páramo, M.; González-López, L.; González-Mercado, M.G.; Ibarra-Cortés, B. Association of polymorphisms of the TNFRSF11B and TNFSF11 genes with bone mineral density in postmenopausal women from western Mexico. Arch. Med. Sci. 2019, 15, 1352–1356. [Google Scholar] [CrossRef]
- Choi, J.Y.; Shin, A.; Park, S.K.; Chung, H.W.; Cho, S.; Shin, C.S.; Kim, H.; Lee, K.H.; Kang, C.; Cho, D.Y.; et al. Genetic Polymorphisms of OPG, RANK, and ESR1 and Bone Mineral Density in Korean Postmenopausal Women. Calcif. Tissue Int. 2005, 77, 152–159. [Google Scholar] [CrossRef]
- Moffett, S.P.; Oakley, J.I.; Cauley, J.A.; Lui, L.Y.; Ensrud, K.E.; Taylor, B.C.; Hillier, T.A.; Hochberg, M.C.; Li, J.; Cayabyab, S.; et al. Osteoprotegerin Lys3Asn Polymorphism and the Risk of Fracture in Older Women. J. Clin. Endocrinol. Metab. 2008, 93, 2002–2008. [Google Scholar] [CrossRef]
- Tu, P.; Duan, P.; Zhang, R.-S.; Xu, D.-B.; Wang, Y.; Wu, H.-P.; Liu, Y.-H.; Si, L. Polymorphisms in genes in the RANKL/RANK/OPG pathway are associated with bone mineral density at different skeletal sites in post-menopausal women. Osteoporos. Int. 2014, 26, 179–185. [Google Scholar] [CrossRef]
- Canto-Cetina, T.; Reyes, L.P.; Herrera, L.G.; Rojano-Mejía, D.; Coral-Vázquez, R.M.; Coronel, A.; Canto, P. Polymorphism of LRP5, but not of TNFRSF11B, is associated with a decrease in bone mineral density in postmenopausal maya-mestizo women. Am. J. Hum. Biol. 2013, 25, 713–718. [Google Scholar] [CrossRef]
- Rojano-Mejía, D.; Coral-Vázquez, R.M.; Espinosa, L.C.; Romero-Hidalgo, S.; López-Medina, G.; del Carmen Aguirre García, M.; Coronel, A.; Ibarra, R.; Canto, P. TNFRSF11B gene haplotype and its association with bone mineral density variations in postmenopausal Mexican-Mestizo women. Maturitas 2012, 71, 49–54. [Google Scholar] [CrossRef]
| Parameter | A Control Group (n = 375) | B Osteopenia (n = 110) | C Osteoporosis (n = 317) | p (ANOVA) | p (Tukey HSD) |
|---|---|---|---|---|---|
| BMD L2–L4 (g/cm2) | <0.001 | C-A–<0.001 | |||
| mean ± SD | 1.20 ± 0.10 | 0.98 ± 0.05 | 0.82 ± 0.07 | B-A–<0.001 | |
| median (min–max) | 1.19 (1.08–1.47) | 0.97 (0.90–1.07) | 0.83 (0.63–0.90) | B-C–<0.001 | |
| BMD L2–L4 YA (%) | <0.001 | C-A–<0.001 | |||
| mean ± SD | 100.45 ± 8.03 | 81.71 ± 4.43 | 68.26 ± 5.34 | B-A–<0.001 | |
| median (min–max) | 99.00 (90.00–123.00) | 81.00 (75.00–89.00) | 69.00 (53.00–75.00) | B-C–<0.001 | |
| BMD L2–L4 AM (%) | <0.001 | C-A–<0.001 | |||
| mean ± SD | 108.52 ± 10.23 | 89.24 ± 6.62 | 78.10 ± 7.15 | B-A–<0.001 | |
| median (min–max) | 107.00 (91.00–133.00) | 89.00 (74.00–108.00) | 78.00 (60.00–92.00) | B-C–<0.001 | |
| T-score | <0.001 | C-A–<0.001 | |||
| mean ± SD | 0.05 ± 0.90 | −1.80 ± 0.43 | −3.16 ± 0.54 | B-A–<0.001 | |
| median (min–max) | −0.17 (−0.97–3.13) | −1.89 (−2.49)–(−1.05) | −3.05 (-4.73)–(−2.50) | B-C–<0.001 | |
| Z-score | <0.001 | C-A–<0.001 | |||
| mean ± SD | 0.64 ± 1.11 | −0.84 ± 0.66 | −1.62 ± 0.72 | B-A–<0.001 | |
| median (min–max) | 0.56 (−1.85–2.65) | −0.88 (−2.36–0.77) | −1.62 (−3.13–0.98) | B-C–<0.001 |
| Parameter | A Control Group (n = 375) | B Osteopenia (n = 110) | C Osteoporosis (n = 317) | p (ANOVA) | p (Tukey HSD) |
|---|---|---|---|---|---|
| Patient age (years) | 0.007 | C-A–0.058 | |||
| mean ± SD | 53.49 ± 8.23 | 53.19 ± 8.20 | 56.45 ± 8.83 | B-A–0.972 | |
| median (min–max) | 55.00 (51.00–71.00) | 53.00 (52.00–77.00) | 57.00 (51.00–78.00) | B-C–0.010 | |
| Height (cm) | <0.001 | C-A–0.002 | |||
| mean ± SD | 163.17 ± 5.98 | 162.81 ± 5.02 | 160.18 ± 5.12 | B-A–0.905 | |
| median (min–max) | 164.00 (152.00–180.00) | 163.00 (150.00–175.00) | 160.00 (150.00–175.00) | B-C–0.002 | |
| Body mass (kg) | <0.001 | C-A–<0.001 | |||
| mean ± SD | 68.71 ± 12.22 | 65.51 ± 11.14 | 60.93 ± 9.16 | B-A–0.148 | |
| median (min–max) | 66.00 (50.00–100.00) | 65.00 (41.00–114.00) | 61.00 (43.00–85.00) | B-C–0.011 | |
| BMI (kg/m2) | 0.004 | C-A–0.003 | |||
| mean ± SD | 25.84 ± 4.55 | 24.72 ± 3.97 | 23.70 ± 3.10 | B-A–0.157 | |
| median (min–max) | 24.76 (18.33–37.18) | 24.98 (17.30–43.43) | 23.63 (17.10–31.63) | B-C–0.168 | |
| First menstruation (years) | 0.654 | C-A–0.630 | |||
| mean ± SD | 13.38 ± 1.88 | 13.12 ± 2.39 | 12.94 ± 2.16 | B-A–0.854 | |
| median (min–max) | 14.00 (9.00–16.00) | 13.00 (9.00–18.00) | 13.00 (9.00–18.00) | B-C–0.894 | |
| Last menstruation (years) | 0.057 | C-A–0.060 | |||
| mean ± SD | 50.17 ± 4.39 | 49.34 ± 4.59 | 48.06 ± 5.08 | B-A–0.646 | |
| median (min–max) | 50.00 (41.00–58.00) | 50.00 (38.00–60.00) | 49.00 (34.00–60.00) | B-C–0.237 | |
| Reproductive period (years) | 0.724 | C-A–0.766 | |||
| mean ± SD | 36.38 ± 5.35 | 36.20 ± 4.93 | 35.62 ± 5.01 | B-A–0.986 | |
| median (min–max) | 37.00 (27.00–48.00) | 36.50 (23.00–49.00) | 36.00 (24.00–48.00) | B-C–0.795 | |
| Years since menopause | 0.001 | C-A–0.013 | |||
| mean ± SD | 7.03 ± 5.59 | 7.18 ± 6.02 | 10.63 ± 5.75 | B-A–0.992 | |
| median (min–max) | 6.50 (1.00–23.00) | 6.00 (0.00–25.00) | 10.00 (1.00–22.00) | B-C–0.003 | |
| Number of pregnancies | 0.852 | C-A–0.994 | |||
| mean ± SD | 1.94 ± 1.22 | 1.84 ± 1.13 | 1.92 ± 1.31 | B-A–0.867 | |
| median (min–max) | 2.00 (0.00–6.00) | 2.00 (0.00–6.00) | 2.00 (0.00–7.00) | B-C–0.902 | |
| Newborn’s weight (g) | 0.005 | C-A–0.009 | |||
| mean ± SD | 3628.95 ± 480.75 | 3226.79 ± 411.07 | 3141.25 ± 536.32 | B-A–0.014 | |
| median (min–max) | 3600 (2460–5100) | 3200 (2500–4500) | 3000 (2470–4500) | B-C–0.828 |
| Rsnumber | Control Group N = 375 | Osteopenia N = 110 | Osteoporosis N = 317 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MAF | HWE p | Missing (%) | MAF | HWE p | Missing (%) | MAF | HWE p | Missing (%) | |
| rs3102735 | G = 14.2 | 0.28 | 1.6 | G = 12.6 | 0.37 | 1.8 | G = 14.9 | 0.51 | 0.6 |
| rs3134069 | G = 7.3 | 1.00 | 3.7 | G = 5.3 | 1.00 | 4.6 | G = 6.6 | 0.45 | 15.8 |
| rs2073617 | C = 45.9 | 0.40 | 2.4 | C = 43.2 | 0.16 | 5.5 | T = 48.5 | 0.57 | 2.8 |
| rs2073618 | C = 45.8 | 0.25 | 1.1 | C = 44.4 | 0.56 | 1.8 | C = 45.4 | 0.31 | 0.9 |
| rs7844539 | C = 12.9 | 0.31 | 1.1 | C = 15.4 | 1.00 | 1.8 | C = 14.0 | 1.00 | 2.86 |
| SNP | Group | Alleles | Percentage of People Surveyed | Chi2 | Pearson’s p | OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| 163A>G rs3102735 | A | G | 0.98 | 0.360 | 0.548 | 0.87 (0.55–1.37) | |
| osteopenia | 187 (0.87) | 27 (0.13) | |||||
| control | 633 (0.86) | 105 (0.14) | |||||
| 245T>G rs3134069 | T | G | 0.96 | 1.061 | 0.302 | 0.70 (0.36–1.38) | |
| osteopenia | 197 (0.95) | 11 (0.05) | |||||
| control | 669 (0.93) | 53 (0.07) | |||||
| 950T>C rs2073617 | T | C | 0.97 | 0.472 | 0.491 | 0.90 (0.66–1.22) | |
| osteopenia | 117 (0.57) | 89 (0.43) | |||||
| control | 396 (0.54) | 336 (0.46) | |||||
| 1181G>C rs2073618 | G | C | 0.99 | 0.136 | 0.711 | 0.94 (0.70–1.28) | |
| osteopenia | 119 (0.56) | 95 (0.44) | |||||
| control | 402 (0.54) | 340 (0.46) | |||||
| 6890A>C rs7844539 | A | C | 0.98 | 0.401 | 0.526 | 1.23 (0.65–2.34) | |
| osteopenia | 181 (0.85) | 33 (0.15) | |||||
| control | 648 (0.87) | 96 (0.13) | |||||
| SNP | Model | Control | Osteopenia | OR (95% PU) | p | AIC |
|---|---|---|---|---|---|---|
| 163A>G rs3102735 | AA | 274 (74.3) | 83 (77.6) | 1.00 | 0.752 | 512.8 |
| AG | 85 (23.0) | 21 (19.6) | 0.82 (0.48–1.40) | |||
| GG | 10 (2.7) | 3 (2.8) | 0.99 (0.27–3.68) | |||
| dominant | 95 (25.7) | 24 (22.4) | 0.83 (0.50–1.39) | 0.482 | 510.8 | |
| recessive | 359 (97.3) | 104 (97.2) | 1.04 (0.28–3.83) | 0.958 | 511.3 | |
| overdominant | 284 (77.0) | 86 (80.4) | 0.82 (0.48–1.39) | 0.451 | 510.8 | |
| log additive | 369 (77.5) | 107 (22.5) | 0.88 (0.57–1.36) | 0.557 | 511.0 | |
| 245T>G rs3134069 | TT | 310 (85.9) | 93 (89.4) | 1.00 | 0.704 | 498.6 |
| TG | 49 (13.6) | 11 (10.6) | 0.75 (0.37–1.50) | |||
| GG | 2 (0.6) | 0 (0.0) | ― | |||
| dominant | 51 (14.1) | 11 (10.6) | 0.72 (0.36–1.44) | 0.337 | 497.4 | |
| recessive | 359 (99.4) | 104 (100.0) | ― | 1.000 | 497.3 | |
| overdominant | 312 (86.4) | 93 (89.4) | 0.75 (0.38–1.51) | 0.413 | 497.6 | |
| log additive | 361 (77.6) | 104 (22.4) | 0.70 (0.36–1.38) | 0.704 | 497.2 | |
| 950T>C rs2073617 | TT | 111 (30.3) | 37 (35.9) | 1.00 | 0.504 | 498.4 |
| TC | 174 (47.5) | 43 (41.7) | 0.74 (0.45–1.22) | |||
| CC | 81 (22.1) | 23 (22.3) | 0.85 (0.47–1.54) | |||
| dominant | 255 (69.7) | 66 (64.1) | 0.78 (0.49–1.23) | 0.284 | 496.6 | |
| recessive | 285 (77.9) | 80 (77.7) | 1.01 (0.60–1.71) | 0.966 | 497.8 | |
| overdominant | 192 (52.5) | 60 (58.3) | 0.79 (0.51–1.23) | 0.297 | 496.7 | |
| log additive | 366 (78.0) | 103 (22.0) | 0.90 (0.67–1.22) | 0.505 | 497.3 | |
| 1181G>C rs2073618 | GG | 103 (27.8) | 31 (29.0) | 1.00 | 0.920 | 514.2 |
| GC | 196 (52.8) | 57 (53.3) | 0.97 (0.59–1.59) | |||
| CC | 72 (19.4) | 19 (17.8) | 0.88 (0.46–1.67) | |||
| dominant | 268 (72.2) | 76 (71.0) | 0.94 (0.59–1.52) | 0.807 | 512.3 | |
| recessive | 299 (80.6) | 88 (82.2) | 0.90 (0.51–1.57) | 0.700 | 512.2 | |
| overdominant | 175 (47.2) | 50 (46.7) | 1.02 (0.66–1.57) | 0.936 | 512.3 | |
| log additive | 371 (77.6) | 107 (22.4) | 0.94 (0.68–1.29) | 0.702 | 512.2 | |
| 6890A>C rs7844539 | AA | 282 (75.8) | 76 (71.0) | 1.00 | 0.795 | 227.7 |
| AC | 84 (22.6) | 29 (27.1) | 1.28 (0.61–2.67) | |||
| CC | 6 (1.6) | 2 (1.9) | 1.24 (0.11–14.02) | |||
| dominant | 90 (24.2) | 31 (29.0) | 1.28 (0.62–2.61) | 0.499 | 225.7 | |
| recessive | 366 (98.4) | 105 (98.1) | 1.16 (0.10–13.08) | 0.903 | 226.1 | |
| overdominant | 288 (77.4) | 78 (72.9) | 1.27 (0.61–2.65) | 0.513 | 225.7 | |
| log additive | 372 (36.7) | 107 (63.3) | 1.24 (0.64–2.38) | 0.518 | 225.7 |
| SNP | Group | Alleles | Percentage of People with Non-Missing Genotypes | Chi2 | Pearson’s p | OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| 163A>G rs3102735 | A | G | 0.988 | 0.131 | 0.717 | 1.057 (0.782~1.428) | |
| osteoporosis | 536(0.85) | 94(0.15) | |||||
| control | 633(0.86) | 105(0.14) | |||||
| 245T>G rs3134069 | T | G | 0.918 | 0.155 | 0.693 | 0.894 (0.514~1.556) | |
| osteoporosis | 508(0.93) | 36(0.07) | |||||
| control | 669(0.93) | 53(0.07) | |||||
| 950T>C rs2073617 | T | C | 0.973 | 4.139 | 0.041 | 1.249 (1.008~1.548) | |
| osteoporosis | 299 (0.49) | 317 (0.51) | |||||
| control | 396 (0.54) | 336 (0.46) | |||||
| 1181G>C rs2073618 | G | C | 0.989 | 0.026 | 0.870 | 0.982 (0.793~1.216) | |
| osteoporosis | 343(0.55) | 285(0.45) | |||||
| control | 402(0.54) | 340(0.46) | |||||
| 6890A>C rs7844539 | A | C | 0.962 | 0.100 | 0.750 | 1.101 (0.606~2.002) | |
| osteoporosis | 505 (0.86) | 83 (0.14) | |||||
| control | 108 (0.87) | 16 (0.13) | |||||
| SNP | Model | Control | Osteoporosis | OR (95% PU) | p | AIC |
|---|---|---|---|---|---|---|
| 163A>G rs3102735 | AA | 274 (74.3) | 226 (71.7) | 1.00 | 0.358 | 947.9 |
| AG | 85 (23.0) | 84 (26.7) | 1.20 (0.84–1.70) | |||
| GG | 10 (2.7) | 5 (1.6) | 0.61 (0.20–1.80) | |||
| dominant | 95 (25.7) | 89 (28.3) | 1.14 (0.81–1.59) | 0.461 | 947.4 | |
| recessive | 359 (97.3) | 310 (98.4) | 0.58 (0.20–1.71) | 0.312 | 946.9 | |
| overdominant | 284 (77.0) | 231 (73.3) | 1.21 (0.86–1.72) | 0.273 | 946.8 | |
| log additive | 369 (53.9) | 315 (46.1) | 1.06 (0.78–1.43) | 0.718 | 947.8 | |
| 245T>G rs3134069 | TT | 310 (85.9) | 119 (87.5) | 1.00 | 0.846 | 589.0 |
| TG | 49 (13.6) | 16 (11.8) | 0.85 (0.47–1.55) | |||
| GG | 2 (0.6) | 1 (0.7) | 1.30 (0.12–14.50) | |||
| dominant | 51 (14.1) | 17 (12.5) | 0.87 (0.48–1.56) | 0.635 | 587.1 | |
| recessive | 359 (99.4) | 135 (99.3) | 1.33 (0.12–14.78) | 0.820 | 587.3 | |
| overdominant | 312 (86.4) | 120 (88.2) | 0.85 (0.46–1.55) | 0.590 | 587.0 | |
| log additive | 361 (72.6) | 136 (27.4) | 0.90 (0.52–1.55) | 0.693 | 587.2 | |
| 950T>C rs2073617 | TT | 111 (30.3) | 75 (24.4) | 1.00 | 0.135 | 931.4 |
| TC | 174 (47.5) | 149 (48.4) | 1.27 (0.88–1.83) | |||
| CC | 81 (22.1) | 84 (27.3) | 1.53 (1.01–2.34) | |||
| dominant | 255 (69.7) | 233 (75.6) | 1.35 (0.96–1.90) | 0.083 | 930.4 | |
| recessive | 285 (77.9) | 224 (72.7) | 1.32 (0.93–1.88) | 0.122 | 931.0 | |
| overdominant | 192 (52.5) | 159 (51.6) | 1.03 (0.76–1.40) | 0.829 | 933.3 | |
| log additive | 366 (54.3) | 308 (45.7) | 1.24 (1.00–1.53) | 0.046 | 929.4 | |
| 1181G>C rs2073618 | GG | 103 (27.8) | 89 (28.3) | 1.00 | 0.985 | 950.8 |
| GC | 196 (52.8) | 165 (52.5) | 0.97 (0.69–1.38) | |||
| CC | 72 (19.4) | 60 (19.1) | 0.96 (0.62–1.50) | |||
| dominant | 268 (72.2) | 225 (71.7) | 0.97 (0.70–1.36) | 0.866 | 948.8 | |
| recessive | 299 (80.6) | 254 (80.9) | 0.98 (0.67–1.44) | 0.921 | 948.9 | |
| overdominant | 175 (47.2) | 149 (47.5) | 0.99 (0.73–1.34) | 0.941 | 948.9 | |
| log additive | 371 (54.2) | 314 (45.8) | 0.98 (0.79–1.22) | 0.866 | 948.8 | |
| 6890A>C rs7844539 | AA | 282 (75.8) | 217 (73,8) | 1.00 | 0.948 | 290.4 |
| AC | 84 (22.6) | 71 (24.2) | 1.09 (0.55–2.15) | |||
| CC | 6 (1.6) | 6 (2.0) | 1.30 (0.14–11.89) | |||
| dominant | 90 (24.2) | 77 (26.0) | 1.10 (0.57–2.14) | 0.773 | 288.5 | |
| recessive | 366 (98.4) | 288 (98.0) | 1.27 (0.14–11.59) | 0.828 | 288.5 | |
| overdominant | 288 (77.4) | 223 (76.0) | 1.08 (0.55–2.13) | 0.821 | 288.5 | |
| log additive | 372 (54.0) | 294 (46.0) | 1.10 (0.61–2.00) | 0.750 | 288.4 |
| SNP1 | SNP2 | D′ | LOD | r2 | Distance in bp |
|---|---|---|---|---|---|
| rs7844539 | rs2073618 | 0.193 | 0.52 | 0.008 | 25,327 |
| rs7844539 | rs2073617 | 0.712 | 6.47 | 0.077 | 25,558 |
| rs7844539 | rs3134069 | 1.0 | 1.08 | 0.01 | 26,263 |
| rs7844539 | rs3102735 | 0.373 | 8.5 | 0.124 | 26,345 |
| rs2073618 | rs2073617 | 0.539 | 42.57 | 0.224 | 231 |
| rs2073618 | rs3134069 | 0.696 | 4.89 | 0.043 | 936 |
| rs2073618 | rs3102735 | 0.089 | 0.18 | 0.001 | 1018 |
| rs2073617 | rs3134069 | 0.751 | 9.3 | 0.048 | 705 |
| rs2073617 | rs3102735 | 0.254 | 2.25 | 0.012 | 787 |
| rs3134069 | rs3102735 | 0.876 | 41.12 | 0.346 | 82 |
| Haplotype * | Group Turnout | Osteopenia vs. Control | Osteoporosis vs. Control | ||
|---|---|---|---|---|---|
| Control Group | Osteopenia | Osteoporosis | Chi-Squared p | Chi-Squared p | |
| CTTA | 0.346 | 0.380 | 0.358 | 0.5531 | 0.7774 |
| GCTA | 0.324 | 0.352 | 0.407 | 0.5191 | 0.0078 (0.0467) |
| GTTA | 0.121 | 0.143 | 0.076 | 0.3454 | 0.0171 (0.0981) |
| GTTG | 0.063 | 0.041 | 0.062 | 0.4069 | 0.8409 |
| CCTA | 0.055 | 0.001 | 0.011 | 0.0083 (0.0608) | 0.0022 (0.0132) |
| CCGG | 0.048 | 0.060 | 0.064 | 0.6116 | 0.3317 |
| GCTG | 0.014 | 0.025 | 0.011 | 0.8498 | 0.5419 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przerwa, F.; Uzar, I.; Bogacz, A.; Kotrych, K.; Sulikowski, T.; Wolek, M.; Kamiński, A.; Ziętek, P.; Czerny, B. Osteoprotegerin Gene as a Biomarker in the Development of Osteoporosis in Postmenopausal Women. Biomedicines 2023, 11, 3218. https://doi.org/10.3390/biomedicines11123218
Przerwa F, Uzar I, Bogacz A, Kotrych K, Sulikowski T, Wolek M, Kamiński A, Ziętek P, Czerny B. Osteoprotegerin Gene as a Biomarker in the Development of Osteoporosis in Postmenopausal Women. Biomedicines. 2023; 11(12):3218. https://doi.org/10.3390/biomedicines11123218
Chicago/Turabian StylePrzerwa, Filip, Izabela Uzar, Anna Bogacz, Katarzyna Kotrych, Tadeusz Sulikowski, Marlena Wolek, Adam Kamiński, Paweł Ziętek, and Bogusław Czerny. 2023. "Osteoprotegerin Gene as a Biomarker in the Development of Osteoporosis in Postmenopausal Women" Biomedicines 11, no. 12: 3218. https://doi.org/10.3390/biomedicines11123218







