Primary Arterial Hypertension and Drug-Induced Hypertension in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review
Abstract
:1. Introduction
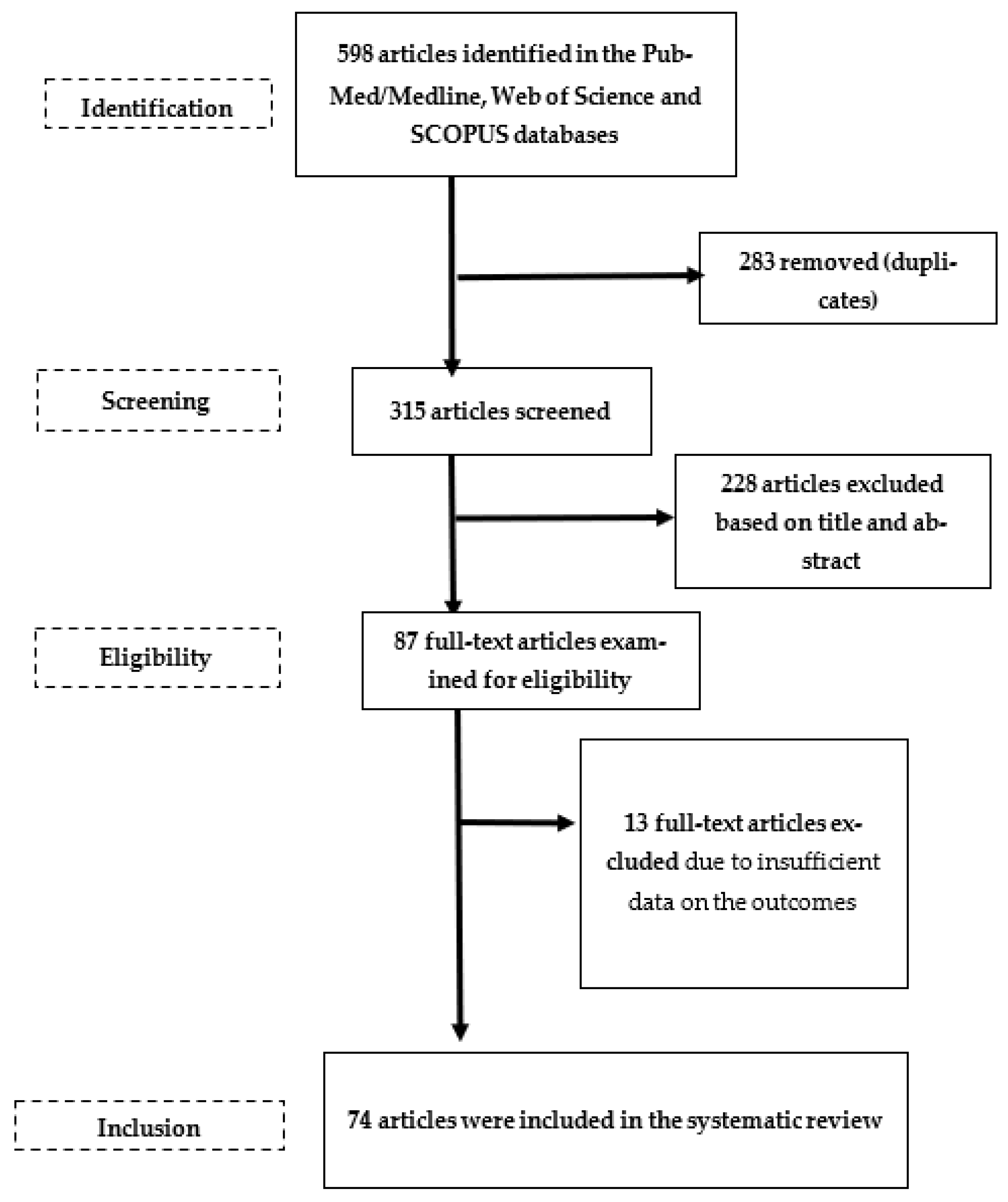
2. Materials and Methods
3. Results
3.1. Hypertension: Just a Comorbidity in Myeloproliferative Neoplasms?
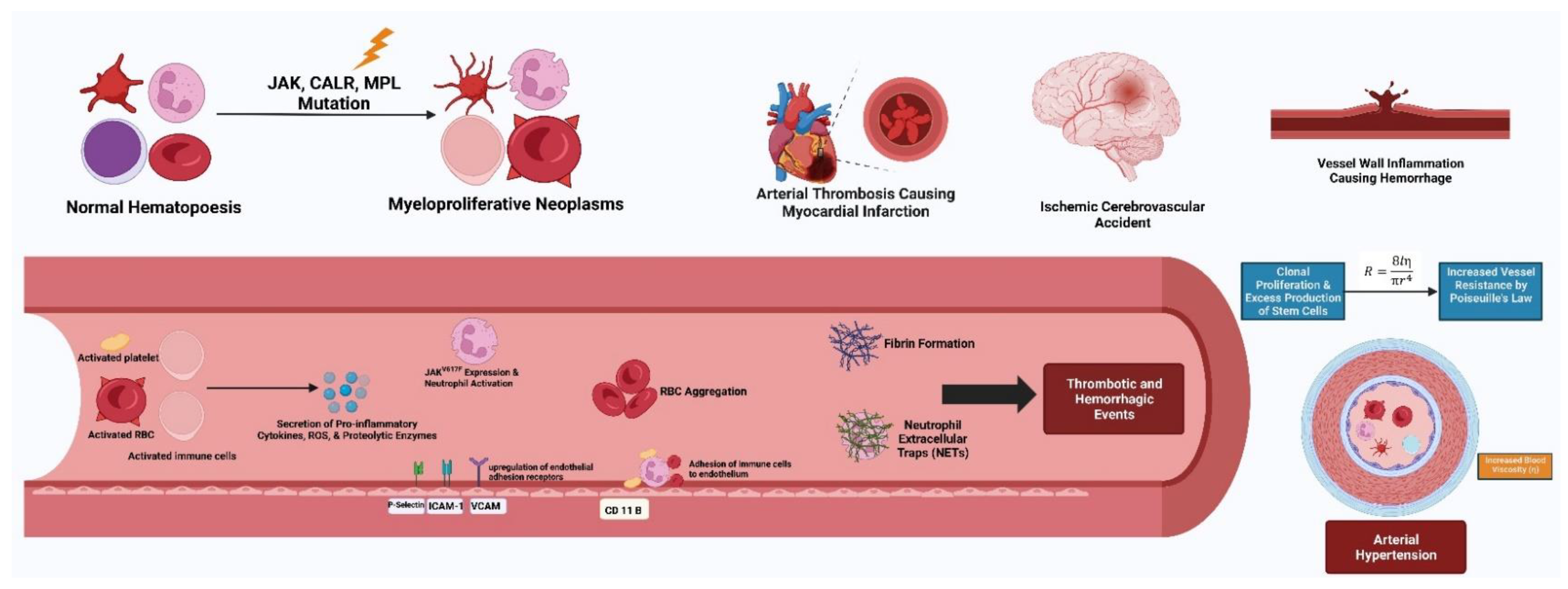
3.2. Hypertension and Thrombosis in Myeloproliferative Neoplasms
3.3. Hypertension Treatment Strategies in Myeloproliferative Neoplasms
3.4. Drug-Induced Hypertension and the Effect of Hematological Treatment on Hypertension as a Cardiovascular Risk Factor
4. Discussion
4.1. Hypertension Is the Most Common Comorbidity in Myeloproliferative Neoplasms
4.2. Relationship of Hypertension and Other Cardiovascular Risk Factors on Thrombotic Events in Myeloproliferative Neoplasms
4.3. Hypertension Treatment Strategies in Myeloproliferative Neoplasms
4.4. Drug-Induced Hypertension and the Effect of Hematological Treatment on Hypertension as a Cardiovascular Risk Factor
4.5. Caveats and Future Research Directions in Unravelling the Interplay between Hypertension and Myeloproliferative Neoplasms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASXL1 | additional sex comb-like transcriptional regulator 1 gene |
| BP | blood pressure |
| CALR | calreticulin gene |
| CHIP | clonal hematopoiesis of indeterminate potential |
| CI | confidence interval |
| DBP | diastolic BP |
| COVID-19 | Coronavirus Disease 2019 |
| CVRF | cardiovascular risk factor |
| DNA | deoxyribonucleic acid |
| DNMT3A | DNA methyltransferase 3 alpha gene |
| ET | essential thrombocythemia |
| ET-MF | post-essential thrombocythemia myelofibrosis |
| HTN | hypertension |
| IDH1/2 | isocitrate dehydrogenase 1 or 2 gene |
| IPSET | International Prognostic Score for Essential Thrombocythemia (score) |
| JAK2 | Janus kinase 2 gene |
| JAK2V617F | JAK2 gene with valine to phenylalanine substitution on codon 617 mutation |
| MBP | mean BP |
| MF | myelofibrosis |
| MINORS | methodological index for non-randomized observational studies |
| MMAT | Mixed Methods Appraisal Tool |
| MOST | Myelofibrosis and Essential Thrombocythemia Observational STudy |
| MPL | myeloproliferative leukemia protein gene |
| MPNs | myeloproliferative neoplasms |
| NOS | nitric oxide synthase |
| OR | odds ratio |
| PMF | primary myelofibrosis |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| PV | polycythemia vera |
| PV-MF | post-polycythemia vera myelofibrosis |
| REVEAL | the Prospective Observational Study of Patients With Polycythemia Vera in US Clinical Practices |
| SBP | systolic BP |
| SMF | secondary myelofibrosis |
| STAT3 | signal transducer and activator of transcription 3 |
| T2DM | type 2 diabetes mellitus |
| TET2 | tet methylcytosine dioxygenase 2 gene |
References
- Rungjirajittranon, T.; Owattanapanich, W.; Ungprasert, P.; Siritanaratkul, N.; Ruchutrakool, T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer 2019, 19, 184. [Google Scholar] [CrossRef]
- McMullin, M.F.; Anderson, L.A. Aetiology of Myeloproliferative Neoplasms. Cancers 2020, 12, 1810. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.; Aly, M. Effect of Charlson comorbidity index on vascular events and survival in Philadelphia chromosome-negative myeloproliferative neoplasms. Egypt. J. Haematol. 2021, 46, 111. [Google Scholar]
- Yokokawa, T.; Misaka, T.; Kimishima, Y.; Wada, K.; Minakawa, K.; Sugimoto, K.; Ishida, T.; Morishita, S.; Komatsu, N.; Ikeda, K.; et al. Crucial role of hematopoietic JAK2 V617F in the development of aortic aneurysms. Haematologica 2021, 106, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, P.; Klisiewicz, A.; Prejbisz, A.; Sikorska, A.; Wójcicki, J.; Lewandowski, J.; Kaszuba, A.M.; Kabat, M.; Kordybach-Prokopiuk, M.; Więcek, A.; et al. Reduced left ventricular strain is related to blood parameters in patients with polycythemia vera. Int. J. Cardiol. 2017, 226, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Finazzi, G.; Falanga, A. Myeloproliferative neoplasms and thrombosis. Blood 2013, 122, 2176–2184. [Google Scholar] [CrossRef]
- Moisa, C.; Găman, M.-A.; Diaconu, C.C.; Gaman, A.M. Oxidative Stress Levels, JAK2V617F Mutational Status and Thrombotic Complications in Patients with Essential Thrombocythemia. Rev. Chim. 2019, 70, 2822–2825. [Google Scholar] [CrossRef]
- Rodríguez, A.; Fortuño, A.; Gómez-Ambrosi, J.; Zalba, G.; Díez, J.; Frühbeck, G. The Inhibitory Effect of Leptin on Angiotensin II-Induced Vasoconstriction in Vascular Smooth Muscle Cells Is Mediated via a Nitric Oxide-Dependent Mechanism. Endocrinology 2007, 148, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhang, Y.; Pang, J.; Zhang, S.; Yu, Q.; He, L.; Wagner, K.U.; Zhou, Z.; Wang, C.Y. Loss of Jak2 Impairs Endothelial Function by Attenuating Raf-1/MEK1/Sp-1 Signaling Along with Altered eNOS Activities. Am. J. Pathol. 2013, 183, 617–625. [Google Scholar] [CrossRef]
- Wojcicki, J.; Sikorska, A.; Kaszuba, A.; Harazny, J.; Lewandowski, J.; Sinski, M.; Binczyk, E.; Szymanek, K.; Prejbisz, A.; Szaflik, J.; et al. [PP.12.17] Evaluation of Selected Parameters of Neuro-Hormonal Activity and Retinal and Intrarenal Perfusion in Patients with Polycythemia Vera. J. Hypertens. 2016, 34 (Suppl. S2), e189. [Google Scholar] [CrossRef]
- Lewandowski, J.; Sinski, M.; Sikorska, A.; Kaszuba, A.; Wojcicki, J.; Prejbisz, A.; Florczak, E.; Kabat, M.; Januszewicz, M.; Gaciong, Z.; et al. [PP.29.14] Sympathetic Activity is Decreased in Patients with Polycythemia Vera as Compared with Patients with Essential Hypertension. J. Hypertens. 2017, 35 (Suppl. S2), e325. [Google Scholar] [CrossRef]
- Gecht, J.; Tsoukakis, I.; Kricheldorf, K.; Stegelmann, F.; Klausmann, M.; Griesshammer, M.; Schulz, H.; Hollburg, W.; Göthert, J.R.; Sockel, K.; et al. Kidney Dysfunction Is Associated with Thrombosis and Disease Severity in Myeloproliferative Neoplasms: Implications from the German Study Group for MPN Bioregistry. Cancers 2021, 13, 4086. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.; Kuliszkiewicz-Janus, M.; Rymer, W.; Jaźwiec, B.; Małecki, R. Treatment of Essential Thrombocythemia with Anagrelide Is Associated with an Increased Risk of Worsened Kidney Function. Pharmacology 2021, 106, 316–322. [Google Scholar] [CrossRef]
- Papageorgiou, L.; Elalamy, I.; Vandreden, P.; Gerotziafas, G.T. Thrombotic and Hemorrhagic Issues Associated with Myeloproliferative Neoplasms. Clin. Appl. Thromb. 2022, 28, 10760296221097969. [Google Scholar] [CrossRef] [PubMed]
- Leiva, O.; Hobbs, G.; Ravid, K.; Libby, P. Cardiovascular Disease in Myeloproliferative Neoplasms. JACC CardioOncology 2022, 4, 166–182. [Google Scholar] [CrossRef]
- Dai, H.; Younis, A.; Kong, J.D.; Bragazzi, N.L.; Wu, J. Trends and Regional Variation in Prevalence of Cardiovascular Risk Factors and Association With Socioeconomic Status in Canada, 2005-2016. JAMA Netw. Open 2021, 4, e2121443. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Pluye, P.; Robert, E.; Cargo, M.; Bartlett, G.; O’cathain, A.; Griffiths, F.; Boardman, F.; Gagnon, M.P.; Rousseau, M.C. Proposal: A Mixed Methods Appraisal Tool for Systematic Mixed Studies Reviews; McGill University: Montréal, QC, Canada, 2011; Volume 2. [Google Scholar]
- Mitra, D.; Kaye, J.A.; Piecoro, L.T.; Brown, J.; Reith, K.; Mughal, T.I.; Sarlis, N.J. Symptom Burden and Splenomegaly in Patients with Myelofibrosis in the United States: A Retrospective Medical Record Review. Cancer Med. 2013, 2, 889–898. [Google Scholar] [CrossRef]
- Parasuraman, S.; Yu, J.; Paranagama, D.; Shrestha, S.; Wang, L.; Baser, O.; Scherber, R. Cytoreductive treatment patterns among US veterans with polycythemia vera. BMC Cancer 2018, 18, 528. [Google Scholar] [CrossRef] [Green Version]
- Grunwald, M.R.; Stein, B.L.; Boccia, R.V.; Oh, S.T.; Paranagama, D.; Parasuraman, S.; Colucci, P.; Mesa, R. Clinical and Disease Characteristics From REVEAL at Time of Enrollment (Baseline): Prospective Observational Study of Patients With Polycythemia Vera in the United States. Clin. Lymphoma Myeloma Leuk. 2018, 18, 788–795.e2. [Google Scholar] [CrossRef] [PubMed]
- Paranagama, D.; Colucci, P.; Evans, K.A.; Bonafede, M.; Parasuraman, S. Are patients with high-risk polycythemia vera receiving cytoreductive medications? A retrospective analysis of real-world data. Exp. Hematol. Oncol. 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Accurso, V.; Santoro, M.; Mancuso, S.; Contrino, A.D.; Casimio, P.; Sardo, M.; Raso, S.; Di Piazza, F.; Perez, A.; Bono, M.; et al. Cardiovascular Risk in Essential Thrombocythemia and Polycythemia Vera: Thrombotic Risk and Survival. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020008. [Google Scholar] [CrossRef]
- Yacoub, A.; Lyons, R.; Verstovsek, S.; Shao, R.; Chu, D.T.; Agrawal, A.; Sivaraman, S.; Colucci, P.; Paranagama, D.; Mascarenhas, J. Disease and Clinical Characteristics of Patients With a Clinical Diagnosis of Essential Thrombocythemia Enrolled in the MOST Study. Clin. Lymphoma Myeloma Leuk. 2021, 21, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Deo, P.; Sachdeva, M.U.S.; Bose, P.; Lad, D.; Prakash, G.; Khadwal, A.; Varma, N.; Varma, S.; Malhotra, P. Aberrant expression of cytokines in polycythemia vera correlate with the risk of thrombosis. Blood Cells Mol. Dis. 2021, 89, 102565. [Google Scholar] [CrossRef]
- Yap, Y.Y.; Law, K.B.; Sathar, J.; Lau, N.S.; Goh, A.S.; Chew, T.K.; Lim, S.M.; Menon, P.; Guan, Y.K.; Bin Husin, A.; et al. The epidemiology and clinical characteristics of myeloproliferative neoplasms in Malaysia. Exp. Hematol. Oncol. 2018, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.Z.; Raza, N.; Nasir, M.I.; Zaidi, S.M.H. Frequency of Zygosity in Jak-2 Positive Patients with Polycythemia Vera-Pakistan’s Perspective. Asian Pac. J. Cancer Prev. 2021, 22, 559–564. [Google Scholar] [CrossRef]
- Mancuso, S.; Accurso, V.; Santoro, M.; Raso, S.; Contrino, A.D.; Perez, A.; Di Piazza, F.; Florena, A.M.; Russo, A.; Siragusa, S. The Essential Thrombocythemia, Thrombotic Risk Stratification, and Cardiovascular Risk Factors. Adv. Hematol. 2020, 2020, 9124821. [Google Scholar] [CrossRef] [Green Version]
- Fattizzo, B.; Giannotta, J.A.; Sciumè, M.; Cattaneo, D.; Bucelli, C.; Fracchiolla, N.S.; Onida, F.; Baldini, L.; Barcellini, W.; Iurlo, A. Reply to “COVID-19 in persons with haematological cancers”: A focus on myeloid neoplasms and risk factors for mortality. Leukemia 2020, 34, 1957–1960. [Google Scholar] [CrossRef]
- Morrissey, H.; Ball, P.; Mandal, A.; Nevil, A.; Paneesha, S.; Basu, S.; Karim, F.; Hossain, I.; Phillips, N.; Khawaja, J.; et al. COVID-19 in haematology patients: A multicentre West Midlands clinical outcomes analysis on behalf of the West Midlands Research Consortium. Br. J. Haematol. 2021, 192, e11–e14. [Google Scholar] [CrossRef]
- Delluc, A.; Lacut, K.; Pan-Petesch, B.; Galinat, H.; Lippert, E.; Ianotto, J.-C. Statin exposure and thrombosis risk in patients with myeloproliferative neoplasms. Thromb. Res. 2018, 167, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Catani, L.; Testoni, N.; Ottaviani, E.; Polverelli, N.; Fiacchini, M.; De Vivo, A.; Salmi, F.; Lucchesi, A.; Baccarani, M.; et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: Safety of cytoreductive therapy. Am. J. Hematol. 2009, 84, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Nakagawa, N.; Suzuki, A.; Kabara, M.; Matsuki, M.; Shindo, M.; Iwasaki, S.; Ogawa, Y.; Hasebe, N. Novel Detection of CALR-Mutated Cells in Myeloproliferative Neoplasm-Related Glomerulopathy With Interstitial Extramedullary Hematopoiesis: A Case Report. Am. J. Kidney Dis. 2019, 74, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Patino-Alonso, C.; Gómez-Sánchez, M.; Hernández-Rivas, J.M.; González-Porras, J.R.; Bastida-Bermejo, J.M.; Martín, A.-A.; Rodríguez-Sánchez, E.; Recio-Rodríguez, J.I.; González-Sánchez, J.; Maderuelo-Fernández, J.A.; et al. Vascular target organ damage in patients with Philadelphia negative myeloproliferative syndrome: A propensity score analysis. Med. Clin. 2021, 158, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Oakley, C.; Ross, D.M.; Simon, S. Sudden onset vision loss: An atypical presentation of giant cell arteritis and myeloproliferative neoplasm. Med. J. Aust. 2021, 214, 14–15.e1. [Google Scholar] [CrossRef] [PubMed]
- Person, F.; Meyer, S.C.; Hopfer, H.; Menter, T. Renal post-mortem findings in myeloproliferative and myelodysplastic/myeloproliferative neoplasms. Virchows Arch. 2021, 479, 1013–1020. [Google Scholar] [CrossRef]
- Lucijanic, M.; Krecak, I.; Galusic, D.; Sedinic, M.; Holik, H.; Perisa, V.; Peric, M.M.; Zekanovic, I.; Stoos-Veic, T.; Pejsa, V.; et al. Higher serum uric acid is associated with higher risks of thrombosis and death in patients with primary myelofibrosis. Wien. Klin. Wochenschr. 2021, 134, 1–7. [Google Scholar] [CrossRef]
- Akdi, A.; Özeke, Ö.; Karanfil, M.; Ertem, A.G.; Yayla, Ç.; Demirtaş, K.; Güney, T.; Ünal, S.; Selçuk, M.T. Diurnal Rhythm of Blood Pressure in Patients with Polycythemia Vera. Blood Press. Monit. 2020, 25, 69–74. [Google Scholar] [CrossRef]
- Jóźwik-Plebanek, K.; Dobrowolski, P.; Lewandowski, J.; Narkiewicz, K.; Sikorska, A.; Siński, M.; Eisenhofer, G.; Schmieder, R.E.; Januszewicz, M.; Windyga, J.; et al. Blood pressure profile, sympathetic nervous system activity and subclinical target organ damage in patients with polycythemia vera. Pol. Arch. Intern. Med. 2020, 130, 607–614. [Google Scholar] [CrossRef]
- Rusak, T.; Misztal, T.; Piszcz, J.; Tomasiak, M. Nitric oxide scavenging by cell-free hemoglobin may be a primary factor determining hypertension in polycythemic patients. Free. Radic. Res. 2014, 48, 230–238. [Google Scholar] [CrossRef]
- Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; Vannucchi, A.M.; Antonioli, E.; et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood 2011, 117, 5857–5859. [Google Scholar] [CrossRef] [PubMed]
- Buxhofer-Ausch, V.; Olcaydu, D.; Gisslinger, B.; Schalling, M.; Frantal, S.; Thiele, J.; Müllauer, L.; Kvasnicka, H.-M.; Watzke, H.; Kralovics, R.; et al. Decanucleotide insertion polymorphism of F7 significantly influences the risk of thrombosis in patients with essential thrombocythemia. Eur. J. Haematol. 2014, 93, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, É.; Marton, I.; Kotosz, B.; Borbényi, Z. Contribution of cardiovascular risk factors in the thrombotic complications of essential thrombocythaemia: A Hungarian single-institute retrospective analysis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1258–1263. [Google Scholar] [PubMed]
- Pósfai,, È.; Marton, I.; Kiss-László, Z.; Kotosz, B.; Széll, M.; Borbényi, Z. Thrombosis and risk factors in female patients with a rare acquired thrombophilia: Chronic myeloproliferative disorder—Polycythaemia vera and essential thrombocythaemia. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3810–3818. [Google Scholar]
- Horvat, I.; Boban, A.; Zadro, R.; Antolic, M.R.; Serventi-Seiwerth, R.; Roncevic, P.; Radman, I.; Sertic, D.; Vodanovic, M.; Pulanic, D.; et al. Influence of Blood Count, Cardiovascular Risks, Inherited Thrombophilia, and JAK2 V617F Burden Allele on Type of Thrombosis in Patients With Philadelphia Chromosome Negative Myeloproliferative Neoplasms. Clin. Lymphoma Myeloma Leuk. 2018, 19, 53–63. [Google Scholar] [CrossRef]
- Leković, D.; Gotic, M.; Milic, N.; Miljic, P.; Mitrović, M.; Čokić, V.; Elezovic, I. The importance of cardiovascular risk factors for thrombosis prediction in patients with essential thrombocythemia. Med. Oncol. 2014, 31, 1–6. [Google Scholar] [CrossRef]
- Lekovic, D.; Gotic, M.; Sefer, D.; Mitrovic-Ajtic, O.; Cokic, V.; Milic, N. Predictors of survival and cause of death in patients with essential thrombocythemia. Eur. J. Haematol. 2015, 95, 461–466. [Google Scholar] [CrossRef]
- Schwarz, J.; Ovesná, P.; Černá, O.; Kissová, J.; Soukupová, J.M.; Brychtová, Y.; Doubek, M.; Červinek, L.; Cmunt, E.; Dulíček, P.; et al. Thrombosis in thrombocythemic Ph- myeloproliferations is associated with higher platelet count prior to the event: Results of analyses of prothrombotic risk factors from a registry of patients treated with anagrelide. Eur. J. Haematol. 2015, 96, 98–106. [Google Scholar] [CrossRef]
- Accurso, V.; Santoro, M.; Mancuso, S.; Siragusa, S. Cardiovascular risk factor in MPN patients. J. Thromb. Thrombolysis 2020, 50, 640–641. [Google Scholar] [CrossRef]
- Cucuianu, A.; Stoia, M.; Farcas, A.D.; Dima, D.; Zdrenghea, M.; Paţiu, M.; Olinic, D.; Petrov, L. Arterial stenosis and atherothrombotic events in polycythemia vera and essential thrombocythemia. Rom. J. Intern. Med. 2006, 44, 397–406. [Google Scholar]
- Barbui, T.; Vannucchi, A.M.; Carobbio, A.; Rumi, E.; Finazzi, G.; Gisslinger, H.; Ruggeri, M.; Randi, M.L.; Cazzola, M.; Rambaldi, A.; et al. The effect of arterial hypertension on thrombosis in low-risk polycythemia vera. Am. J. Hematol. 2016, 92, E5–E6. [Google Scholar] [CrossRef]
- Benevolo, G.; Elli, E.M.; Bartoletti, D.; Latagliata, R.; Tiribelli, M.; Heidel, F.H.; Cavazzini, F.; Bonifacio, M.; Crugnola, M.; Binotto, G.; et al. Impact of comorbidities and body mass index on the outcome of polycythemia vera patients. Hematol. Oncol. 2021, 39, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Birgegard, G.; Besses, C.; Griesshammer, M.; Gugliotta, L.; Harrison, C.N.; Hamdani, M.; Wu, J.; Achenbach, H.; Kiladjian, J.-J. Treatment of essential thrombocythemia in Europe: A prospective long-term observational study of 3649 high-risk patients in the Evaluation of Anagrelide Efficacy and Long-term Safety study. Haematologica 2018, 103, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cerquozzi, S.; Barraco, D.; Lasho, T.; Finke, C.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; Tefferi, A. Risk factors for arterial versus venous thrombosis in polycythemia vera: A single center experience in 587 patients. Blood Cancer J. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.; Alvarez-Larrán, A.; Arellano-Rodrigo, E.; Granell, M.; Domingo, A.; Montserrat, E. Frequency and risk factors for thrombosis in idiopathic myelofibrosis: Analysis in a series of 155 patients from a single institution. Leukemia 2006, 20, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Navarro, L.M.; Trufelli, D.C.; Bonito, D.R.; Del Giglio, A.; Bollmann, P.W. Application of prognostic score IPSET-thrombosis in patients with essential thrombocythemia of a Brazilian public service. Rev. Assoc. Med. Bras. 2016, 62, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Shih, L.-Y.; Lin, T.-L.; Lai, C.-L.; Dunn, P.; Wu, J.-H.; Wang, P.-N.; Kuo, M.-C.; Lee, L.-C. Predictive values of X-chromosome inactivation patterns and clinicohematologic parameters for vascular complications in female patients with essential thrombocythemia. Blood 2002, 100, 1596–1601. [Google Scholar] [CrossRef]
- Bucalossi, A.; Marotta, G.; Bigazzi, C.; Galieni, P.; Dispensa, E. Reduction of Antithrombin III, Protein C, and Protein S Levels and Activated Protein C Resistance in Polycythemia Vera and Essential Thrombocythemia Patients with Thrombosis. Am. J. Hematol. 1996, 52, 14–20. [Google Scholar] [CrossRef]
- Landolfi, R.; Di Gennaro, L.; Barbui, T.; De Stefano, V.; Finazzi, G.; Marfisi, R.; Tognoni, G.; Marchioli, R.; European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP). Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007, 109, 2446–2452. [Google Scholar] [CrossRef] [Green Version]
- Finazzi, G. A prospective analysis of thrombotic events in the European collaboration study on low-dose aspirin in polycythemia (ECLAP). Pathol. Biol. 2004, 52, 285–288. [Google Scholar] [CrossRef]
- Bazzan, M.; Tamponi, G.; Schinco, P.; Vaccarino, A.; Foli, C.; Gallone, G.; Pileri, A. Thrombosis-free survival and life expectancy in 187 consecutive patients with essential thrombocythemia. Ann. Hematol. 1999, 78, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Cortelazzo, S.; Viero, P.; Finazzi, G.; D’Emilio, A.; Rodeghiero, F.; Barbui, T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J. Clin. Oncol. 1990, 8, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, R.; Juvonen, E.; Ikkala, E.; Oksanen, K.; Anttila, P.; Ruutu, T. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann. Hematol. 2001, 80, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Finazzi, G.; Vannucchi, A.M.; De Stefano, V. Targeting myeloid cells to prevent recurrent stroke in general population: The lesson of hydroxyurea in myeloproliferative neoplasms. Blood Cancer J. 2018, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Košťál, M.; Schwarz, J.; Ovesná, P.; Penka, M.; Dulíček, P.; for CZEMP–Czech Group. Ph- Myeloproliferative neoplasms Ph− myeloproliferative neoplasms and the related risk factors for stroke occurrence: Results from a registry of patients treated with Anagrelide. J. Thromb. Thrombolysis 2020, 51, 112–119. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, V.; Carobbio, A.; Di Lazzaro, V.; Guglielmelli, P.; Iurlo, A.; Finazzi, M.C.; Rumi, E.; Cervantes, F.; Elli, E.M.; Randi, M.L.; et al. Benefit-risk profile of cytoreductive drugs along with antiplatelet and antithrombotic therapy after transient ischemic attack or ischemic stroke in myeloproliferative neoplasms. Blood Cancer J. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, L.; Huang, X.; Fan, C.; Zhao, H.; Li, Z.; Shen, H.; Chen, J.; Duan, J. Clinical Characteristics and Management of Cerebral Venous Sinus Thrombosis in Patients with Essential Thrombocythemia. Neuropsychiatr. Dis. Treat. 2021, 17, 1195–1206. [Google Scholar] [CrossRef]
- Robertson, B.; Urquhart, C.; Ford, I.; Townend, J.; Watson, H.G.; Vickers, M.A.; Greaves, M. Platelet and coagulation activation markers in myeloproliferative diseases: Relationships with JAK2 V6I7 F status, clonality, and antiphospholipid antibodies: Platelet Activation and JAK2 Mutation Status. J. Thromb. Haemost. 2007, 5, 1679–1685. [Google Scholar] [CrossRef]
- Barbui, T.; Masciulli, A.; Ghirardi, A.; Carobbio, A. ACE inhibitors and cytoreductive therapy in polycythemia vera. Blood 2017, 129, 1226–1227. [Google Scholar] [CrossRef]
- Krečak, I.; Perić, M.M.; Zekanović, I.; Holik, H.; Coha, B.; Gverić-Krečak, V.; Lucijanić, M. Beneficial effect of ACE inhibitors on kidney function in polycythemia vera. Wien. Klin. Wochenschr. 2021, 133, 808–815. [Google Scholar] [CrossRef]
- Gorokhovskaya, G.N.; Гopoxoвcкaя, Г.H.; Zavyalova, A.I.; Зaвьялoвa, A.И.; Martynov, A.I.; Mapтынoв, A.И. Diroton effects on 24-h fluctuations of arterial pressure as shown bymonitoring in hypertensive patients with polycythemia vera. Ter. Arkh. 2003, 78, 26–32. [Google Scholar]
- Rao, R.; Kulkarni, S.; Wilkinson, I.B. Two Cases of Severe Hypertension in JAK2 Mutation-Positive Myeloproliferative Neoplasms. Case Rep. Vasc. Med. 2020, 2020, 8887423. [Google Scholar] [CrossRef] [PubMed]
- Najman, A.; Andre, K.; Gorin, N.-C. Influence des polypathologies sur le traitement des hémopathies malignes. Bull. Cancer 2009, 96, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Mollé, N.; Krichevsky, S.; Kermani, P.; Silver, R.T.; Ritchie, E.; Scandura, J. Ruxolitinib can cause weight gain by blocking leptin signaling in the brain via JAK2/STAT3. Blood 2020, 135, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Sapre, M.; Tremblay, D.; Wilck, E.; James, A.; Leiter, A.; Coltoff, A.; Koshy, A.G.; Kremyanskaya, M.; Hoffman, R.; Mascarenhas, J.O.; et al. Metabolic Effects of JAK1/2 Inhibition in Patients with Myeloproliferative Neoplasms. Sci. Rep. 2019, 9, 16609. [Google Scholar] [CrossRef] [Green Version]
- Passamonti, F.; Griesshammer, M.; Palandri, F.; Egyed, M.; Benevolo, G.; Devos, T.; Callum, J.; Vannucchi, A.M.; Sivgin, S.; Bensasson, C.; et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): A randomised, open-label, phase 3b study. Lancet Oncol. 2016, 18, 88–99. [Google Scholar] [CrossRef]
- Mascarenhas, J.O.; Rampal, R.K.; Kosiorek, H.E.; Bhave, R.; Hexner, E.; Wang, E.S.; Gerds, A.; Abboud, C.N.; Kremyanskaya, M.; Berenzon, D.; et al. Phase 2 study of ruxolitinib and decitabine in patients with myeloproliferative neoplasm in accelerated and blast phase. Blood Adv. 2020, 4, 5246–5256. [Google Scholar] [CrossRef]
- Rampal, R.K.; Mascarenhas, J.O.; Kosiorek, H.E.; Price, L.; Berenzon, D.; Hexner, E.; Abboud, C.N.; Kremyanskaya, M.; Weinberg, R.S.; Salama, M.E.; et al. Safety and efficacy of combined ruxolitinib and decitabine in accelerated and blast-phase myeloproliferative neoplasms. Blood Adv. 2018, 2, 3572–3580. [Google Scholar] [CrossRef] [Green Version]
- Gowin, K.; Kosiorek, H.; Dueck, A.; Mascarenhas, J.; Hoffman, R.; Reeder, C.; Camoriano, J.; Tibes, R.; Gano, K.; Palmer, J.; et al. Multicenter phase 2 study of combination therapy with ruxolitinib and danazol in patients with myelofibrosis. Leuk. Res. 2017, 60, 31–35. [Google Scholar] [CrossRef]
- Griesshammer, M.; Saydam, G.; Palandri, F.; Benevolo, G.; Egyed, M.; Callum, J.; Devos, T.; Sivgin, S.; Guglielmelli, P.; Bensasson, C.; et al. Ruxolitinib for the treatment of inadequately controlled polycythemia vera without splenomegaly: 80-week follow-up from the RESPONSE-2 trial. Ann. Hematol. 2018, 97, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Kiladjian, J.-J.; Guglielmelli, P.; Griesshammer, M.; Saydam, G.; Masszi, T.; Durrant, S.; Passamonti, F.; Jones, M.; Zhen, H.; Li, J.; et al. Efficacy and safety of ruxolitinib after and versus interferon use in the RESPONSE studies. Ann. Hematol. 2018, 97, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Kiladjian, J.-J.; Zachee, P.; Hino, M.; Pane, F.; Masszi, T.; Harrison, C.N.; Mesa, R.; Miller, C.B.; Passamonti, F.; Durrant, S.; et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020, 7, e226–e237. [Google Scholar] [CrossRef] [PubMed]
- Foran, J.; Ravandi, F.; Wierda, W.; Garcia-Manero, G.; Verstovsek, S.; Kadia, T.; Burger, J.; Yule, M.; Langford, G.; Lyons, J.; et al. A Phase I and Pharmacodynamic Study of AT9283, a Small-Molecule Inhibitor of Aurora Kinases in Patients With Relapsed/Refractory Leukemia or Myelofibrosis. Clin. Lymphoma Myeloma Leuk. 2014, 14, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaldi, A.; Iurlo, A.; Vannucchi, A.M.; Martino, B.; Guarini, A.; Ruggeri, M.; von Bubnoff, N.; De Muro, M.; McMullin, M.F.; Luciani, S.; et al. Long-term safety and efficacy of givinostat in polycythemia vera: 4-year mean follow up of three phase 1/2 studies and a compassionate use program. Blood Cancer J. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Gotic, M.; Egyed, M.; Gercheva, L.; Warzocha, K.; Kvasnicka, H.M.; Achenbach, H.; Wu, J. Cardiovascular Safety of Anagrelide Hydrochloride versus Hydroxyurea in Essential Thrombocythaemia. Cardiovasc. Toxicol. 2021, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Gugliotta, L.; Tieghi, A.; Tortorella, G.; Scalzulli, P.R.; Ciancia, R.; Lunghi, M.; Cacciola, E.; Cacciola, R.; Candoni, A.; Crugnola, M.; et al. Low impact of cardiovascular adverse events on anagrelide treatment discontinuation in a cohort of 232 patients with essential thrombocythemia. Leuk. Res. 2011, 35, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, G.; Piccin, A.; Tieghi, A.; Marcheselli, L.; Steurer, M.; Gastl, G.; Codeluppi, K.; Fama, A.; Santoro, U.; Birtolo, C.; et al. Anagrelide Treatment and Cardiovascular Monitoring in Essential Thrombocythemia. A Prospective Observational Study. Leuk. Res. 2015, 39, 592–598. [Google Scholar]
- Kimishima, Y.; Misaka, T.; Yokokawa, T.; Wada, K.; Ueda, K.; Sugimoto, K.; Minakawa, K.; Nakazato, K.; Ishida, T.; Oshima, M.; et al. Clonal hematopoiesis with JAK2V617F promotes pulmonary hypertension with ALK1 upregulation in lung neutrophils. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Frederiksen, H.; Szépligeti, S.K.; Bak, M.; Ghanima, W.; Hasselbalch, H.C.; Christiansen, C.F. Vascular Diseases In Patients With Chronic Myeloproliferative Neoplasms—Impact Of Comorbidity. Clin. Epidemiol. 2019, 11, 955–967. [Google Scholar] [CrossRef] [Green Version]
- Kingston, H.W.F.; Ghose, A.; Rungpradubvong, V.; Satitthummanid, S.; Herdman, M.T.; Plewes, K.; Ishioka, H.; Leopold, S.J.; Sinha, I.; Intharabut, B.; et al. Cell-Free Hemoglobin Is Associated With Increased Vascular Resistance and Reduced Peripheral Perfusion in Severe Malaria. J. Infect. Dis. 2019, 221, 127–137. [Google Scholar] [CrossRef]
- Mrug, M.; Stopka, T.; Julian, B.A.; Prchal, J.F. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J. Clin. Investig. 1997, 100, 2310–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, H.; Lal, S.M. A Prospective, Randomized, Open Labeled Crossover Trial of Fosinopril and Theophylline in Post Renal Transplant Erythrocytosis. Ren. Fail. 2003, 25, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Filho, G.A. Use of Aminophylline and Enalapril in Posttransplant Polycythemia. Transplantation 1998, 65, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Sugihara, T.; Tomiyama, T.; Kitano, Y.; Yawata, Y.; Osawa, G. Polycythaemia vera: Response to treatment with angiotensin-converting enzyme inhibitor. Eur. J. Haematol. 1996, 57, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Park, P.G.; Song, K.-I. IgA Nephropathy in a Patient with Polycythemia vera. Am. J. Nephrol. 2002, 22, 397–401. [Google Scholar] [CrossRef]
- Lucijanic, M.; Galusic, D.; Krecak, I.; Sedinic, M.; Holik, H.; Perisa, V.; Peric, M.M.; Zekanovic, I.; Stoos-Veic, T.; Kusec, R. Reduced renal function strongly affects survival and thrombosis in patients with myelofibrosis. Ann. Hematol. 2020, 99, 2779–2785. [Google Scholar] [CrossRef]
- Krečak, I.; Holik, H.; Martina, M.P.; Zekanović, I.; Coha, B.; Gverić-Krečak, V. Chronic kidney disease could be a risk factor for thrombosis in essential thrombocythemia and polycythemia vera. Int. J. Hematol. 2020, 112, 377–384. [Google Scholar] [CrossRef]
- Gargallo, P.; Molero, M.; Bilbao, C.; Stuckey, R.; Carrillo-Cruz, E.; Hermosín, L.; Pérez-López, O.; Jiménez-Velasco, A.; Soria, E.; Lázaro, M.; et al. Next-Generation DNA Sequencing-Based Gene Panel for Diagnosis and Genetic Risk Stratification in Onco-Hematology. Cancers 2022, 14, 1986. [Google Scholar] [CrossRef]
- Kjær, L. Clonal Hematopoiesis and Mutations of Myeloproliferative Neoplasms. Cancers 2020, 12, 2100. [Google Scholar] [CrossRef]
- Găman, M.-A.; Cozma, M.-A.; Dobrică, E.-C.; Crețoiu, S.M.; Găman, A.M.; Diaconu, C.C. Liquid Biopsy and Potential Liquid Biopsy-Based Biomarkers in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review. Life 2021, 11, 677. [Google Scholar] [CrossRef]
- Delic, S.; Rose, D.; Kern, W.; Nadarajah, N.; Haferlach, C.; Haferlach, T.; Meggendorfer, M. Application of an NGS-based 28-gene panel in myeloproliferative neoplasms reveals distinct mutation patterns in essential thrombocythaemia, primary myelofibrosis and polycythaemia vera. Br. J. Haematol. 2016, 175, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-F.; Li, J.; Xu, L.-L.; Găman, M.-A.; Zou, Z.-Y. Allogeneic stem cell transplantation in the treatment of acute myeloid leukemia: An overview of obstacles and opportunities. World J. Clin. Cases 2023, 11, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Gianelli, U.; Thiele, J.; Orazi, A.; Gangat, N.; Vannucchi, A.M.; Tefferi, A.; Kvasnicka, H.M. International Consensus Classification of myeloid and lymphoid neoplasms: Myeloproliferative neoplasms. Virchows Arch. 2022, 482, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Melaccio, A.; Vacca, A.; Ria, R. The bone marrow niche landscape: A journey through aging, extrinsic and intrinsic stressors in the haemopoietic milieu. J. Cancer Metastasis Treat. 2022, 8, 9. [Google Scholar] [CrossRef]
- Bhuria, V.; Baldauf, C.K.; Schraven, B.; Fischer, T. Thromboinflammation in Myeloproliferative Neoplasms (MPN)—A Puzzle Still to Be Solved. Int. J. Mol. Sci. 2022, 23, 3206. [Google Scholar] [CrossRef]
- Gaman, A.M.; Moisa, C.; Diaconu, C.C.; Gaman, M.A. Crosstalk between Oxidative Stress, Chronic Inflammation and Disease Progression in Essential Thrombocythemia. Rev. Chim. 2019, 70, 3486–3489. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Xu, L.; Găman, M.-A.; Zou, Z. The genesis and evolution of acute myeloid leukemia stem cells in the microenvironment: From biology to therapeutic targeting. Cell Death Discov. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Tofolean, I.T.; Ganea, C.; Ionescu, D.; Filippi, A.; Garaiman, A.; Goicea, A.; Gaman, M.-A.; Dimancea, A.; Baran, I. Cellular determinants involving mitochondrial dysfunction, oxidative stress and apoptosis correlate with the synergic cytotoxicity of epigallocatechin-3-gallate and menadione in human leukemia Jurkat T cells. Pharmacol. Res. 2016, 103, 300–317. [Google Scholar] [CrossRef]
- Fisher, D.A.C.; Fowles, J.S.; Zhou, A.; Oh, S.T. Inflammatory Pathophysiology as a Contributor to Myeloproliferative Neoplasms. Front. Immunol. 2021, 12, 683401. [Google Scholar] [CrossRef]
- Hasselbalch, H.C.; Bjørn, M.E. MPNs as Inflammatory Diseases: The Evidence, Consequences, and Perspectives. Mediat. Inflamm. 2015, 2015, 102476. [Google Scholar] [CrossRef] [Green Version]
- Gaulin, C.; Kelemen, K.; Yi, C.A. Molecular Pathways in Clonal Hematopoiesis: From the Acquisition of Somatic Mutations to Transformation into Hematologic Neoplasm. Life 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Bartels, S.; Vogtmann, J.; Schipper, E.; Büsche, G.; Schlue, J.; Lehmann, U.; Kreipe, H. Combination of myeloproliferative neoplasm driver gene activation with mutations of splice factor or epigenetic modifier genes increases risk of rapid blastic progression. Eur. J. Haematol. 2021, 106, 520–528. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Study Location | MPN Subtype | Number of Patients | Main Results |
|---|---|---|---|---|
| Mitra et al. (2013) [20] | USA | PMF, SMF | 180 | ↑ BP in 52% of PMF/SMF patients with splenomegaly, 50% in those without HTN = most common comorbidity in PMF/SMF |
| Parasuraman et al. (2018) [21] | USA | PV | 7718 | HTN = most prevalent comorbidity (~72%) (n = 5531) of PV patients enrolled in the United States Veterans Health Administration database |
| Grunwald et al. (2018) [22] | USA | PV | 2510 | HTN = most prevalent comorbidity (~71%) (n = 1772) of PV subjects enrolled in The Prospective Observational Study of Patients With Polycythemia Vera in US Clinical Practices (REVEAL) |
| Paranagama et al. (2018) [23] | USA | PV | 2856 | HTN in ~57 (n = 1630) of PV cases ↑ BP more prevalent in high-risk vs. low-risk PV (~65% vs. ~43%, p < 0.001) |
| Accurso et al. (2020) [24] | Italy | PV, ET, PMF, SMF | 603 | ↑BP = #1 CVRF in MPNs ↑ BP in ~63% of PV, ~64% of ET, ~15% of pre-fibrotic PMF, ~63% of PMF/SMF |
| Yacoub et al. (2021) [25] | USA | ET | 1207 | ↑ BP in 56% of the 1207 ET Myelofibrosis and Essential Thrombocythemia Observational STudy (MOST), HTN #1 comorbidity in ET |
| Jain et al. (2021) [26] | India | PV | 52 | HTN #1 comorbidity in PV (n = 24/52 patients, 46%) |
| Yap et al. (2018) [27] | Malaysia | PV, ET, PMF | 1010 | association of HTN with JAK2V617F (OR = 1.688; 95% CI = 1.234–2.310; p = 0.001) and with the diagnosis of PV and ET HTN #1 comorbidity in MPN subjects, i.e., PV > ET > PMF > MPN unclassifiable > hypereosinophilic syndrome (~58% vs. ~42% vs. ~41% vs. ~36% vs. ~33%, p < 0.001) |
| Shah et al. (2021) [28] | Pakistan | PV | 51 | ~90% of PV cases display HTN HTN associated with patients’ age (p = 0.033) |
| Mancuso et al. (2020) [29] | Italy | ET | 233 | HTN #1 CVRF in ET ~40% of ET cases display 1 CVRF, whereas ~35% have multiple CVRFs IPSET-thrombosis score calculation reclassifies several ET cases from low-risk of thrombotic events to intermediate or high-risk thrombotic complications occur mainly in ET with associated CVRFs |
| Fattizzo et al. (2020) [30] | Italy | NS | 16 | HTN #1 comorbidity (56%) in myeloid cancers (50% of which were MPNs or MPN/MDS) who developed COVID-19 HTN had no impact on survival in this setting |
| Morrissey et al. (2020) [31] | UK | NS | NS | HTN #1 comorbidity (41%) in COVID-19 patients with malignant (including MPNs) and benign blood disorders of whom 55% died HTN not linked with adverse outcomes |
| Delluc et al. (2018) [32] | France | PV, ET | 192 | HTN #1 comorbidity in MPNs (57.3%) HTN more likely in PV and ET cases on statin treatment (~85% vs. ~51%, p < 0.001) |
| Palandri et al. (2009) [33] | Italy | ET | 386 | HTN occurred in 48% of ET cases HTN independently predicted poor survival in ET |
| Wojcicki et al. (2016) [10] Lewandowski et al. (2017) [11] | Poland | PV | 20 12 | ↑ BP in ¾ of PV subjects when examined in the investigator’s offices or on ambulatory BP measurements less notable ↓ in BP during the night ↓ heart rate values per 24 h (heart rate during the day was particularly ↓ in PV) ↓ microneurography-evaluated muscle sympathetic nervous activity (muscle activity per minute particularly ↓ in PV and positively associated with the heart rate during the night, r = 0.617, p < 0.05) ↓ serum free epinephrine, ↓ aldosterone concentrations (p = 0.048) in HTN + PV versus HTN non-PV patients no differences regarding the morphology of the retina between the two groups ↓ decreased retinal microperfusion (p = 0.032) in PV negatively associated with number of erythrocytes in PV (r = −0.44, p = 0.012) ↑ resistive index of the kidney arteries in PV versus non-PV HTN (p = 0.033) |
| Maruyama et al. (2019) [34] | Japan | PMF | 1 | HTN may aggravate MPN-related glomerulopathy via nephrosclerosis with development of polar vasculosis and exudative lesions |
| Patino-Alonso et al. (2021) [35] | Spain | PV, ET, PMF | 57 | similar values for SBP and DBP between MPNs (n = 57) and controls ↑ risk of carotid artery injury in MPNs (OR = 2.382, 1.066–5.323, p = 0.034) ↑ albumin-to-creatinine ratio in MPNs |
| Rana et al. (2021) [36] | Australia | NS | 1 | MPN and HTN association can contribute to the occlusion of the central artery of the retina and present as sudden loss of vision |
| Gecht et al. (2021) [37] | Germany | PV, ET, PMF, SMF | 1420 | 49%o of 1420 MPN cases had HTN ↑ BP more common in PV vs. ET or vs. PMF/SMF (~63% vs. ~46% and ~63% vs. ~39%, respectively, p < 0.0001) MPN subjects have eGFR = 60–89 mL/min/1.73 m2 (~57%), followed by eGFR < 60 mL/min/1.73 m2 (~22%) and eGFR ≥ 90 mL/min/1.73 m2 (~21%) more PMF/SMF subjects had eGFR < 60 mL/min/1.73 m2 vs. PV or vs. ET (~28% vs. ~20%, p = 0.005 for both) HTN = risk factor (OR = 2.419, 95% CI 1.879–3.114, p < 0.0001) for kidney dysfunction, particularly in subjects with eGFR < 60 mL/min/1.73 m2 vs. eGFR ≥ 90 mL/min/1.73 m2 (OR = 6.220, 95% CI 1.501–25.779, p = 0.01) and vs. eGFR = 60–89 mL/min/1.73 m2 (OR = 3.429, 95% CI 1.207–9.739, p = 0.02) in the univariate regression analysis HTN = risk factor for kidney dysfunction (OR = 2.004, 95% CI 1.440–2.789, p < 0.0001) following multiple regression analysis HTN = risk factor for thrombotic (OR = 1.838, 95% CI 1.398–2.418, p < 0.0001 in the univariate and OR = 1.800, 95% CI 1.349–2.401, p = 0.01 in the multivariate regression) but not for bleeding complications (OR = 1.302, 95% CI 0.678–2.499, p > 0.05) HTN = predictor for thrombotic events in ET (OR = 1.913, 95% CI 1.099–3.330, p = 0.02) and PMF/SMF (OR = 1.960, 95% CI 1.067–3.601, p = 0.03) |
| Kwiatkowski et al. (2021) [13] | Poland | ET | 310 | ET subjects display a degree of kidney dysfunction even before initiation of risk-adapted treatment (hydroxyurea or anagrelide) the decision to prescribe these drugs is associated with ↑ in creatinine levels ET with concomitant HTN cases had ↑ creatinine concentrations before the commencement of treatment (p < 0.001) and experienced ↑ in creatinine levels after cytoreduction with hydroxyurea or anagrelide (0.91 mg/dL pre-treatment versus 0.96 mg/dL post-treatment, p < 0.001) ET patients on antihypertensive agents (OR = 2.20, 95% CI: 1.26–3.85, p = 0.006) and anagrelide (OR = 13.01, 95% CI: 6.27–27.01, p < 0.001), as well as harboring CALR mutations (OR = 1.95, 95% CI: 1.10–3.45, p = 0.02), were more likely to experience kidney dysfunction |
| Person et al. (2021) [37] | Switzerland | PV, ET, PMF | 57 | post-mortem evaluation of kidneys in MPNs did not delineate proof of HTN or T2DM-related nephropathy in subjects who exhibited diffuse glomerulosclerosis |
| Lucijanic et al. (2022) [38] | Croatia | PMF, SMF | 173 | PMF/SMF with high vs. low uric acid levels at diagnosis more likely to suffer from HTN (PMF: ~71% vs. ~52%, p = 0.03; SMF: ~65% vs. ~30%, p = 0.01) |
| Akdi et al. (2020) [39] | Turkey | PV | 50 | PV cases likely to be non-dippers (~64% vs. ~37%, p = 0.007) and display lower nocturnal fall in SBP, DBP and MBP (−6.91 ± 8.92 mmHg vs. −11.65 ± 7.68 mmHg, p = 0.005; −11.31 ± 12.18 mmHg vs. −16.28 ± 12.03 mmHg, p = 0.042; −9.31 ± 10.41 mmHg vs. −14.06 ± 9.41 mmHg, p = 0.018) vs. hypertensive non-PV counterparts average-24 h and daytime SBP and DBP similar between the two groups nighttime SBP and DBP elevated in PV (125.32 ± 17.24 mmHg vs. 118.93 ± 12.20 mmHg, p = 0.034; 73.74 ± 12.16 mmHg vs. 69.49 ± 8.52 mmHg, p = 0.044, respectively) nocturnal falls in SBP (r = 0.305, p = 0.002 and r = 0.354, p < 0.001), DBP (r = 0.197, p = 0.048 and r = 0.236, p = 0.017) and MBP (r = 0.246, p = 0.013 and r = 0.293, p = 0.003) positively associated with hemoglobin and hematocrit levels |
| Dobrowolski et al. (2017) [5] | Poland | PV | 23 | no significant differences in office or 24 h SBP, DBP, heart rate or use of antihypertensive agents in PV vs. healthy counterparts signs of systolic and diastolic dysfunction of the heart by the assessment of several echocardiographic parameters, i.e., septal and lateral systolic velocities (8.7 ± 1.3 vs. 7.2 ± 2.4, p = 0.04 and 9.3 ± 1.2 vs. 7.7 ± 2.4, p = 0.04, respectively) and global longitudinal, circumferential and radial strains (−20.1 ± 4.3% vs. −18.1 ± 3.1%, p = 0.01; −19.7 ± 1.1% vs. −16.7 ± 2.7%, p = 0.001; 37.5 ± 8.7% vs. 29.6 ± 12.8%, p = 0.05, respectively) elevated in controls, whereas the isovolumic relaxation time was increased in PV (110.9 ± 24.9 ms vs. 83.5 ± 12.9 ms, p = 0.0001) hemoglobin levels were negatively associated with the global longitudinal and circumferential strains (β = −0.488, p = 0.0001; β = −0.537, p = 0.005) in PV hematocrit value was positively linked only with the global longitudinal strain (β = 0.408, p = 0.001) in PV red blood cell count was negatively correlated with the isovolumic relaxation time (β = −0.463, p = 0.05) in PV |
| Jóźwik-Plebanek et al. (2020) [40] | Poland | PV | 20 | HTN affected 75% of PV cases who displayed lower 24 h SBP and 24 h DBP (p = 0.003 and p = 0.01, respectively) versus comparators All other office or ambulatory BP measurements similar in PV and controls ↓ metanephrine and aldosterone in the plasma (p < 0.001 and p = 0.008, respectively), ↑ potassium levels (p < 0.001), ↓ free normetanephrine, metanephrine and norepinephrine in the urine (p = 0.03, p = 0.007 and p = 0.03, respectively) in PV number of erythrocytes (p < 0.001), leukocytes (p = 0.001), platelets (p < 0.001), hemoglobin (p < 0.001) and hematocrit (p = 0.02) ↑ in PV ↓ mean corpuscular volume, mean corpuscular hemoglobin concentration and mean corpuscular hemoglobin were lower (p < 0.001 for all) PV exhibited reduced capillary blood flow in the retina (p = 0.08) which was inversely associated with hemoglobin levels and erythrocyte numbers in PV (r = −0.57; p = 0.001 and r = −0.40, p = 0.02, respectively aldosterone concentrations negatively correlated with the aforementioned red cell parameters (r = −0.33, p = 0.04 for both) although HR baroreflex control was not different in PV and healthy counterparts, daytime, nighttime and 24 h ABPM, in addition to muscle adrenergic nerve activity (p = 0.007 for bursts/min and p = 0.04 for bursts/100 heartbeats) were ↓ in PV |
| Rusak et al. (2013) [41] | Poland | PV | 73 | PV subjects exhibited higher blood viscosity (p < 0.01), SBP (p < 0.05), DBP (p < 0.05), MAP (p < 0.05), cell-free hemoglobin (p < 0.01) and nitrite/nitrate (p < 0.01) versus healthy comparators especially when PV and HTN co-occur cell-free hemoglobin values were positively associated with MAP (r = +0.49, p < 0.05), nitrite/nitrate (r = +0.46, p < 0.05), hematocrit (r = +0.47, p < 0.05) and blood viscosity (r = +0.39, p < 0.05) isovolemic erythrocytapheresis decreased several biochemical parameters; however, it barely influenced the cell-free hemoglobin—hematocrit, cell-free hemoglobin—blood viscosity associations which continued to display a positive trend line and remained statistically significant (p < 0.05) |
| Author and Year | Study Location | MPN Subtype | Number of Patients | Main Results |
|---|---|---|---|---|
| Carobbio et al. (2011) [42] | International cohort: Italy, Austria, Germany, USA | ET | 891 | HTN, T2DM or smoking (at least one CVRF) were predictors of both major thrombotic events (HR = 1.56, 95% CI = 1.03–2.36, p = 0.038) HTN, T2DM or tobacco use were predictors of major arterial thrombotic events (HR = 1.91, 95% CI = 1.19–3.07, p = 0.007), namely acute myocardial infarction, ischemic stroke, cerebral transient ischemic attacks or peripheral arterial thrombosis, but not of the occurrence of major venous thrombosis (HR = 0.77, 95% CI = 0.33–1.83, p = 0.556), namely venous thromboembolism |
| Buxhofer-Ausch et al. (2014) [43] | Austria | ET, PMF | 167 | HTN prevalence similar in both subgroups (50% vs. 44%, p = 0.48) HTN = risk factor for thrombosis (univariate model: HR = 3.43, range 1.12–10.52, p = 0.03; multivariate model: HR = 3.33, range 0.90–12.29, p = 0.07), in particular arterial thrombosis (only in the univariate model: HR = 3.76, range 1.05–13.48, p = 0.04; multivariate model: HR = 2.79, range 7.06–11.02, p = 0.14) in ET HTN did not impact the occurrence of venous thrombosis (HR = 2.09, range 0.19–23.11, p = 0.55) in ET |
| Pósfai et al. (2015) [44] | Hungary | ET | 101 | HTN = #1 comorbidity (46.5%) in ET HTN not linked to the occurrence of thrombosis in the logistic regression analysis co-existence of two or more CVRFs out of HTN, dyslipidemia, diabetes or smoking was linked to the development of thrombotic events (p = 0.02) thrombosis-free survival lower in ET with ≥ 1 CVRF vs. those without CVRFs (p = 0.01) and in ET patients with one CVRF vs. ≥ 2 CVRFs (p = 0.002) |
| Pósfai et al. (2014) [45] | Hungary | ET | 128 | HTN = predisposing factor (p = 0.001) to the development of thrombotic complications in females with ET of whom ~55% (n = 70) had elevated BP ≥2 CVRFs = linked with elevated probability of suffering a thrombotic event in women diagnosed with ET (RR = 4.728, 95% CI 1.312–17.040, p = 0.01) |
| Horvat et al. (2018) [46] | Hungary | PV, ET, PMF | 258 | HTN and presence of ≥1 CVRF = risk factors for thrombotic events (OR = 2.8, 95% CI 1.6–5.0, p < 0.001; OR = 3.2, 95% CI 1.7–6.3, p = 0.001, respectively), especially arterial thrombosis (OR = 3.3, 95% CI 1.7–6.3, p < 0.001; OR = 5.7, 95% CI 2.3–13.9, p < 0.001, respectively) in PV (n = 70) and PMF (n = 54) the presence of ≥1 CVRF but not HTN alone predicted the development of arterial thrombotic complications (OR = 7.9, 95% CI 1.0–64.9, p = 0.049; OR = 12.2, 95% CI 0.7–225.3, p = 0.044, respectively) both HTN and the presence of ≥1 CVRF were risk factors not only for overall thrombosis (OR = 3.8, 95% CI 1.6–8.7, p = 0.003; OR = 5.1, 95% CI 1.8–14.1, p = 0.001, respectively), but also for arterial (OR = 2.8, 95% CI 1.2–6.5, p = 0.021; OR = 3.9, 95% CI 1.4–11.1, p = 0.009, respectively) and venous (OR = 30.3, 95% CI 1.7–532.4, p < 0.001; OR = 17.1, 95% CI 1.0–300.8, p = 0.005) thrombosis separately in ET |
| Lekovic et al. (2014) [47] | Serbia | ET | 244 | ~58% of ET cases had HTN development of both arterial and global thrombosis associated with HTN (p = 0.01 and p = 0.001, respectively), CVRFs in general (p = 0.01 and p = 0.002, respectively) and number of CVRFs (p < 0.001 and p < 0.001, respectively) |
| Lekovic et al. (2015) [48] | Serbia | ET | 244 | CVRFs (HTN, T2DM and dyslipidemia) and combination of CVRFs and tobacco use were less common in the patients who were still alive at the time of the analysis (~62% versus ~78%, p = 0.05 and ~21% versus ~41%, p = 0.01, respectively) presence of CVRFs (HR = 2.33) and CVRFs + tobacco use (HR = 2.08) linked with shorter overall survival in ET novel assessment tool for the prognosis of ET, namely the Cardio-IPSET prognostic model which takes into consideration the following factors: age, history of thrombotic events, leukocyte count and the presence of CVRFs (HTN, T2DM, dyslipidemia, and smoking) ~75% of deaths in ET attributed to cardiovascular causes |
| Schwarz et al. (2015) [49] | Czech Republic | PV, ET, PMF | 1179 | HTN = predictor of overall thrombosis (p = 0.003), major thrombosis (p = 0.022) and arterial thrombosis (p < 0.001); however, not of microvascular events or venous thrombotic events based on the univariate analysis in MPNs treated with anagrelide in the multivariate regression analysis, HTN was the best predictor of arterial thrombotic events (OR = 1.813, 95% CI 1.295–2.538, p = 0.001) |
| Accurso et al. (2020) [50] | Italy | PV, ET | 403 | HTN = #1 cardiovascular comorbidity in PV and ET (~64%) an elevated percentage of PV vs. ET cases (~39% vs. ~27%, p = 0.014) experienced thrombotic complications CVRFs associated with decreased survival in PV (p = 0.014) and ET (p = 0.036) |
| Cucuianu et al. (2006) [51] | Romania | PV, ET | 37 | ~31% of the patients had HTN association of HTN, platelet count > 600,000 platelets/mmc and hematocrit > 55% was linked with higher incidence of thrombotic events (p = 0.02) in PV |
| Barbui et al. (2017) [52] | International cohort: Italy, Austria, USA | PV | 604 | HTN impacts the incidence of thrombosis in low-risk PV (n = 525). Thrombosis-free survival higher in low-risk PV patients who did not suffer from HTN (IR = 0.85, 95% CI 0.57-1.25 vs. IR = 2.05, 95% CI 1.34-3.14, p = 0.025) Compared to another ET cohort (n = 891), HTN was more prevalent in PV (OR = 1.38, p = 0.022) and BP values positively correlated with hematocrit levels |
| Benevolo et al. (2021) [53] | Italy | PV | 861 | HTN (HR = 1.77, 95% CI 1.03–3.06, p = 0.04) and previous history of thrombosis (HR = 2.10, 95% CI 1.21–3.60, p = 0.01) elevate risk of thrombosis in PV |
| Birgegård et al. (2018) [54] | International cohort: Sweden, Italy, France, UK, USA, Germany, Spain, Switzerland | ET | 3649 | post-hoc multivariate analysis of the Evaluation of Anagrelide Efficacy and Long-term Safety study, long-term research with prospective observational design which recruited high-risk ET cases 34% of ET cases had elevated BP (#1 CVRF in ET) HTN = predictor of major hemorrhages (HR = 1.33, 95% CI 1.04–1.69, p = 0.02) and thrombohemorrhagic complications (HR = 1.69, 95% CI 1.02-2.79, p = 0.04) |
| Cerquozzi et al. (2017) [55] | USA | PV | 587 | 42% of PV cases had HTN rate of arterial and venous thrombotic complications was elevated in subjects with elevated BP (52% vs. 38%, p = 0.004 and 44% vs. 30%, p = 0.009, respectively). Individuals with PV had lower thrombosis-free survival (HR = 1.7, 95% CI 1.1–2.6, p = 0.02) in the univariate but not in the multivariate analysis |
| Cervantes et al. (2006) [56] | Spain | PMF, SMF | 155 | patients with any CVRF (HTN, T2DM, hypercholesterolemia, use of cigarettes) were at an elevated risk for thrombosis (OR = 14.9, 95% CI 2.5–87, p = 0.003) and had lower thrombosis-free survival (~83% vs. 97%, p = 0.02) |
| Navarro et al. (2015) [57] | Brazil | ET | 46 | association between CVRFs and thrombosis (p = 0.01), namely arterial (p = 0.03) and not venous (p > 0.05) thrombotic complications |
| Shih et al. (2002) [58] | Taiwan | ET | 89 | assessment of thrombosis in women with ET and with/without clonal/polyclonal X-chromosome inactivation patterns Thrombosis but not hemorrhage was more common in ET subjects with vs. without HTN (p = 0.002 and p = 0.287, respectively) After adjustment for HTN and age, the risk of thrombotic events was 7 times more elevated in ET individuals with clonal X-chromosome inactivation patterns vs. those without |
| Bucalossi et al. (1996) [59] | Italy | PV, ET | 81 | similar prevalence of HTN in PV and ET with/without thrombosis |
| Landolfi et al. (2007) [60] | International cohort | PV | 1638 | assessment of 1638 subjects from the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) HTN did not emerge as a predictor for major/arterial/venous thrombosis, AMI, TIA, stroke or peripheral arterial thrombosis |
| Finazzi (2004) [61] | International cohort | PV | 1630 | European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) analysis cumulative incidence rate of cardiovascular events (i.e., cardiovascular death and non-fatal thrombotic events) = 5.5 events/100 persons per year Thrombosis = main cause of death Age > 65 years, history of thrombosis = predictors of cardiovascular events smoking, HTN, congestive heart failure = risk factors for thrombosis Platelet counts, myelosuppressive drugs = no association with the risk of cardiovascular events Antiplatelet treatment = only variable associated with lower risk of thrombosis |
| Bazzan et al. (1999) [62] Cortelazzo et al. (1990) [63] | Italy | ET | 187 100 | HTN did not impact thrombosis-free survival and life expectancy |
| Jantunen et al. (2001) [64] | Finland | ET | 132 | cigarette use = more common risk factor for thrombosis versus HTN (24.3% versus 20.5%) male gender (p < 0.001) and tobacco consumption (p = 0.01) = risk factors for thrombotic complications, whereas HTN did not (p = 0.34) |
| Barbui et al. (2018) [65] | International cohort | PV, ET, PMF | 597 | HTN = more common occurrence in MPNs who developed ischemic stroke versus those with transient ischemic attacks HTN = prognostic factor in recurrence of stroke (HR = 4.24) |
| Košťál et al. (2020) [66] | Czech Republic | PV, ET, PMF | 1442 | HTN = more common individuals who experienced a stroke or a TIA (~53% vs. ~41%), HTN = risk factor for such complications based on the univariate analysis model (OR = 1.604, 95% CI = 1.219–2.111, p = 0.001) but not on the multivariate logistic models on data with imputed missing values (OR = 1.170, 95% CI 0.845–1.619, p = 0.344 for treated and untreated subjects; OR = 0.918, 95% CI = 0.55–1.534, p = 0.745 for subjects not receiving cytoreductive agents |
| De Stefano et al. (2018) [67] | International cohort | PV, ET, PMF | 597 | assessment of MPNs with history of stroke or TIA similar HTN frequency (stroke vs. TIA = 57% vs. 52%, p > 0.05) HTN = independent risk factor for the recurrence of ischemic stroke in MPNs (HR = 4.24, 95% CI 1.23–14.7) Cytoreduction decreased the risk of stroke re-occurrence by 76% |
| Jiao et al. (2021) [68] | China | ET | 91 | HTN more prevalent (~32% vs. ~4%, p = 0.003) in ET without CVST |
| Robertson et al. (2007) [69] | UK | PV, ET, PMF | 118 | compared to subjects with HTN, individuals diagnosed with MPNs display elevated concentrations of soluble p-selectin (p < 0.001), particularly if they harbor the JAK2V617F mutation (p = 0.006 between JAK2V617F-positive and JAK2V617F-negative cases), and D-dimers (p = 0.03), but similar soluble E-selectin, thrombin–antithrombin complexes, prothrombin fragments or antiphospholipid antibodies soluble p-selectin levels were similar in MPN patients who experienced thrombotic events versus those who did not |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Găman, M.-A.; Kipkorir, V.; Srichawla, B.S.; Dhali, A.; Găman, A.M.; Diaconu, C.C. Primary Arterial Hypertension and Drug-Induced Hypertension in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review. Biomedicines 2023, 11, 388. https://doi.org/10.3390/biomedicines11020388
Găman M-A, Kipkorir V, Srichawla BS, Dhali A, Găman AM, Diaconu CC. Primary Arterial Hypertension and Drug-Induced Hypertension in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review. Biomedicines. 2023; 11(2):388. https://doi.org/10.3390/biomedicines11020388
Chicago/Turabian StyleGăman, Mihnea-Alexandru, Vincent Kipkorir, Bahadar S. Srichawla, Arkadeep Dhali, Amelia Maria Găman, and Camelia Cristina Diaconu. 2023. "Primary Arterial Hypertension and Drug-Induced Hypertension in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review" Biomedicines 11, no. 2: 388. https://doi.org/10.3390/biomedicines11020388








