Overcoming EGFR Resistance in Metastatic Colorectal Cancer Using Vitamin C: A Review
Abstract
:1. Introduction
2. Methods and Search Strategy
3. Findings
3.1. Mechanisms of EGFR Resistance in mCRC
3.2. The Role of High Dose Vitamin C in Cancer
3.2.1. Vitamin C Bioavailability and Requirements
3.2.2. High Dose Vitamin C in Cancer Clinical Trials
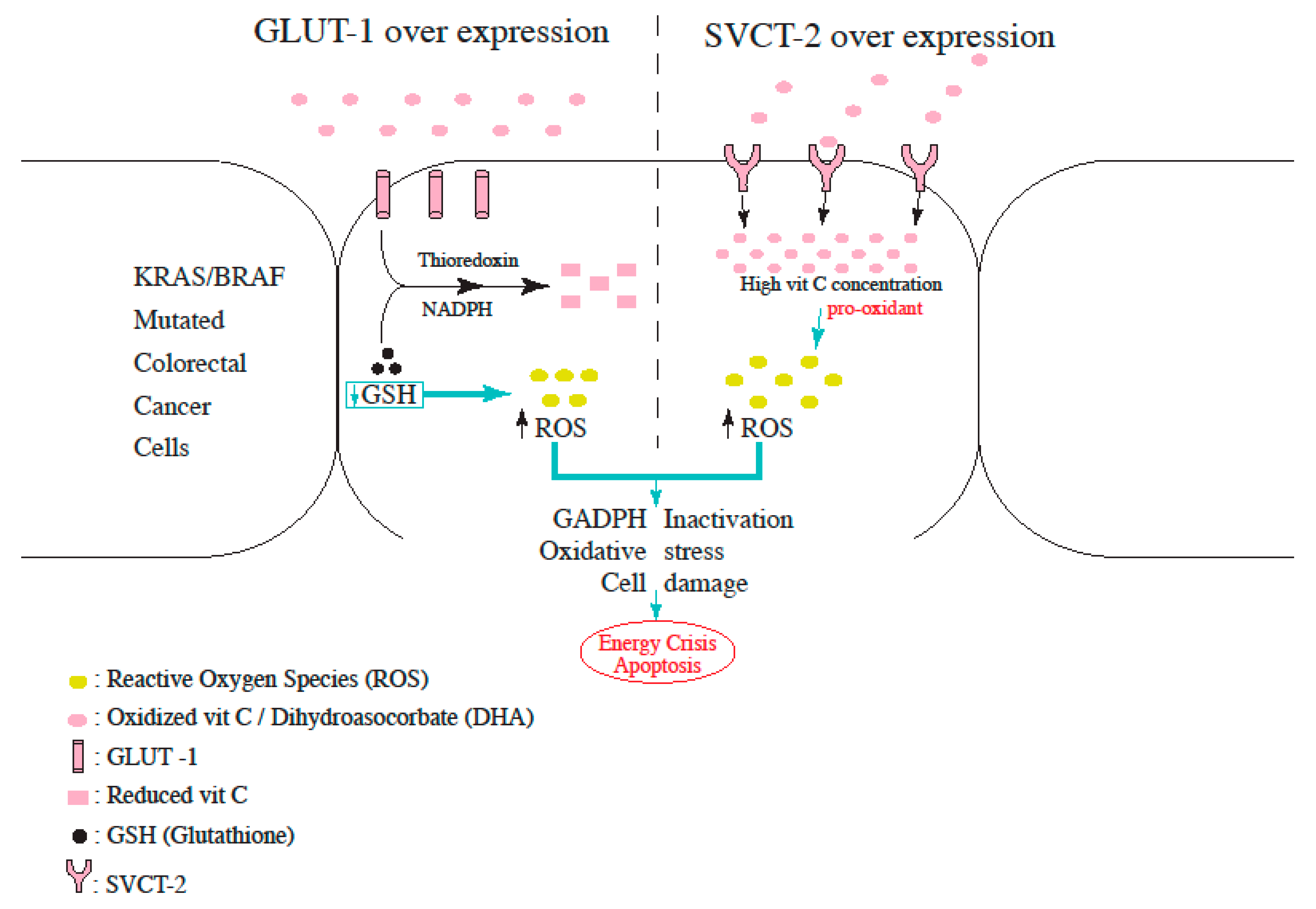
3.2.3. Role of Vitamin C in KRAS and BRAF Mutated Colorectal Cancer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. CR 2021, 40, 343. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Z.; Lv, W.; Pan, H. The Role of Anti-EGFR Monoclonal Antibody in mCRC Maintenance Therapy. Front. Mol. Biosci. 2022, 9, 870395. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- El Halabi, I.; Bejjany, R.; Nasr, R.; Mukherji, D.; Temraz, S.; Nassar, F.J.; El Darsa, H.; Shamseddine, A. Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2752. [Google Scholar] [CrossRef]
- Dachs, G.U.; Gandhi, J.; Wohlrab, C.; Carr, A.C.; Morrin, H.R.; Pullar, J.M.; Bayer, S.B.; Eglinton, T.W.; Robinson, B.A.; Vissers, M.C.M. Vitamin C Administration by Intravenous Infusion Increases Tumor Ascorbate Content in Patients With Colon Cancer: A Clinical Intervention Study. Front. Oncol. 2021, 10, 600715. [Google Scholar] [CrossRef]
- Arrington, A.K.; Heinrich, E.L.; Lee, W.; Duldulao, M.; Patel, S.; Sanchez, J.; Garcia-Aguilar, J.; Kim, J. Prognostic and Predictive Roles of KRAS Mutation in Colorectal Cancer. Int. J. Mol. Sci. 2012, 13, 12153–12168. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: Underlying mechanisms and reversal strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef]
- Lorenzato, A.; Magrì, A.; Matafora, V.; Audrito, V.; Arcella, P.; Lazzari, L.; Montone, M.; Lamba, S.; Deaglio, S.; Siena, S.; et al. Vitamin C Restricts the Emergence of Acquired Resistance to EGFR-Targeted Therapies in Colorectal Cancer. Cancers 2020, 12, 685. [Google Scholar] [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care Off. Publ. Tufts Univ. 2002, 5, 66–74. [Google Scholar] [CrossRef]
- German Nutrition Society. New Reference Values for Vitamin C Intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. [Google Scholar] [CrossRef]
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J. Korean Med. Sci. 2007, 22, 7–11. [Google Scholar] [CrossRef]
- Mansoor, F.; Kumar, S.; Rai, P.; Anees, F.; Kaur, N.; Devi, A.; Kumar, B.; Memon, M.K.; Khan, S. Impact of Intravenous Vitamin C Administration in Reducing Severity of Symptoms in Breast Cancer Patients During Treatment. Cureus 2021, 13, e14867. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kiziltan, H.S. Palliative Vitamin C Application in Patients with Radiotherapy-Resistant Bone Metastases: A Retrospective Study. Nutr. Cancer 2015, 67, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar]
- Wang, F.; He, M.-M.; Wang, Z.-X.; Li, S.; Jin, Y.; Ren, C.; Shi, S.-M.; Bi, B.-T.; Chen, S.-Z.; Lv, Z.-D. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef]
- Bruckner, H.; Hirschfeld, A.; Gurell, D.; Lee, K. Broad safety impact of high-dose ascorbic acid and induction chemotherapy for high-risk pancreatic cancer. J. Clin. Oncol. 2017, 35, e15711. [Google Scholar] [CrossRef]
- Welsh, J.L.; Wagner, B.A.; Van’t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J., 2nd; et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef]
- Riordan, H.D.; Casciari, J.J.; González, M.J.; Riordan, N.H.; Miranda-Massari, J.R.; Taylor, P.; Jackson, J.A. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. Puerto Rico Health Sci. J. 2005, 24, 269–276. [Google Scholar]
- Cho, S.; Chae, J.S.; Shin, H.; Shin, Y.; Song, H.; Kim, Y.; Yoo, B.C.; Roh, K.; Cho, S.; Kil, E.J.; et al. Hormetic dose response to (L)-ascorbic acid as an anti-cancer drug in colorectal cancer cell lines according to SVCT-2 expression. Sci. Rep. 2018, 8, 11372. [Google Scholar] [CrossRef]
- Vitiello, P.P.; De Falco, V.; Giunta, E.F.; Ciardiello, D.; Cardone, C.; Vitale, P.; Zanaletti, N.; Borrelli, C.; Poliero, L.; Terminiello, M.; et al. Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience. Cancers 2019, 11, 1504. [Google Scholar] [CrossRef]
- Michela, B. Liquid Biopsy: A Family of Possible Diagnostic Tools. Diagnostics 2021, 11, 1391. [Google Scholar] [CrossRef]
- Hirahata, T.; Ul Quraish, R. Liquid Biopsy: A Distinctive Approach to the Diagnosis and Prognosis of Cancer. Cancer Inform. 2022, 21, 11769351221076062. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Gunningham, S.P.; Morrison, M.J.; Dachs, G.U.; Currie, M.J. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free. Radic. Biol. Med. 2007, 42, 765–772. [Google Scholar] [CrossRef]
- Campbell, E.J.; Vissers, M.C.M.; Wohlrab, C.; Hicks, K.O.; Strother, R.M.; Bozonet, S.M.; Robinson, B.A.; Dachs, G.U. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free. Radic. Biol. Med. 2016, 99, 451–462. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- De Grooth, H.J.; Manubulu-Choo, W.P.; Zandvliet, A.S.; Spoelstra-de Man, A.M.E.; Girbes, A.R.; Swart, E.L.; Oudemans-van Straaten, H.M. Vitamin C Pharmacokinetics in Critically Ill Patients: A Randomized Trial of Four IV Regimens. Chest 2018, 153, 1368–1377. [Google Scholar] [CrossRef]
- Gaianigo, N.; Melisi, D.; Carbone, C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers 2017, 9, 122. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Aguilera, O.; Muñoz-Sagastibelza, M.; Torrejón, B.; Borrero-Palacios, A.; del Puerto-Nevado, L.; Martínez-Useros, J.; Rodriguez-Remirez, M.; Zazo, S.; García, E.; Fraga, M.; et al. Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget 2016, 7, 47954–47965. [Google Scholar] [CrossRef]
- Linardou, H.; Dahabreh, I.J.; Kanaloupiti, D.; Siannis, F.; Bafaloukos, D.; Kosmidis, P.; Papadimitriou, C.A.; Murray, S. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: A systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008, 9, 962–972. [Google Scholar] [CrossRef]
- Jung, S.-A.; Lee, D.-H.; Moon, J.-H.; Hong, S.-W.; Shin, J.-S.; Hwang, I.Y.; Shin, Y.J.; Kim, J.H.; Gong, E.-Y.; Kim, S.-M.; et al. L-Ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free. Radic. Biol. Med. 2016, 95, 200–208. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers. Int. J. Mol. Sci. 2015, 16, 22976–22988. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Zhang, Y.; Yuan, X.; He, M.; Fang, W.; Zhang, Y.; Wang, W.; Hu, X.; Ma, Z.; et al. SO-17 A randomized, open-label, multicenter, phase III study of high-dose vitamin C plus FOLFOX +/− bevacizumab versus FOLFOX +/− bevacizumab as first-line treatment in patients with unresectable metastatic colorectal cancer. Ann. Oncol. 2022, 33, S364. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: The phase 2 CHRONOS trial. Nat Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef]

| Study | Design and Population | Aim | Intervention | Results | Notes |
|---|---|---|---|---|---|
| Wang (2019) NCT02969681 [25] | -Phase I open label single center dose escalation speed expansion -30 mCRC and 6 mGC | Determine maximum tolerated dose of AA w/mFOLFOX or FOLFIRI +/− bevacizumab | Part 1 (dose escalation): AA (0.4, 0.6, 0.8, 1, 1.2 and 1.5 g/kg) on days 1 to 3 of FOLFOX or FOLFIRI every 14 d. Part 2 (dose expansion): AA given 1.5 g/kg or MTD on days 1 to 3. Tx duration: 12 cycles or progression or side effects. | No DLT in part 1 or part 2 and MTD not reached → 1.5 g/kg chosen as RP2D. Disease control rate 95.5%. No difference in efficacy between wt and m KRAS/BRAF CRC. Median PFS of the entire cohort 8.8 m. | The current study showed markedly decreased all-grade and grade ≥ 3 bone marrow and gastrointestinal toxic effects compared with previous trials investigating the same chemotherapeutic regimens in mCRC or mGC. |
| Monti 2012 [26] | -Phase I open label dose escalation -A total of 14 stage IV pancreatic cancer patients receiving gemcitabine and erlotinib (not previously treated) | Primary: safety Secondary: response to tx | First cohort received 50 g IV AA per infusion, second cohort received 75 g/infusion, and third cohort received 100 g/infusion. A cycle consisted of three infusions per week performed on separate days, for 8 weeks. | A total of 9 patients completed the study. Side effects: mild headache and nausea from osmotic load that resolve; 8 serious adverse events recorded but related to gemcitabine/disease progression; 8 patients had dec in tumor size, 7 patients had stable disease, and 2 progressed. | Med PFS 89 days and med OS 182 d (comparable to gem/erlo alone). |
| Bruckner 2017 (abstract) [27] | -Phase II trial, open label -A total of 26 patients with advanced pancreatic cancer | High dose AA (75–100 g) 1–2 x/week with GFLIP Q2w until progression. | Decreased rate of severe toxicity. | ||
| Welsh (2013) [28] | -Phase I single institution, prospective, open label -A total of 9 patients with stage IV pancreatic cancer receiving gemcitabine | Safety and tolerability of AA with gemcitabine | Twice weekly (50–125 g) IV AA and concurrent gemcitabine until DLT or progression. Target peak AA level > 350 mg/dl. | A total of 6/9 patients maintained/improved PS. PFS 26 +/− 7 weeks and OS 12 m. Adverse events related to AA were rare and included diarrhea and dry mouth. Adverse events were less severe when compared to published data for gemcitabine alone. | |
| Stephenson [20] | -Phase I, single center, non-comparative dose escalation -A total of 17 patients with advanced cancers not responsive to standard tx | Safety and tolerability of pharmacokinetics of high dose IV AA as monotherapy in advanced tumors | A total of 5 cohorts of 3 patients receiving dose escalation (30 g/m2 and inc by 20) until MTD | No objective tumor response. Side effects were mild and possibly related to treatment. Some patients had improved qol score at 3 and 4 weeks. | Dose of 70 to 80 g/m2 appears to be optimal for future studies. |
| Hoffer (2008) [19] | -Phase I, single center, dose escalating. -A total of 24 patients with advanced cancers, pretreated. They did not receive chemo with AA. | Document the safety and clinical consequences of i.v. ascorbic acid administrated in a dose sufficient to sustain plasma ascorbic acid concentrations >10 mmol/l for several hours | Cohorts receiving fixed doses of 0.4, 0.6, 0.9, and 1.5 g/kg for 4 weeks cycle | Mild clinical toxicity occurred, all consistent with the SE attending the rapid infusion of any high-osmolarity solution. Preventable by encouraging patients to drink fluids. No objective tumor response, but 2 patients in the 0.6 group had stable disease. AA could be promising when combined with cytotoxic agents. | 1.5 g/kg (infused > 90–120 min 3 x/w) was adopted as the recommend dose for future phase II trials |
| Riordan [29] | -Pilot study -24 late stage terminal cancer patients | Clinical safety of high dose AA | Continuous infusions of 150 to 710 mg/kg/day for up to eight weeks | Most SE were mild and 2 were grade 3 possibly related to AA: kidney stone and hypoK. One patient had stable disease and continued the treatment for 48 weeks. AA is relatively safe, provided the patient does not have a history of kidney stone. | |
| Sartore-Bianchi (2022) [49] | -Open-label, single-arm phase 2 clinical trial -A total of 52 patients with tissue-RAS WT tumors after a previous treatment with anti-EGFR-based regimens underwent an interventional ctDNA-based screening. | Exploiting blood-based identification of RAS/BRAF/EGFR mutations levels to tailor a chemotherapy-free anti-EGFR rechallenge with panitumumab | A total of 36 patients were molecularly eligible for panitumumab rechallenge. Of these, 27 received the drug as per trial protocol, 6 did not meet clinical inclusion criteria, and 3 were treated otherwise as per physician choice | Of 27 enrolled patients, 8 (30%) achieved partial response and 17 (63%) disease control, including 2 unconfirmed responses. These clinical results favorably compare with standard third-line treatments and show that interventional liquid biopsies can be effectively and safely exploited in a timely manner to guide anti-EGFR rechallenge therapy with panitumumab in patients with mCRC. | |
| Wang (2022) [48] | -Randomized, open labeled, multicenter phase II -A total of 442 histologically confirmed mCRC patients with normal glucose-6-phosphate dehydrogenase status and no prior treatment for metastatic disease | Compare the efficacy and safety of high-dose vitamin C plus FOLFOX +/− bevacizumab versus FOLFOX +/− bevacizumab as first-line treatment in patients with metastatic colorectal cancer (mCRC) | A total of 442 patients were randomized into a control (FOLFOX +/− bevacizumab) and an experimental (high-dose vitamin C (1.5 g/kg/d, intravenously for 3 h from D1 to D3) plus FOLFOX +/− bevacizumab) group | In prespecified subgroup analyses, patients with RAS mutation had significantly longer Progression Free Survival (median PFS, 9.2 vs. 7.8 months; HR, 0.67; 95% CI, 0.50–0.91; p = 0.01) with vitamin C added to chemotherapy than with chemotherapy only. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machmouchi, A.; Chehade, L.; Temraz, S.; Shamseddine, A. Overcoming EGFR Resistance in Metastatic Colorectal Cancer Using Vitamin C: A Review. Biomedicines 2023, 11, 678. https://doi.org/10.3390/biomedicines11030678
Machmouchi A, Chehade L, Temraz S, Shamseddine A. Overcoming EGFR Resistance in Metastatic Colorectal Cancer Using Vitamin C: A Review. Biomedicines. 2023; 11(3):678. https://doi.org/10.3390/biomedicines11030678
Chicago/Turabian StyleMachmouchi, Ahmad, Laudy Chehade, Sally Temraz, and Ali Shamseddine. 2023. "Overcoming EGFR Resistance in Metastatic Colorectal Cancer Using Vitamin C: A Review" Biomedicines 11, no. 3: 678. https://doi.org/10.3390/biomedicines11030678








