Measuring Drug Therapy Effect on Osteoporotic Fracture Risk by Trabecular Bone Lacunarity: The LOTO Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Characterization of Trabecular Bone Microarchitecture
2.3. Main Endpoint Outcome
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
3.2. TBLβ as an Index of Osteoporosis Therapy Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anonymous. Consensus development conference. Diagnosis, prophylaxis and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.M.; Cooper, C. Epidemiology of osteoporosis. Best Pract. Res. Clin. Rheumatol. 2002, 16, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Borgstrom, F.; De Laet, C.; Johansson, H.; Johnell, O.; Jonsson, B.; Oden, A.; Zethraeus, N.; Pfleger, B.; Khaltaev, N. Assessment of fracture risk. Osteoporos. Int. 2005, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, G.; Muscarella, S.; Bazzocchi, A. Integrated imaging approach to osteoporosis: State-of-the-art review and update. Radiographics 2011, 31, 1343–1364. [Google Scholar] [CrossRef]
- Lindsay, R.; Silverman, S.L.; Cooper, C.; Hanley, D.A.; Barton, I.; Broy, S.B.; Licata, A.; Benhamou, L.; Geusens, P.; Flowers, K.; et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001, 285, 320–323. [Google Scholar] [CrossRef]
- Helgason, B.; Perilli, E.; Schileo, E.; Taddei, F.; Brynjolfsson, S.; Viceconti, M. Mathematical relationships between bone density and mechanical properties: A literature review. Clin. Biomec. 2008, 23, 135–146. [Google Scholar] [CrossRef]
- Seeman, E. Is a change in bone mineral density a sensitive and specific surrogate of anti-fracture efficacy? Bone 2007, 41, 308–317. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Giannini, S. Bone microarchitecture as an important determinant of bone strength. J. Endocrinol. Investig. 2004, 27, 99–105. [Google Scholar] [CrossRef]
- Laib, A.; Newitt, D.C.; Lu, Y.; Majumdar, S. New model-independent measures of trabecular bone structure applied to in vivo high resolution MR images. Osteoporos. Int. 2002, 13, 130–136. [Google Scholar] [CrossRef]
- Krug, R.; Burghardt, A.J.; Majumdar, S.; Link, T.M. High-resolution Imaging Techniques for the Assessment of Osteoporosis. Radiol. Clin. N. Am. 2010, 48, 601–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, J.F.; Genant, H.K. Bone mass and architecture determination: State of the art. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 737–764. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.S.; Link, T.M. Advances in osteoporosis imaging. Eur. J. Radiol. 2009, 71, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, J.; Goldenstein, J.; Carballido-Gamio, J.; Kazakia, G.; Burghardt, A.J.; Rodriguez, A.; Krug, R.; de Papp, A.E.; Link, T.M.; Majumdar, S. Longitudinal evaluation of the effects of alendronate on MRI bone microarchitecture in postmenopausal osteopenic women. Bone 2011, 48, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE). JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnut, C.H., 3rd; Majiumdar, S.; Newitt, D.C.; Shields, A.; Van Pelt, J.; Laschansky, E.; Azria, M.; Kriegman, A.; Olson, M.; Eriksen, E.F.; et al. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: Results from the QUEST study. J. Bone Miner. Res. 2005, 20, 1548–1561. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.H.; Liu, X.S.; Vasilic, B.; Wehrli, F.W.; Benito, M.; Rajapakse, C.S.; Snyder, P.J.; Guo, X.E. In vivo microMRI-based finite element and morphological analyses of tibial trabecular bone in eugonadal and hypogonadal men before and after testosterone treatment. J. Bone Miner. Res. 2008, 23, 1426–1434. [Google Scholar] [CrossRef]
- Zaia, A.; Eleonori, R.; Maponi, P.; Rossi, R.; Murri, R. Medical imaging and osteoporosis: Fractal’s lacunarity analysis of trabecular bone in MR images. In Proceedings of the Eighteenth IEEE Symposium on Computer-Based Medical Systems (CBMS 2005), Dublin, Ireland, 23–24 June 2005; pp. 3–8. [Google Scholar] [CrossRef]
- Zaia, A.; Eleonori, R.; Maponi, P.; Rossi, R.; Murri, R. MR imaging and osteoporosis: Fractal lacunarity analysis of trabecular Bone. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 484–489. [Google Scholar] [CrossRef]
- Zaia, A.; Rossi, R.; Egidi, N.; Maponi, P. Fractal’s lacunarity analysis of trabecular bone in MR images. In Computational Vision and Medical Image Processing; Tavares, J., Jorge, N., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 421–426. [Google Scholar]
- Zaia, A. Fractal lacunarity of trabecular bone and magnetic resonance imaging: New perspectives for osteoporotic fracture risk assessment. World J. Orthop. 2015, 6, 221–235. [Google Scholar] [CrossRef]
- Zaia, A.; Rossi, R.; Galeazzi, R.; Maponi, P.; Scendoni, P. Fractal lacunarity of trabecular bone in vertebral MRI to predict osteoporotic fracture risk in over-fifties women. The LOTO study. BMC Musculoskelet. Disord. 2021, 22, 108. [Google Scholar] [CrossRef]
- Zaia, A. Osteoporosis and fracture risk: New perspectives for early diagnosis and treatment assessment. In Osteoporosis: Etiology, Diagnosis and Treatment; Mattingly, B.E., Pillare, A.C., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2009; pp. 267–290. [Google Scholar]
- Genant, H.K.; Jergas, M.; Palermo, L.; Nevitt, M.; Valentin, R.S.; Black, D.; Cummings, S.R. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J. Bone Miner. Res. 1996, 11, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.; Cloitre, M. Characterizing the lacunarity of random and deterministic fractal sets. Phys. Rev. A 1991, 44, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A.; El-Hajj, F.G.; Bauer, D.C.; Camacho, P.M.; Clarke, B.L.; Clines, G.A.; Compston, J.E.; Drake, M.T.; Edwards, B.J.; Favus, M.J.; et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016, 31, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Glüer, C.C.; de Villiers, T.; Baber, R.; et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J. Bone Oncol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Beaudoin, C.; Jean, S.; Moore, L.; Gamache, P.; Bessette, L.; Ste-Marie, L.-G.; Brown, J.P. Number, location, and time since prior fracture as predictors of future fracture in the elderly from the general population. J. Bone Miner. Res. 2018, 33, 1956–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Sui, L.; Gai, P.; Li, G.; Qi, X.; Jiang, X. The efficacy and safety of vertebral fracture prevention therapies in post-menopausal osteoporosis treatment: Which therapies work best? a network meta-analysis. Bone Joint Res. 2017, 6, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Yong, E.; Logan, S. Menopausal osteoporosis: Screening, prevention and treatment. Singapore Med. J. 2021, 62, 159–166. [Google Scholar] [CrossRef]
- Davis, S.; Martyn-St James, M.; Sanderson, J.; Stevens, J.; Goka, E.; Rawdin, A.; Sadler, S.; Wong, R.; Campbell, F.; Stevenson, M.; et al. A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess 2016, 20, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Peng, W.; Yang, C.; Zheng, J. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: A meta-analysis. Int. J. Surg. 2019, 66, 1–11. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Bacha, D.S.; Gharios, C.; Ajjour, S.; Assaad, M.; Jabbour, Y.; Kahale, F.; Bassatne, A.; Antoun, S.; Akl, E.A.; et al. Vitamin D Supplementation and Fractures in Adults: A Systematic Umbrella Review of Meta-Analyses of Controlled Trials. J. Clin. Endocrinol. Metab. 2022, 107, 882–898. [Google Scholar] [CrossRef]
- Thanapluetiwong, S.; Chewcharat, A.; Takkavatakarn, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Vitamin D supplement on prevention of fall and fracture: A Meta-analysis of Randomized Controlled Trials. Medicine 2020, 99, e21506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zeng, X.; Wang, J.; Liu, L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B. Vitamin D and muscle function. J. Steroid. Biochem. Mol. Biol. 2017, 173, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K.; et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594. [Google Scholar] [CrossRef]
- Chavassieux, P.; Chapurlat, R.; Portero-Muzy, N.; Roux, J.P.; Garcia, P.; Brown, J.P.; Libanati, C.; Boyce, R.W.; Wang, A.; Grauer, A. Bone–Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women With Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis After 2 and 12 Months of Treatment. J. Bone Miner. Res. 2019, 34, 1597–1608. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [Green Version]
- Silverman, S.L.; Cummings, S.R.; Watts, N.B. Consensus Panel of the ASBMR, ISCD, and NOF. Recommendations for the clinical evaluation of agents for treatment of osteoporosis: Consensus of an expert panel representing the American Society for Bone and Mineral Research (ASBMR), the International Society for Clinical Densitometry (ISCD), and the National Osteoporosis Foundation (NOF). J. Bone Miner. Res. 2008, 23, 159–165. [Google Scholar] [CrossRef]
- Siris, E.S.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.J.; Harris, S.T.; Jan de Beur, S.M.; Khosla, S.; Lane, N.E.; et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Lentle, B.; Cheung, A.M.; Hanley, D.; Leslie, W.D.; Lyons, D.; Papaioannou, A.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hodsman, A.B.; et al. Osteoporosis Canada 2010 guidelines for the assessment of fracture risk. Can. Assoc. Radiol. J. 2011, 62, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A.; Oden, A.; Johnell, O.; Johansson, H.; De Laet, C.; Brown, J.; Burckhardt, P.; Cooper, C.; Christiansen, C.; Cummings, S.; et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos. Int. 2007, 18, 1033–1046. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johansson, H.; Harvey, N.C.; McCloskey, E.V. A brief history of FRAX. Arch. Osteoporos. 2018, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Trémollieres, F.A.; Pouillès, J.M.; Drewniak, N.; Laparra, J.; Ribot, C.A.; Dargent-Molina, P. Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: Sensitivity of the WHO FRAX tool. J. Bone. Miner. Res. 2010, 25, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Kužma, M.; Hans, D.; Koller, T.; Némethová, E.; Jackuliak, P.; Killinger, Z.; Resch, H.; Payer, J. Less strict intervention thresholds for the FRAX and TBS-adjusted FRAX predict clinical fractures in osteopenic postmenopausal women with no prior fractures. J. Bone Miner. Metab. 2018, 36, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Bousson, V.; Bergot, C.; Sutter, B.; Levitz, P.; Cortet, B.; Scientific Committee of the Groupe de Recherche et d’Information sur les Ostéoporoses. Trabecular bone score (TBS): Available knowledge, clinical relevance, and future prospects. Osteoporos. Int. 2012, 23, 1489–1501. [Google Scholar] [CrossRef]
- Harvey, N.C.; Glüer, C.C.; Binkley, N.; McCloskey, E.V.; Brandi, M.-L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.M.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Guo, Y.; Zhang, X.; Mei, Y.; Feng, Y.; Zhang, X. Bone susceptibility mapping with MRI is an alternative and reliable biomarker of osteoporosis in postmenopausal women. Eur. Radiol. 2018, 28, 5027–5034. [Google Scholar] [CrossRef]
- Wu, H.Z.; Zhang, X.F.; Han, S.M.; Cao, L.; Wen, J.X.; Wu, W.J.; Gao, B.L. Correlation of bone mineral density with MRI T2* values in quantitative analysis of lumbar osteoporosis. Arch. Osteoporos. 2020, 15, 18. [Google Scholar] [CrossRef]
- Sollmann, N.; Loffler, M.T.; Kronthaler, S.; Bohm, C.; Dieckmeyer, M.; Ruschke, S.; Kirschke, J.S.; Carballido-Gamio, J.; Karampinos, D.C.; Krug, R.; et al. MRI-based quantitative osteoporosis imaging at the spine and femur. J. Magn. Reason. Imaging 2021, 54, 12–35. [Google Scholar] [CrossRef]
- Cohen, A.; Stein, E.M.; Recker, R.R.; Lappe, J.M.; Dempster, D.W.; Zhou, H.; Cremers, S.; McMahon, D.J.; Nickolas, T.L.; Müller, R.; et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J. Clin. Endocrinol. Metab. 2013, 98, 1971–1981. [Google Scholar] [CrossRef] [Green Version]
- Mandelbrot, B.B. A Fractal’s Lacunarity, and how it can be Tuned and Measured. In Fractals in Biology and Medicine; Nonnenmacher, T.F., Losa, G.A., Weibel, E.R., Eds.; Birkhauser Press: Basel, Switzerland, 1993; pp. 8–21. [Google Scholar]
- Dougherty, G.; Henebry, G.M. Lacunarity analysis of spatial pattern in CT images of vertebral trabecular bone for assessing osteoporosis. Med. Eng. Phys. 2002, 24, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Zaia, A.; Rossi, R.; Galeazzi, R.; Scendoni, P. Lacunarity of trabecular bone microarchitecture, TBLβ, as a predictor of bone fragility fracture and potential index of osteoporosis treatment efficacy. The LOTO study [Abstract]. Ann. Rheum. Dis. 2017, 76 (Suppl. S2), 690. [Google Scholar] [CrossRef] [Green Version]
| Characteristic | Overall N = 279 | VF− N = 191 | VF+ N = 88 |
|---|---|---|---|
| Age, years | |||
| Mean ± SD | 60 ± 7 | 59 ± 7 | 63 ± 7 |
| <65, n (%) | 212 (76.0) | 153 (80.1) | 59 (67.0) |
| ≥65, n (%) | 67 (24.0) | 38 (19.9) | 29 (33.0) |
| Bone mineral density, T-score | |||
| Lumbar spine L1-L4 | |||
| Mean ± SD | −2.4 ± 0.9 | −2.4 ± 0.9 | −2.5 ± 0.8 |
| >−2.5, n (%) | 133 (47.7) | 87 (45.5) | 46 (52.3) |
| ≤−2.5, n (%) | 146 (52.3) | 104 (54.5) | 42 (47.7) |
| Trabecular bone architecture, TBLβ | |||
| Lumbar spine L4 | |||
| Median (interquartile range) | 43 (26–87) | 51 (27–103) | 31 (23–60) |
| >40, n (%) | 143 (51.3) | 113 (59.2) | 30 (34.1) |
| ≤40, n (%) | 136 (48.7) | 78 (40.8) | 58 (65.9) |
| Osteoporosis medications | |||
| none, n (%) | 156 (55.9) | 121 (63.4) | 35 (39.8) |
| any medication, n (%) | 123 (44.1) | 70 (36.6) | 53 (60.2) |
| Treatment | Overall N = 123 | VF− N = 70 | VF+ N = 53 | |||
|---|---|---|---|---|---|---|
| Vitamin D/calcium supplements, n (%) a | 95 | (77.2) | 55 | (78.6) | 40 | (75.5) |
| Vitamin D/calcium supplements alone, n (%) a | 44 | (35.8) | 25 | (35.7) | 19 | (35.8) |
| Drug therapy, n (%) a | 79 | (64.2) | 45 | (64.3) | 34 | (64.1) |
| Bisphosphonates, n (%) b | 70 | (88.6) | 43 | (95.6) | 27 | (79.4) |
| alendronate, n (%) c | 24 | (34.3) | 16 | (37.2) | 8 | (29.6) |
| clodronate, n (%) c | 20 | (28.6) | 9 | (20.9) | 11 | (40.7) |
| ibandronate, n (%) c | 6 | (8.6) | 4 | (9.3) | 2 | (7.4) |
| neridronate, n (%) c | 3 | (4.3) | 2 | (4.6) | 1 | (3.7) |
| risedronate, n (%) c | 17 | (24.3) | 12 | (27.9) | 5 | (18.5) |
| Other, n (%) b | 9 | (11.4) | 2 | (4.4) | 7 | (20.6) |
| strontium ranelate, n (%) c | 7 | (77.8) | 1 | (50.0) | 6 | (85.7) |
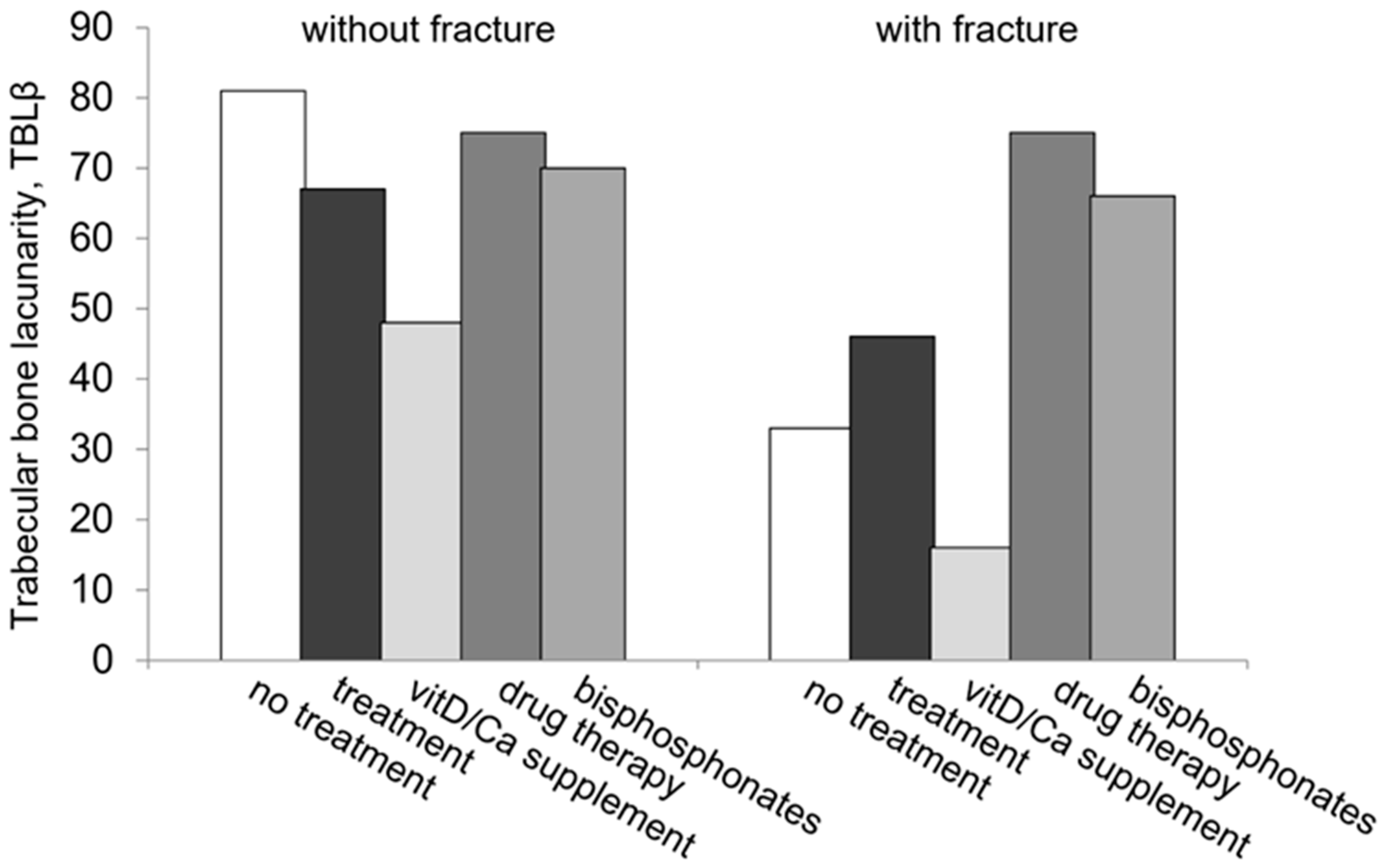
| Subjects | TBLβ | ||||
|---|---|---|---|---|---|
| n VF−/VF+ (%) | VF− | VF+ | p | ||
| Overall | 191/88 | (100) | 51 (27–103) | 31 (23–60) | 0.004 |
| Untreated | 121/35 | (55.9) | 52 (28–110) | 30 (23–56) | 0.027 |
| Treated (any medication) | 70/53 | (44.1) | 50 (26–93) | 31 (23–69) | 0.08 |
| Vit D/calcium supplements alone a | 25/19 | (35.8) | 45 (18–66) | 27 (23–39) | 0.005 |
| Drug therapy a | 45/34 | (73.2) | 52 (26–101) | 37 (25–101) | 0.31 |
| Bisphosphonates b | 43/27 | (88.6) | 50 (26–100) | 39 (24–125) | 0.40 |
| Alendronate c | 16/8 | (34.3) | 50 (28–93) | 41 (19–152) | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaia, A.; Maponi, P.; Sallei, M.; Galeazzi, R.; Scendoni, P. Measuring Drug Therapy Effect on Osteoporotic Fracture Risk by Trabecular Bone Lacunarity: The LOTO Study. Biomedicines 2023, 11, 781. https://doi.org/10.3390/biomedicines11030781
Zaia A, Maponi P, Sallei M, Galeazzi R, Scendoni P. Measuring Drug Therapy Effect on Osteoporotic Fracture Risk by Trabecular Bone Lacunarity: The LOTO Study. Biomedicines. 2023; 11(3):781. https://doi.org/10.3390/biomedicines11030781
Chicago/Turabian StyleZaia, Annamaria, Pierluigi Maponi, Manuela Sallei, Roberta Galeazzi, and Pietro Scendoni. 2023. "Measuring Drug Therapy Effect on Osteoporotic Fracture Risk by Trabecular Bone Lacunarity: The LOTO Study" Biomedicines 11, no. 3: 781. https://doi.org/10.3390/biomedicines11030781





