Assessment of the Mutation Profile of Tonsillar Squamous Cell Carcinomas Using Targeted Next-Generation Sequencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Samples
2.2. DNA Extraction
2.3. Detection of HPV
2.4. Immunohistochemistry
2.5. Library Preparation and Sequencing
2.6. Data Analyses, TCGA Data, and Gene Set Enrichment Analysis (GSEA)
2.7. Statistical Analyses
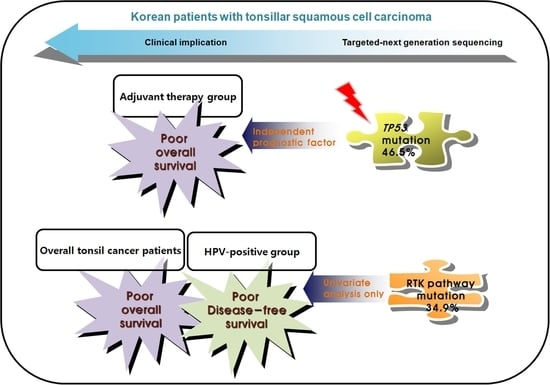
3. Results
3.1. Patient Characteristics
3.2. Molecular Profiling in Overall TSCCs
3.3. Comparisons between HPV-Positive and -Negative TSCCs
3.4. Impact of Gene Mutations and Pathway Mutations on Clinicopathological Features
3.5. Prognostic Genetic Factors in Overall Tonsil Cancers
3.6. Prognostic Genetic Factors in HPV-Positive TSCCs
3.7. Prognostic Genetic Factors in Patients Who Received Adjuvant Therapy following Surgery
3.8. Comparisons with TCGA Data
3.9. Identification of Gene Signatures Using GSEA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taberna, M.; Mena, M.; Pavon, M.A.; Alemany, L.; Gillison, M.L.; Mesia, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Luginbuhl, A.; Sanders, M.; Spiro, J.D. Prevalence, morphology, and prognosis of human papillomavirus in tonsillar cancer. Ann. Otol. Rhinol. Laryngol. 2009, 118, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, O.; Moorthy, R.; Lyne, O.; Black, M.; Mitchell, D.; Wiseberg, J. A 20-year retrospective study of tonsil cancer incidence and survival trends in South East England: 1987–2006. Clin. Otolaryngol. 2011, 36, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.J.; Schartinger, V.H.; Wuerdemann, N.; Langer, C.; Mollenhoff, K.; Collin, L.; Sutton, L.; Riedl, D.; Kreuter, A.; Lechner, M.; et al. Awareness of Human Papillomavirus (HPV) and HPV Vaccination amongst the General Population in Germany: Lack of Awareness and Need for Action. Oncol. Res. Treat. 2022, 45, 561–567. [Google Scholar] [CrossRef]
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.; Moore, M.G. HPV-related oropharyngeal cancer: A review on burden of the disease and opportunities for prevention and early detection. Hum. Vaccin. Immunother. 2019, 15, 1920–1928. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Rubin Grandis, J.; Takata, T.; Slootweg, P.J. International Agency for Research on Cancer. In WHO Classification of Head and Neck Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 133–146. [Google Scholar]
- Doweck, I.; Robbins, K.T.; Mendenhall, W.M.; Hinerman, R.W.; Morris, C.; Amdur, R. Neck level-specific nodal metastases in oropharyngeal cancer: Is there a role for selective neck dissection after definitive radiation therapy? Head Neck 2003, 25, 960–967. [Google Scholar] [CrossRef]
- Slootweg, P.J.; Eveson, J.W. Tumours of the oral cavity and oropharynx. In World Health Organization Classification of Tumours: Head and Neck Tumours; Barnes, L., Eveson, J.W., Reichart, P., Sidransky, D., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2005; pp. 163–207. [Google Scholar]
- Throat Cancer: Squamous Cell Carcinoma of the Tonsil. Available online: https://healthengine.com.au/info/throat-cancer-squamous-cell-carcinoma-of-the-tonsil (accessed on 25 January 2023).
- Kuo, Y.Y.; Chu, P.Y.; Chang, S.Y.; Wang, Y.F.; Tsai, T.L.; Yang, M.H.; Wang, L.W.; Tai, S.K. Treatment selection for tonsillar squamous cell carcinoma. J. Chin. Med. Assoc. JCMA 2013, 76, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Koo, B.S.; Kang, S.; Park, K.; Kim, H.; Lee, K.R.; Lee, M.J.; Kim, J.M.; Choi, E.C.; Cho, N.H. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int. J. Cancer 2007, 120, 1418–1425. [Google Scholar] [CrossRef]
- Amin, M.B.; American Joint Committee on Cancer; American Cancer Society. AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S.B., Gress, D.M., Meyer, L.R., Eds.; American Joint Committee on Cancer; Springer: Chicago, IL, USA, 2017; pp. 113–135. [Google Scholar]
- Banerjee, S.; Kundu, D.; Mukherjee, M.; Kumar Mait, P. Early stage squamous cell carcinoma of the tonsil presenting with multiple organ metastases including skin and brain after successful local treatment. J. Cancer Metastasis Treat. 2015, 1, 31–33. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.H.; Perez-Ordonez, B.; Weinreb, I.; Hope, A.; Massey, C.; Waldron, J.N.; Kim, J.; Bayley, A.J.; Cummings, B.; Cho, B.C.; et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral. Oncol. 2013, 49, 79–85. [Google Scholar] [CrossRef]
- Califano, J.; van der Riet, P.; Westra, W.; Nawroz, H.; Clayman, G.; Piantadosi, S.; Corio, R.; Lee, D.; Greenberg, B.; Koch, W.; et al. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996, 56, 2488–2492. [Google Scholar] [CrossRef]
- Gillison, M.L.; Akagi, K.; Xiao, W.; Jiang, B.; Pickard, R.K.L.; Li, J.; Swanson, B.J.; Agrawal, A.D.; Zucker, M.; Stache-Crain, B.; et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome. Res. 2019, 29, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Garnaes, E.; Kiss, K.; Andersen, L.; Therkildsen, M.H.; Franzmann, M.B.; Filtenborg-Barnkob, B.; Hoegdall, E.; Krenk, L.; Josiassen, M.; Lajer, C.B.; et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000-2010: The largest registry-based study to date. Int. J. Cancer 2015, 136, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kwon, M.J.; Kang, H.S.; Kim, Y.J.; Kim, N.Y.; Kim, M.J.; Min, K.W.; Choi, K.C.; Nam, E.S.; Cho, S.J.; et al. Targeted next-generation sequencing of well-differentiated rectal, gastric, and appendiceal neuroendocrine tumors to identify potential targets. Hum. Pathol. 2019, 87, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kwon, M.J.; Park, B.; Choi, H.G.; Nam, E.S.; Cho, S.J.; Min, K.W.; Kim, E.S.; Hwang, H.S.; Hong, M.; et al. Negative Prognostic Implication of TERT Promoter Mutations in Human Papillomavirus-Negative Tonsillar Squamous Cell Carcinoma Under the New 8th AJCC Staging System. Indian J. Surg. Oncol. 2021, 12, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, C.F.; Anthony, K.; Krupa, S.; Buchoff, J.; Day, M.; Hannay, T.; Buetow, K.H. PID: The Pathway Interaction Database. Nucleic Acids Res. 2009, 37, D674–D679. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Myers, J.N. TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J. Cell Biochem. 2016, 117, 2682–2692. [Google Scholar] [CrossRef] [Green Version]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Kumar, M. An integrative approach toward identification and analysis of therapeutic targets involved in HPV pathogenesis with a focus on carcinomas. Cancer Biomark. 2022, 36, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Bersani, C.; Sivars, L.; Haeggblom, L.; DiLorenzo, S.; Mints, M.; Ahrlund-Richter, A.; Tertipis, N.; Munck-Wikland, E.; Nasman, A.; Ramqvist, T.; et al. Targeted sequencing of tonsillar and base of tongue cancer and human papillomavirus positive unknown primary of the head and neck reveals prognostic effects of mutated FGFR3. Oncotarget 2017, 8, 35339–35350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, D.; Visser-Grieve, S.; Yang, X. Tumour suppressor genes in chemotherapeutic drug response. Biosci. Rep. 2012, 32, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.P.; Brown-Swigart, L.; Evan, G.I. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 2006, 127, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Ventura, A.; Kirsch, D.G.; McLaughlin, M.E.; Tuveson, D.A.; Grimm, J.; Lintault, L.; Newman, J.; Reczek, E.E.; Weissleder, R.; Jacks, T. Restoration of p53 function leads to tumour regression in vivo. Nature 2007, 445, 661–665. [Google Scholar] [CrossRef]
- Byskata, K.; Lukoseviciute, M.; Tuti, F.; Zupancic, M.; Kostopoulou, O.N.; Holzhauser, S.; Dalianis, T. Targeted Therapy with PI3K, PARP, and WEE1 Inhibitors and Radiotherapy in HPV Positive and Negative Tonsillar Squamous Cell Carcinoma Cell Lines Reveals Synergy while Effects with APR-246 Are Limited. Cancers 2022, 15, 93. [Google Scholar] [CrossRef]
- Kong, A.; Good, J.; Kirkham, A.; Savage, J.; Mant, R.; Llewellyn, L.; Parish, J.; Spruce, R.; Forster, M.; Schipani, S.; et al. Phase I trial of WEE1 inhibition with chemotherapy and radiotherapy as adjuvant treatment, and a window of opportunity trial with cisplatin in patients with head and neck cancer: The WISTERIA trial protocol. BMJ Open 2020, 10, e033009. [Google Scholar] [CrossRef]
- Nair, S.; Bonner, J.A.; Bredel, M. EGFR Mutations in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3818. [Google Scholar] [CrossRef]
- Munirajan, A.K.; Tutsumi-Ishii, Y.; Mohanprasad, B.K.; Hirano, Y.; Munakata, N.; Shanmugam, G.; Tsuchida, N. p53 gene mutations in oral carcinomas from India. Int. J. Cancer 1996, 66, 297–300. [Google Scholar] [CrossRef]
- Beaty, B.T.; Moon, D.H.; Shen, C.J.; Amdur, R.J.; Weiss, J.; Grilley-Olson, J.; Patel, S.; Zanation, A.; Hackman, T.G.; Thorp, B.; et al. PIK3CA Mutation in HPV-Associated OPSCC Patients Receiving Deintensified Chemoradiation. J. Natl. Cancer Inst. 2020, 112, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, K.; Imoto, I.; Pimkhaokham, A.; Hasegawa, S.; Tsuda, H.; Omura, K.; Inazawa, J. PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci. 2006, 97, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.B.; Lee, S.W.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y.; Cho, K.J. Human papillomavirus prevalence and cell cycle related protein expression in tonsillar squamous cell carcinomas of korean patients with clinicopathologic analysis. Korean J. Pathol. 2013, 47, 148–157. [Google Scholar] [CrossRef]
- Fasano, M.; Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Paragliola, F.; Sparano, F.; Iacovino, M.L.; Castrichino, A.; Doria, F.; Sica, A.; et al. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Adv. Med. Oncol. 2021, 13, 1758835920949418. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.M.; Johnson, D.E.; Grandis, J.R. Investigational multitargeted kinase inhibitors in development for head and neck neoplasms. Expert Opin. Investig. Drugs 2019, 28, 351–363. [Google Scholar] [CrossRef]
- Nichols, A.C.; Palma, D.A.; Chow, W.; Tan, S.; Rajakumar, C.; Rizzo, G.; Fung, K.; Kwan, K.; Wehrli, B.; Winquist, E.; et al. High frequency of activating PIK3CA mutations in human papillomavirus-positive oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, H.; McGrath, J.E.; Hughes, B.; Xiu, J.; Brodskiy, P.; Sukari, A.; Darabi, S.; Ikpeazu, C.; Nabhan, C.; Korn, W.M.; et al. Genomic and Molecular Profiling of Human Papillomavirus Associated Head and Neck Squamous Cell Carcinoma Treated with Immune Checkpoint Blockade Compared to Survival Outcomes. Cancers 2021, 13, 6309. [Google Scholar] [CrossRef]
- Reder, H.; Wagner, S.; Wuerdemann, N.; Langer, C.; Sandmann, S.; Braeuninger, A.; Dugas, M.; Gattenloehner, S.; Wittekindt, C.; Klussmann, J.P. Mutation patterns in recurrent and/or metastatic oropharyngeal squamous cell carcinomas in relation to human papillomavirus status. Cancer Med. 2021, 10, 1347–1356. [Google Scholar] [CrossRef]
- Chung, K.; Min, H.K.; Jung, K.Y.; Choi, G.; Choi, J.O. Squamous cell carcinoma of the tonsil--clinical features and treatment results. J. Korean Med. Sci. 1997, 12, 416–420. [Google Scholar] [CrossRef] [Green Version]
- van Rensburg, E.J.; Engelbrecht, S.; van Heerden, W.F.; Kotze, M.J.; Raubenheimer, E.J. Detection of p53 gene mutations in oral squamous cell carcinomas of a black African population sample. Hum. Mutat. 1998, 11, 39–44. [Google Scholar] [CrossRef]
- Sewell, A.; Brown, B.; Biktasova, A.; Mills, G.B.; Lu, Y.; Tyson, D.R.; Issaeva, N.; Yarbrough, W.G. Reverse-phase protein array profiling of oropharyngeal cancer and significance of PIK3CA mutations in HPV-associated head and neck cancer. Clin. Cancer Res. 2014, 20, 2300–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batta, N.; Pandey, M. Mutational spectrum of tobacco associated oral squamous carcinoma and its therapeutic significance. World J. Surg. Oncol. 2019, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.R.; Davis, S.J.; Lang-Kuhs, K.A.; Rohde, S.; Wang, X.; Liu, P.; Dupont, W.D.; Plummer, D., Jr.; Thorstad, W.L.; Chernock, R.D.; et al. Oropharyngeal Squamous Cell Carcinoma With Discordant p16 and HPV mRNA Results: Incidence and Characterization in a Large, Contemporary United States Cohort. Am. J. Surg. Pathol. 2021, 45, 951–961. [Google Scholar] [CrossRef]
- Hong, A.; Jones, D.; Chatfield, M.; Lee, C.S.; Zhang, M.; Clark, J.; Elliott, M.; Harnett, G.; Milross, C.; Rose, B. HPV status of oropharyngeal cancer by combination HPV DNA/p16 testing: Biological relevance of discordant results. Ann. Surg. Oncol. 2013, 20 (Suppl. S3), S450–S458. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Postel-Vinay, S. Immunotherapy for SMARCB1-Deficient Sarcomas: Current Evidence and Future Developments. Biomedicines 2022, 10, 650. [Google Scholar] [CrossRef]







| Variable | Total |
|---|---|
| n = 43 (%) | |
| Sex | |
| Male | 38 (88.4) |
| Female | 5 (11.6) |
| Age (mean, range) | 54 (36–80) |
| Age (y) | |
| ≤60 | 28 (65.1) |
| >60 | 15 (34.9) |
| Smoking history | |
| Low | 21 (48.8) |
| Heavy | 22 (51.2) |
| Alcohol history | |
| Light | 27 (62.8) |
| Heavy | 16 (37.2) |
| HPV status | |
| Negative | 8 (18.6) |
| Positive | 35 (81.4) |
| pT category | |
| 1 | 9 (20.9) |
| 2 | 13 (30.25) |
| 3 | 13 (30.25) |
| 4 | 8 (18.6) |
| pN category | |
| 0 | 8 (18.6) |
| 1 | 18 (41.9) |
| 2 | 8 (18.6) |
| 3 | 9 (20.9) |
| AJCC stage (8th) | |
| I | 15 (34.9) |
| II | 8 (18.6) |
| III | 7 (16.3) |
| IV | 13 (30.2) |
| PD-L1 expression | |
| Negative | 33 (76.7) |
| Positive | 10 (23.3) |
| Adjuvant therapy | |
| Done | 28 (65.1) |
| Not done | 15 (34.9) |
| Pathways | Genes | No. Variant Calls (%) | No. Cases (%) |
|---|---|---|---|
| EGFR pathway | 39 (32.5) | 22 (51.2) | |
| EGFR, ERBB2 | |||
| KRAS, HRAS | |||
| PIK3CA, PTEN, AKT1 | |||
| p53 pathway (genome integrity) | 21 (48.8) | ||
| TP53 | 30 (25.0) | 20 (46.5) | |
| ATM | 2 (1.7) | 2 (4.7) | |
| PI3K pathway | 17 (39.5) | ||
| PIK3CA | 13 (10.8) | 11 (25.6) | |
| PTEN | 9 (7.5) | 8 (18.6) | |
| AKT1 | 1 (0.8) | 1 (2.3) | |
| RTK pathway | 15 (34.9) | ||
| KIT | 3 (2.5) | 3 (7.0) | |
| PDGFRA | 4 (3.3) | 4 (9.3) | |
| EGFR | 9 (7.5) | 7 (16.3) | |
| ERBB2 | 2 (1.7) | 2 (4.7) | |
| FGFR2 | 2 (1.7) | 2 (4.7) | |
| FGFR3 | 1 (0.8) | 1 (2.3) | |
| MET | 1 (0.8) | 1 (2.3) | |
| RET | 1 (0.8) | 1 (2.3) | |
| FLT3 | 1 (0.8) | 1 (2.3) | |
| ALK | 2 (1.7) | 2 (4.7) | |
| RB pathway (cell cycle) | 8 (18.6) | ||
| RB1 | 5 (4.2) | 5 (11.6) | |
| CDKN2A | 3 (2.5) | 3 (7.0) | |
| TGF-β pathway | SMAD4 | 8 (6.8) | 6 (14.0) |
| MAPK pathway | 5 (11.6) | ||
| KRAS | 2 (1.7) | 2 (4.7) | |
| HRAS | 3 (2.5) | 3 (7.0) | |
| Proteolysis | FBXW7 | 5 (4.2) | 5 (11.6) |
| SWI/SNF complex | SMARCB1 | 4 (3.3) | 4 (9.3) |
| Notch pathway | NOTCH1 | 2 (1.7) | 2 (4.7) |
| HIPPO pathway | CDH1 | 2 (1.7) | 2 (4.7) |
| mTOR pathway | STK11 | 1 (0.8) | 1 (2.3) |
| Metabolism | IDH1 | 1 (0.8) | 1 (2.3) |
| IDH2 | 1 (0.8) | 1 (2.3) | |
| Transcription factor/regulator | VHL | 1 (0.8) | 1 (2.3) |
| Other | MPL | 1 (0.8) | 1 (2.3) |
| Parameter | TP53 | PIK3CA | EGFR Pathway | P53 Pathway | PI3K Pathway | RTK Pathway | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MT | WT | p | MT | WT | p | MT | WT | p | MT | WT | p | MT | WT | p | MT | WT | p | |
| n = 20 (%) | n = 23 (%) | n = 11 (%) | n = 32 (%) | n = 22 (%) | n = 21 (%) | n = 21 (%) | n = 22 (%) | n = 17 (%) | n = 26 (%) | n = 15 (%) | n = 28 (%) | |||||||
| Sex | 0.051 | 0.306 | 0.185 | 0.048 | 0.139 | 0.798 | ||||||||||||
| Male | 20 (100) | 18 (78.3) | 11 (100) | 27 (84.4) | 21 (95.5) | 17 (81.0) | 21 (100) | 17 (77.3) | 17 (100) | 21 (80.8) | 13 (86.7) | 25 (89.3) | ||||||
| Female | 0 (0) | 5 (21.7) | 0 (0) | 5 (15.6) | 1 (4.5) | 4 (19.0) | 0 (0.0) | 5 (22.7) | 0 (0.0) | 5 (19.2) | 2 (13.3) | 3 (10.7) | ||||||
| Age (yrs) | 0.512 | 0.719 | 0.526 | 0.666 | 0.745 | 0.235 | ||||||||||||
| ≤60 | 12 (60.0) | 16 (69.6) | 8 (72.7) | 20 (62.5) | 13 (59.1) | 15 (71.4) | 13 (61.9) | 15 (68.2) | 12 (70.6) | 16 (61.5) | 8 (53.3) | 20 (71.4) | ||||||
| >60 | 8 (40.0) | 7 (30.4) | 3 (27.3) | 12 (37.5) | 9 (40.9) | 6 (28.6) | 8 (38.1) | 7 (31.8) | 5 (29.4) | 10 (38.5) | 7 (46.7) | 8 (28.6) | ||||||
| Smoking | 0.033 | 0.736 | 0.763 | 0.015 | 1.000 | 0.526 | ||||||||||||
| Low | 6 (30.0) | 15 (65.2) | 6 (54.5) | 15 (46.9) | 10 (45.5) | 11 (52.4) | 6 (28.6) | 15 (68.2) | 8 (47.1) | 13 (50.0) | 6 (40.0) | 15 (53.6) | ||||||
| Heavy | 14 (70.0) | 8 (34.8) | 5 (45.5) | 17 (53.1) | 12 (54.5) | 10 (47.6) | 15 (71.4) | 7 (31.8) | 9 (52.9) | 13 (50.0) | 9 (60.0) | 13 (46.4) | ||||||
| Alcohol | 0.127 | 0.494 | 0.537 | 0.215 | 1.000 | 0.782 | ||||||||||||
| Light | 10 (50.0) | 17 (73.9) | 8 (72.7) | 19 (59.4) | 15 (68.2) | 12 (57.1) | 11 (52.4) | 16 (72.7) | 11 (64.7) | 16 (61.5) | 9 (60.0) | 18 (64.3) | ||||||
| Heavy | 10 (50.0) | 6 (26.1) | 3 (27.3) | 13 (40.6) | 7 (31.8) | 9 (42.9) | 10 (47.6) | 6 (27.3) | 6 (35.3) | 10 (38.5) | 6 (40.0) | 10 (35.7) | ||||||
| HPV | 0.017 | 0.392 | 0.698 | 0.021 | 0.502 | 0.320 | ||||||||||||
| Negative | 7 (35.0) | 1 (4.3) | 3 (27.3) | 5 (15.6) | 5 (22.7) | 3 (14.3) | 7 (33.3) | 1 (4.5) | 4 (23.5) | 4 (15.4) | 4 (26.7) | 4 (14.3) | ||||||
| Positive | 13 (65.0) | 22 (95.7) | 8 (72.7) | 27 (84.4) | 17 (77.3) | 18 (85.7) | 14 (66.7) | 21 (95.5) | 13 (76.5) | 22 (84.6) | 11 (73.3) | 24 (85.7) | ||||||
| AJCC stage | 0.547 | 0.736 | 0.763 | 0.366 | 1.000 | 0.019 | ||||||||||||
| I–II | 9 (45.0) | 13 (56.5) | 5 (45.5) | 17 (53.1) | 12 (54.5) | 10 (47.6) | 9 (42.9) | 13 (59.1) | 9 (52.9) | 13 (50.0) | 4 (26.7) | 18 (64.3) | ||||||
| III–IV | 11 (55.0) | 10 (43.5) | 6 (54.5) | 15 (46.9) | 10 (45.5) | 11 (52.4) | 12 (57.1) | 9 (40.9) | 8 (47.1) | 13 (50.0) | 11 (73.3) | 10 (35.7) | ||||||
| pT category | 0.763 | 1.000 | 0.131 | 1.000 | 0.537 | 0.347 | ||||||||||||
| T1–T2 | 11 (55.0) | 11 (47.8) | 6 (54.5) | 16 (50.0) | 14 (63.6) | 8 (38.1) | 11 (52.4) | 11 (50.0) | 10 (58.8) | 12 (46.2) | 6 (40.0) | 16 (57.1) | ||||||
| T3–T4 | 9 (45.0) | 12 (52.2) | 5 (45.5) | 16 (50.0) | 8 (36.4) | 13 (61.9) | 10 (47.6) | 11 (50.0) | 7 (41.2) | 14 (53.8) | 9 (60.0) | 12 (42.9) | ||||||
| pN category | 0.038 * | 0.656 | 0.412 | 0.046 | 0.298 | 0.391 | ||||||||||||
| N0 | 6 (30.0) | 1 (4.3) | 1 (9.1) | 6 (18.8) | 5 (22.7) | 2 (9.5) | 6 (28.6) | 1 (4.5) | 4 (23.5) | 3 (11.5) | 1 (6.7) | 6 (21.4) | ||||||
| N+ | 14 (70.0) | 22 (95.7) | 10 (90.9) | 26 (81.2) | 17 (77.3) | 19 (90.5) | 15 (71.4) | 21 (95.5) | 13 (76.5) | 23 (88.5) | 14 (93.3) | 22 (78.6) | ||||||
| PD-L1 | 0.011 | 0.409 | 0.162 | 0.069 | 0.061 | 1.000 | ||||||||||||
| Negative | 19 (95.0) | 14 (60.9) | 10 (90.9) | 23 (71.9) | 19 (86.4) | 14 (66.7) | 19 (90.5 | 14 (63.6) | 16 (94.1) | 17 (65.4) | 12 (80.0) | 21 (75.0) | ||||||
| Positive | 1 (5.0) | 9 (39.1) | 1 (9.1) | 9 (28.1) | 3 (13.6) | 7 (33.3) | 2 (9.5) | 8 (36.4) | 1 (5.9) | 9 (34.6) | 3 (20.0) | 7 (25.0) | ||||||
| Overall Survival | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Sex | 1.105 | 0.894 | 1.337 | 0.695 | ||||
| Female vs. Male | (0.256–4.767) | (0.313–5.710) | ||||||
| Age (y) | 3.146 | 0.011 * | 3.565 | 0.020 * | 2.140 | 0.072 | 2.068 | 0.088 |
| ≤60 vs. >60 | (1.296–7.639) | (1.221–10.412) | (0.935–4.902) | (0.898–4.761) | ||||
| Smoking | 1.427 | 0.433 | 1.126 | 0.777 | ||||
| Low vs. Heavy | (0.587–3.471) | (0.495–2.561) | ||||||
| Alcohol | 1.651 | 0.272 | 1.126 | 0.782 | ||||
| Light vs. Heavy | (0.675–4.041) | (0.485–2.615) | ||||||
| HPV | 0.385 | 0.058 | 1.901 | 0.313 | 0.567 | 0.239 | ||
| Negative vs. Positive | (0.144–1.033) | (0.546–6.618) | (0.220–1.460) | |||||
| AJCC stage | 4.688 | 0.003 * | 5.246 | 0.006 * | 3.386 | 0.008 * | 2.856 | 0.029 * |
| I–II vs. III–IV | (1.687–13.030) | (1.596–17.235) | (1.380–8.306) | (1.110–7.345) | ||||
| Adjuvant therapy | 1.287 | 0.607 | 1.736 | 0.247 | ||||
| Yes vs. No | (0.493–3.356) | (0.683–4.418) | ||||||
| TP53 | 2.221 | 0.088 | 1.781 | 0.265 | 1.436 | 0.390 | ||
| WT vs. MT | (0.887–5.563) | (0.646–4.909) | (0.629–3.280) | |||||
| PIK3CA | 0.787 | 0.642 | 0.892 | 0.811 | ||||
| WT vs. MT | (0.286–2.166) | (0.351–2.267) | ||||||
| RTK pathway | 3.371 | 0.007 * | 1.798 | 0.214 | 2.349 | 0.042 * | 1.584 | 0.295 |
| WT vs. MT | (1.390–8.178) | (0.712–4.539) | (1.033–5.339) | (0.670–3.746) | ||||
| p53 pathway | 1.915 | 0.161 | 1.248 | 0.597 | ||||
| WT vs. MT | (0.772–4.752) | (0.548–2.843) | ||||||
| PI3K pathway | 0.647 | 0.353 | 0.816 | 0.635 | ||||
| WT vs. MT | (0.258–1.622) | (0.352–1.890) | ||||||
| EGFR pathway | 0.669 | 0.372 | 0.781 | 0.556 | ||||
| WT vs. MT | (0.277–1.618) | (0.344–1.776) | ||||||
| Overall Survival | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Sex | 0.658 | 0.585 | 0.833 | 0.809 | ||||
| Female vs. Male | (0.147–2.953) | (0.190–3.653) | ||||||
| Age (y) | 4.942 | 0.003 * | 10.521 | 0.002 * | 3.165 | 0.021 * | 4.378 | 0.010 * |
| ≤60 vs. >60 | (1.705–14.329) | (2.426–45.616) | (1.190–8.420) | (1.429–13.408) | ||||
| Smoking | 1.447 | 0.493 | 1.159 | 0.762 | ||||
| No vs. Current/Former | (0.504–4.159) | (0.446–3.013) | ||||||
| Alcohol | 1.624 | 0.390 | 1.086 | 0.877 | ||||
| Light vs. Heavy | (0.538–4.907) | (0.381–3.093) | ||||||
| AJCC stage | 5.205 | 0.006 * | 9.576 | 0.003 * | 3.809 | 0.009 * | 4.700 | 0.007 * |
| I–II vs. III–IV | (1.622–16.707) | (2.141–42.823) | (1.399–10.370) | (1.520–14.530) | ||||
| TP53 | 1.482 | 0.474 | 0.972 | 0.955 | ||||
| WT vs. MT | (0.505–4.350) | (0.358–2.636) | ||||||
| PIK3CA | 0.428 | 0.268 | 0.606 | 0.432 | ||||
| WT vs. MT | (0.096–1.918) | (0.174–2.113) | ||||||
| P53 pathway | 1.246 | 0.686 | 0.821 | 0.698 | ||||
| WT vs. MT | (0.428–3.631) | (0.303–2.223) | ||||||
| PI3K pathway | 0.357 | 0.115 | 0.587 | 0.318 | ||||
| WT vs. MT | (0.099–1.284) | (0.207–1.670) | ||||||
| RTK pathway | 4.314 | 0.007 * | 1.979 | 0.218 | 2.758 | 0.037* | 1.415 | 0.495 |
| WT vs. MT | (1.489–12.502) | (0.667–5.868) | (1.061–7.173) | (0.522–3.836) | ||||
| EGFR pathway | 0.522 | 0.247 | 0.702 | 0.474 | ||||
| WT vs. MT | (0.174–1.567) | (0.267–1.849) | ||||||
| Overall Survival | Disease-Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Sex | 2.616 | 0.354 | 3.535 | 0.221 | ||||
| Female vs. Male | (0.342–20.020) | (0.468–26.710) | ||||||
| Age (y) | 2.211 | 0.158 | 1.490 | 0.456 | ||||
| ≤60 vs. >60 | (0.735–6.648) | (0.522–4.248) | ||||||
| Smoking | 1.429 | 0.505 | 1.079 | 0876 | ||||
| Low vs. Heavy | (0.500–4.086) | (0.416–2.800) | ||||||
| Alcohol | 2.112 | 0.185 | 1.285 | 0.639 | ||||
| Light vs. Heavy | (0.698–6.388) | (0.451–3.657) | ||||||
| HPV | 0.340 | 0.076 | 1.146 | 0.841 | 0.601 | 0.376 | ||
| Negative vs. Positive | (0.103–1.119) | (0.302–4.355) | (0.195–1.856) | |||||
| AJCC stage | 4.765 | 0.017 * | 4.856 | 0.024 * | 2.868 | 0.049 * | 3.075 | 0.039 * |
| I–II vs. III–IV | (1.319–17.220) | (1.228–19.201) | (1.005–8.187) | (1.057–8.944) | ||||
| TP53 | 4.582 | 0.011 * | 4.348 | 0.022 * | 2.439 | 0.075 | 2.653 | 0.059 |
| WT vs. MT | (1.419–14.789) | (1.242–15.223) | (0.913–6.511) | (0.963–7.309) | ||||
| PIK3CA | 0.759 | 0.643 | 0.871 | 0.796 | ||||
| WT vs. MT | (0.237–2.428) | (0.306–2.483) | ||||||
| P53 pathway | 3.678 | 0.029 * | 2.681 | 0.153 | 1.929 | 0.187 | ||
| WT vs. MT | (1.144–11.827) | (0.693–10.368) | (0.727–5.117) | |||||
| PI3K pathway | 0.663 | 0.463 | 0.906 | 0.842 | ||||
| WT vs. MT | (0.222–1.984) | (0.343–2.391) | ||||||
| RTK pathway | 2.848 | 0.055 | 1.653 | 0.303 | ||||
| WT vs. MT | (0.980–8.279) | (0.636–4.297) | ||||||
| EGFR pathway | 0.598 | 0.344 | 0.745 | 0.546 | ||||
| WT vs. MT | (0.206–1.735) | (0.286–1.941) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.Y.; Lee, J.S.; Wee, J.H.; Kang, J.W.; Kim, E.S.; Koo, T.; Hwang, H.S.; Kim, H.J.; Kang, H.S.; Lim, H.; et al. Assessment of the Mutation Profile of Tonsillar Squamous Cell Carcinomas Using Targeted Next-Generation Sequencing. Biomedicines 2023, 11, 851. https://doi.org/10.3390/biomedicines11030851
Park HY, Lee JS, Wee JH, Kang JW, Kim ES, Koo T, Hwang HS, Kim HJ, Kang HS, Lim H, et al. Assessment of the Mutation Profile of Tonsillar Squamous Cell Carcinomas Using Targeted Next-Generation Sequencing. Biomedicines. 2023; 11(3):851. https://doi.org/10.3390/biomedicines11030851
Chicago/Turabian StylePark, Ha Young, Joong Seob Lee, Jee Hye Wee, Jeong Wook Kang, Eun Soo Kim, Taeryool Koo, Hee Sung Hwang, Hyo Jung Kim, Ho Suk Kang, Hyun Lim, and et al. 2023. "Assessment of the Mutation Profile of Tonsillar Squamous Cell Carcinomas Using Targeted Next-Generation Sequencing" Biomedicines 11, no. 3: 851. https://doi.org/10.3390/biomedicines11030851