Regenerative Potential of Hydroxyapatite-Based Ceramic Biomaterial on Mandibular Cortical Bone: An In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Fabrication
2.1.1. The Synthesis of HAP Powder
2.1.2. The Preparation of the HAP Ceramics
2.2. In Vivo Creation of the Mandibular Defect
2.2.1. Animal Model
2.2.2. Premedication and General Anaesthesia
2.2.3. Clinical Procedures
2.2.4. Post-Operative Care
2.2.5. Animal Euthanasia and General Observations
2.3. Evaluation of the Biomaterial and Newly Formed Bone Tissue
2.3.1. Assessment of Bone Hardness
2.3.2. XRD Phase Analysis, Compressive Strength and Microstructure of Samples
2.3.3. In Vitro Cell Cultivation, Cytotoxicity and Viability Testing
2.3.4. Macroscopic Assessment
2.3.5. Histological and Immunohistochemical Assessment
2.3.6. Radiological Assessment
3. Results
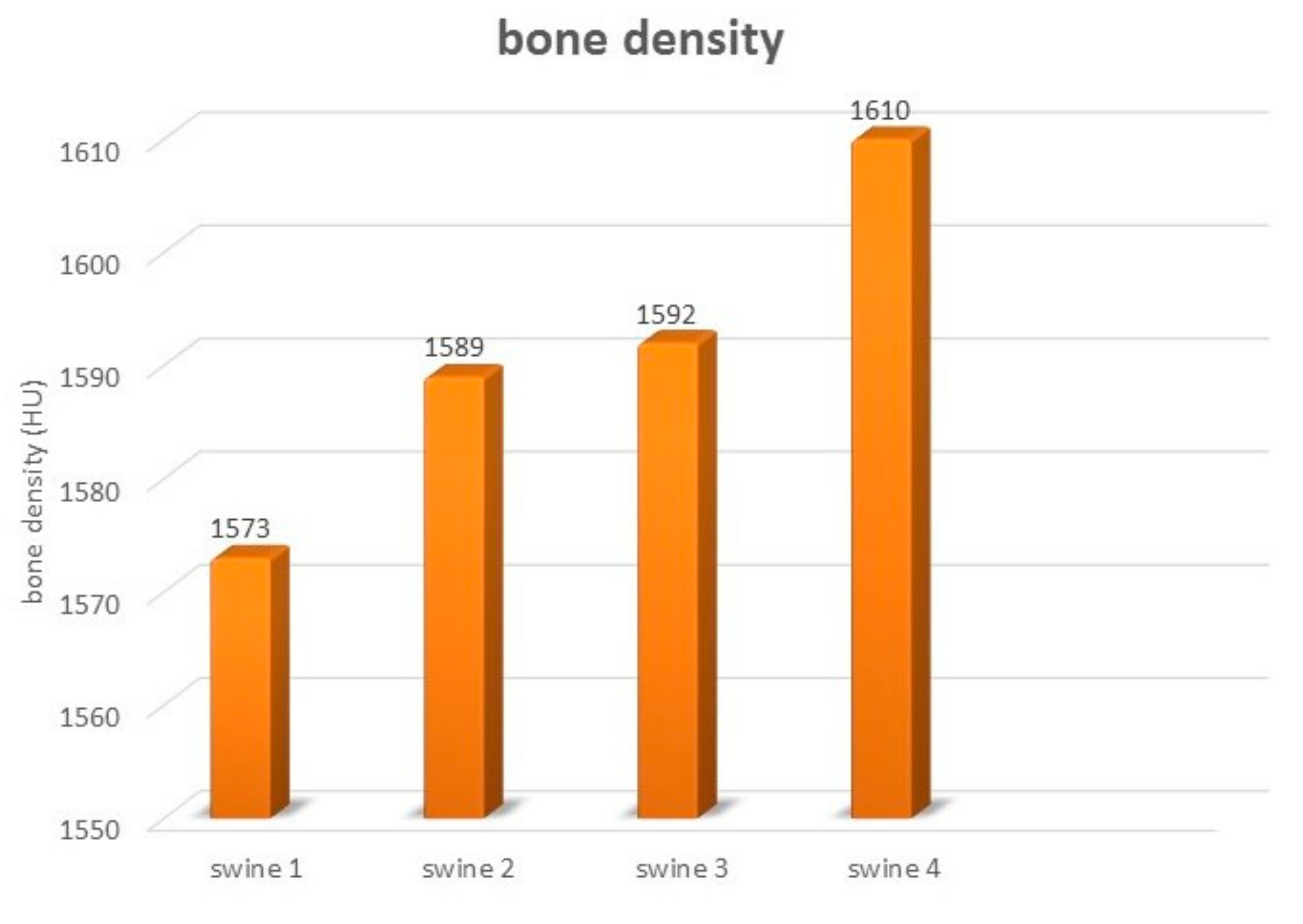
3.1. Bone Hardness
3.2. Microstructure, Compressive Strength and XRD Phase Analysis of Ceramics
3.3. In Vitro Cytotoxicity of Samples and Live/Dead Staining of Cells
3.4. Animal Pig Model
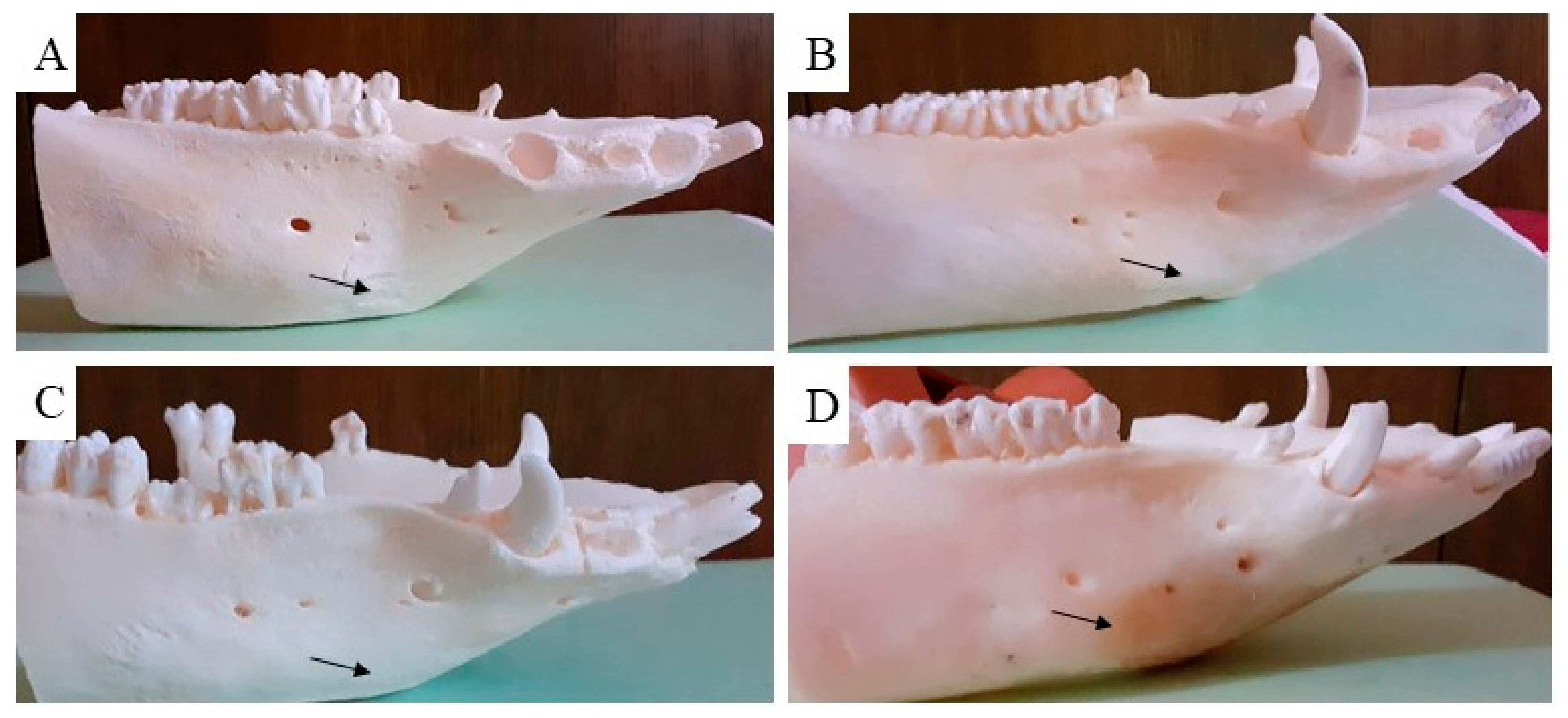
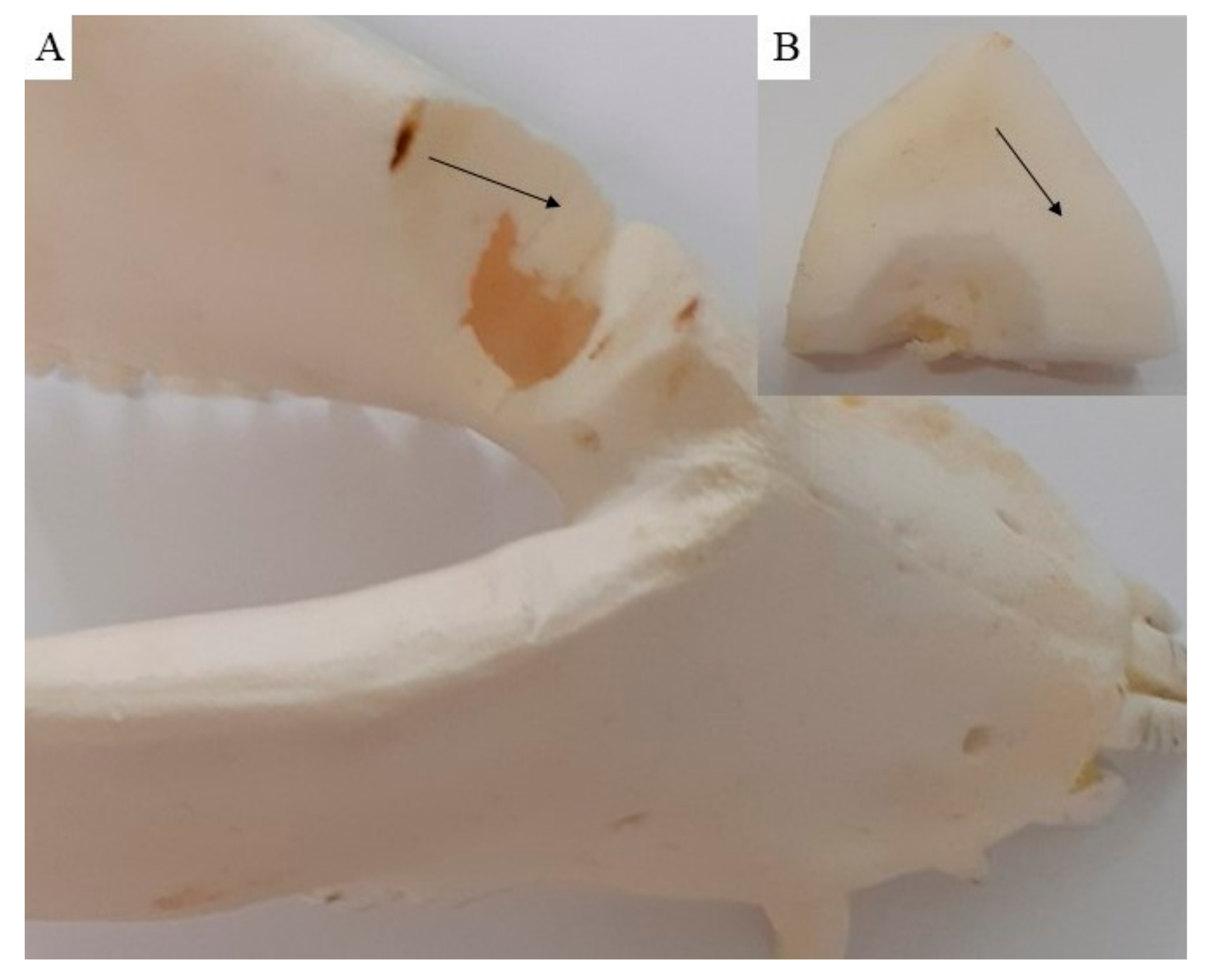
3.5. Macroscopic Assessment
3.6. Histological and Immunohistochemically Assessment
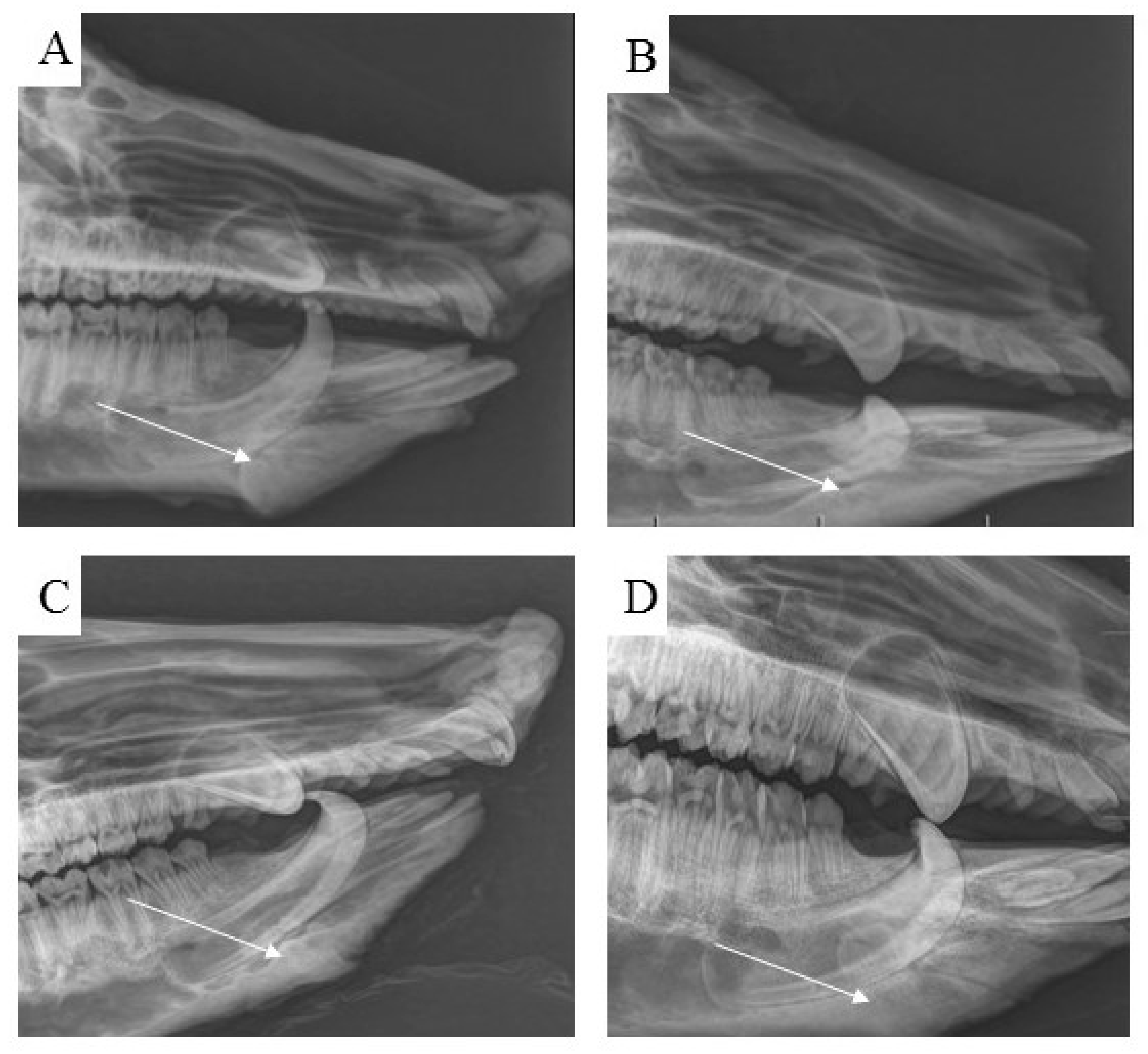
3.7. Radiological Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular Reconstruction: Overview. J. Maxillofac. Oral Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Tuna, E.B.; Ozgen, M.; Cankaya, A.B.; Sen, C.; Gencay, K. Oral Rehabilitation in a Patient with Major Maxillofacial Trauma: A Case management. Case Rep. Dent. 2012, 2012, 267143. [Google Scholar] [CrossRef]
- Passi, D.; Ram, H.; Singh, G.; Malkunje, L. Total avulsion of mandible in maxillofacial trauma. Ann. Maxillofac. Surg. 2014, 4, 115–118. [Google Scholar] [CrossRef]
- Rana, M.; Warraich, R.; Kokemüller, H.; Lemound, J.; Essig, H.; Tavassol, F.; Eckardt, A.; Gellrich, N.C. Reconstruction of mandibular defects-clinical retrospective research over a 10-year period. Head Neck Oncol. 2011, 3, 23. [Google Scholar] [CrossRef]
- Jewer, D.D.; Boyd, J.B.; Manktelow, R.T.; Zuker, R.M.; Rosen, J.B.; Gullane, P.J.; Rotstein, L.D.; Freeman, J.E. Orofacial and mandibular reconstruction with the iliac crest free flap: A review of 60 cases and a new method of classification. Plast. Reconstr. Surg. 1989, 84, 391–405. [Google Scholar] [CrossRef]
- Adelusi, E.A. Classification of Mandibulectomy/Mandibular Defects. J. Oral Maxillofac. Surg. 2019, 2, 1–7. [Google Scholar]
- Tafti, A.K.; Shaterzadeh-Yazdi, M.; Zamiri, B.; Omidi, M. Tissue Engineering in Maxillary Bone Defects. World J. Plast. Surg. 2018, 7, 3–11. [Google Scholar]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2012, 6, 2467–2478. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 15, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Kelm, R.C.; Surmanowicz, P.; Lung, K.E.; Davis, C.M. New technique for removing bone covering miniplates and screws. Br. J. Oral Maxillofac. Surg. 2020, 58, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene Is the New Graphene in Biomedical Applications. Materials 2019, 12, 2301. [Google Scholar] [CrossRef]
- Brierly, G.I.; Tredinnick, S.; Lynham, A.; Woodruff, M.A. Critical Sized Mandibular Defect Regeneration in Preclinical In Vivo Models. Curr. Mol. Biol. Rep. 2016, 2, 83–89. [Google Scholar] [CrossRef]
- Markwardt, J.; Sembdner, P.; Lesche, R.; Jung, R.; Spekl, K.; Mai, R.; Schulz, M.C.; Reitermeier, B. Experimental findings on customized mandibular implants in Göttingen mini pigs A pilot study. Int. J. Sur. 2014, 12, 60–66. [Google Scholar] [CrossRef]
- Mai, R.; Reinstorf, A.; Pilling, E.; Hlawitschka, M.; Jung, R.; Gelinsky, M.; Schneider, M.; Loukola, R.; Pompe, W.; Eckelt, U.; et al. Histilogical study of incorporation and resorption of a bone cement-collagen composite: An in vivo study in the minipig. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 9–14. [Google Scholar] [CrossRef]
- Furko, M.; Balázsi, C. Calcium Phosphate Based Bioactive Ceramic Layers on Implant Materials Preparation, Properties, and Biological Performance. Coatings 2020, 10, 823. [Google Scholar] [CrossRef]
- Medvecký, Ľ.; Kašiarová, M.; Bruncková, H.; Mihalik, J. Statistical evaluation of apatite-like precursor preparation methods from the viewpoint of porous calcium phosphate ceramics. Powder Metall. Prog. 2010, 10, 111–118. [Google Scholar]
- Medvecký, Ľ.; Giretová, M.; Štulajterová, R. Chemical modification of hydroxyapatite ceramic surface by calcium phosphate coatings and invitro osteoblast response. Powder Metall. Prog. 2012, 4, 224–233. [Google Scholar]
- Sun, Z.; Kennedy, K.S.; Tee, B.C.; Damron, J.B.; Allen, M.J. Establishing a critical-size mandibular defect model in growing pigs: Characterization of spontaneous healing. J. Oral Maxillofac. Surg. 2014, 72, 1852–1868. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schütz, M.A.; Duda, G.N.; Schell, H.; Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomater 2019, 30, 2149–2163. [Google Scholar] [CrossRef]
- Bonucci, E.; Ballanti, P. Osteoporosis—Bone remodeling and animal models. Toxicol. Pathol. 2014, 42, 957–969. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Ruehe, B.; Niehues, S.; Heberer, S.; Nelson, K. Miniature pigs as an animal model for implant research: Bone regeneration in critical-size defects. Oral Maxillofac. Implant. 2009, 108, 699–706. [Google Scholar] [CrossRef]
- Stevanovic, M.; Selakovic, D.; Vasovic, M.; Ljujic, B.; Zivanovic, S.; Papic, M.; Zivanovic, M.; Milivojevic, N.; Miovic, M.; Tabakovic, S.Z.; et al. Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study. Molecules 2022, 27, 1694. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.h.; Lin, S.C.; Chiang, C.C.; Chang, M.C.; Lee, O.K.S. Reconstruction of Bone Defect Combined with Massive Loss of Periosteum Using Injectable Human Mesenchymal Stem Cells in Biocompatible Ceramic Scaffolds in a Porcine Animal Model. Stem Cell Int. 2019, 23, 6832952. [Google Scholar] [CrossRef]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies differences in bone composition, density, and quality: Potential implications for in vivo bone research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Abukawa, H.; Zhang, W.; Young, C.S.; Asrican, R.; Vacanti, J.P.; Kaban, L.K.; Troulis, M.J.; Yelick, P.C. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J. Oral Maxillofac. Surg. 2009, 67, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Shusterman, E.M.; Troulis, M.; Holmes, R.; Perrott, D.; Kaban, L.B. Distraction osteogenesis of the porcine mandible: Histomorphometric evaluation of bone. Plast. Reconstr. Surg. 2004, 113, 566–573. [Google Scholar] [CrossRef]
- Adams, C.S.; Mansfield, K.; Perlot, R.L.; Shapiro, I.M. Tissue Engineering Approach for Reconstructing Bone Defects Using Mesenchymal Stem Cells (Review). J. Biol. Chem. 2001, 276, 20316–20322. [Google Scholar] [CrossRef]
- Medvecký, Ľ.; Giretová, M.; Štulajterová, R. In-vitro characterization of surface treated hydroxyapatite ceramic substrates. In Proceedings of the 13th Conference of the European Ceramic Society, Limoges, France, 23–27 June 2013; pp. 120–121. [Google Scholar]
- Keller, J.C.; Collins, J.G.; Niederauer, G.G.; McGee, T.D. In vitro attachment of osteoblast-like cells to osteoceramic materials. Dent. Mater. 1997, 13, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Murison, P.J.; Jones, A.; Mitchard, L.; Burt, R.; Bircha, M.A. Development of perioperative care for pigs undergoing laryngeal transplantation: A case series. Lab. Anim. 2009, 43, 338–343. [Google Scholar] [CrossRef]
- Lüthje, F.L.; Skovgaard, K.; Jensen, H.E.; Jensen, L.K. Pigs are useful for the molecular study of bone inflammation and regeneration in humans. Lab. Anim. 2018, 52, 630–640. [Google Scholar] [CrossRef]
- Kim, T.; Wang See, C.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Tatullo, M.; Zavan, B.; Genovese, F.; Codispoti, B.; Makeeva, I.; Rengo, S.; Fortunato, L.; Spagnuolo, G. Borophene Is a Promising 2D Allotropic Material for Biomedical Devices. Appl. Sci. 2019, 9, 3446. [Google Scholar] [CrossRef]
- Huawei, Q.; Hongya, F.; Zhenyu, H.; Yang, S. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar]
- Kong, Y.O.; Kim, H.E.; Kim, H.W. Phase Conversion of Tricalcium Phosphate Into Ca-Deficient Apatite During Sintering of Hydroxyapatite–Tricalcium Phosphate Biphasic Ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Falisi, G.; Apicella, A.; Apicella, D.; Amantea, M.; Cielo, A.; Bonanome, L.; Palmieri, F.; Santacroce, L.; Giannini, S.; et al. Behaviour of dental pulp stem cells on different types of innovative mesoporous and nanoporous silicon scaffolds with different functionalizations of the surfaces. J. Biol. Regul. Homeost. Agents 2015, 29, 991–997. [Google Scholar] [PubMed]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. Nanometric Bio-Banked MSC-Derived Exosome (nanobiome) as a Novel Approach to Regenerative Medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphatepoly(D,L-lactide-co-glycolide) scafolds. Sci. Rep. 2020, 10, 2062. [Google Scholar] [CrossRef]
- Van Oirschot, B.A.J.A.J.; Geven, E.J.V.; Mikos, A.G.; Van den Beucken, J.J.J.P.; Jansen, J.A. A Mini-Pig Mandibular Defect Model for Evaluation of Craniomaxillofacial Bone Regeneration. Special issue: In vivo models for bone regeneration. Tissue Eng. Part C 2022, 28, 193–201. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Phamracol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wowern, N.; Westergaard, J.; Kollerup, G. Bone mineral content and bone metabolism in young adults with severe periodontitis. J. Clin. Periodontol. 2001, 28, 583–588. [Google Scholar] [CrossRef] [PubMed]









| Biocompatibility | Non-Toxic |
|---|---|
| Bioactivity | Biomaterial supported neo bone formation |
| Osteoinduction | Scaffold supported migration and proliferation of the mesenchymal stem cells |
| Osteoconduction | Biomaterial conducted the new bone formation |
| Biodegradation | Scaffold degradation |
| Bioresorption | Mandible defect was filled by the new regenerated bone |
| Mechanical resistance | Similar elastic and compressive strength to host bone |
| Porosity | Scaffold structure allowed neovascularization and growth of the stem cells |
| Animals | Weight of the Animals (kg) | Material | Place of Defect | Size of Defect (cm) | Euthanasia of Animals after (Months) |
|---|---|---|---|---|---|
| Swine 1 | 258.9 kg | HAP ceramic plate | Cortical bone of the mandible body at the level of the P1–P2 | 1.6 × 0.8 × 0.4 | 3 |
| Swine 2 | 262.4 kg | HAP ceramic plate | Cortical bone of the mandible body at the level of the P1–P2 | 1.6 × 0.8 × 0.4 | 4 |
| Swine 3 | 257.2 kg | HAP ceramic plate | Cortical bone of the mandible body at the level of the P1–P2 | 1.6 × 0.8 × 0.4 | 5 |
| Swine 4 | 263.8 kg | HAP ceramic plate | Cortical bone of the mandible body at the level of the P1–P2 | 1.6 × 0.8 × 0.4 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vdoviaková, K.; Jenca, A.; Jenca, A., Jr.; Danko, J.; Kresáková, L.; Simaiová, V.; Reichel, P.; Rusnák, P.; Pribula, J.; Vrzgula, M.; et al. Regenerative Potential of Hydroxyapatite-Based Ceramic Biomaterial on Mandibular Cortical Bone: An In Vivo Study. Biomedicines 2023, 11, 877. https://doi.org/10.3390/biomedicines11030877
Vdoviaková K, Jenca A, Jenca A Jr., Danko J, Kresáková L, Simaiová V, Reichel P, Rusnák P, Pribula J, Vrzgula M, et al. Regenerative Potential of Hydroxyapatite-Based Ceramic Biomaterial on Mandibular Cortical Bone: An In Vivo Study. Biomedicines. 2023; 11(3):877. https://doi.org/10.3390/biomedicines11030877
Chicago/Turabian StyleVdoviaková, Katarína, Andrej Jenca, Andrej Jenca, Jr., Ján Danko, Lenka Kresáková, Veronika Simaiová, Peter Reichel, Pavol Rusnák, Jozef Pribula, Marko Vrzgula, and et al. 2023. "Regenerative Potential of Hydroxyapatite-Based Ceramic Biomaterial on Mandibular Cortical Bone: An In Vivo Study" Biomedicines 11, no. 3: 877. https://doi.org/10.3390/biomedicines11030877
APA StyleVdoviaková, K., Jenca, A., Jenca, A., Jr., Danko, J., Kresáková, L., Simaiová, V., Reichel, P., Rusnák, P., Pribula, J., Vrzgula, M., Askin, S. J., Giretová, M., Briancin, J., & Medvecký, L. (2023). Regenerative Potential of Hydroxyapatite-Based Ceramic Biomaterial on Mandibular Cortical Bone: An In Vivo Study. Biomedicines, 11(3), 877. https://doi.org/10.3390/biomedicines11030877







