Age-Related Modifications of Electroencephalogram Coherence in Mice Models of Alzheimer’s Disease and Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implantation of Electrodes
2.2. EEG Recording
2.3. EEG Coherence Computation
2.4. Statistics
3. Results
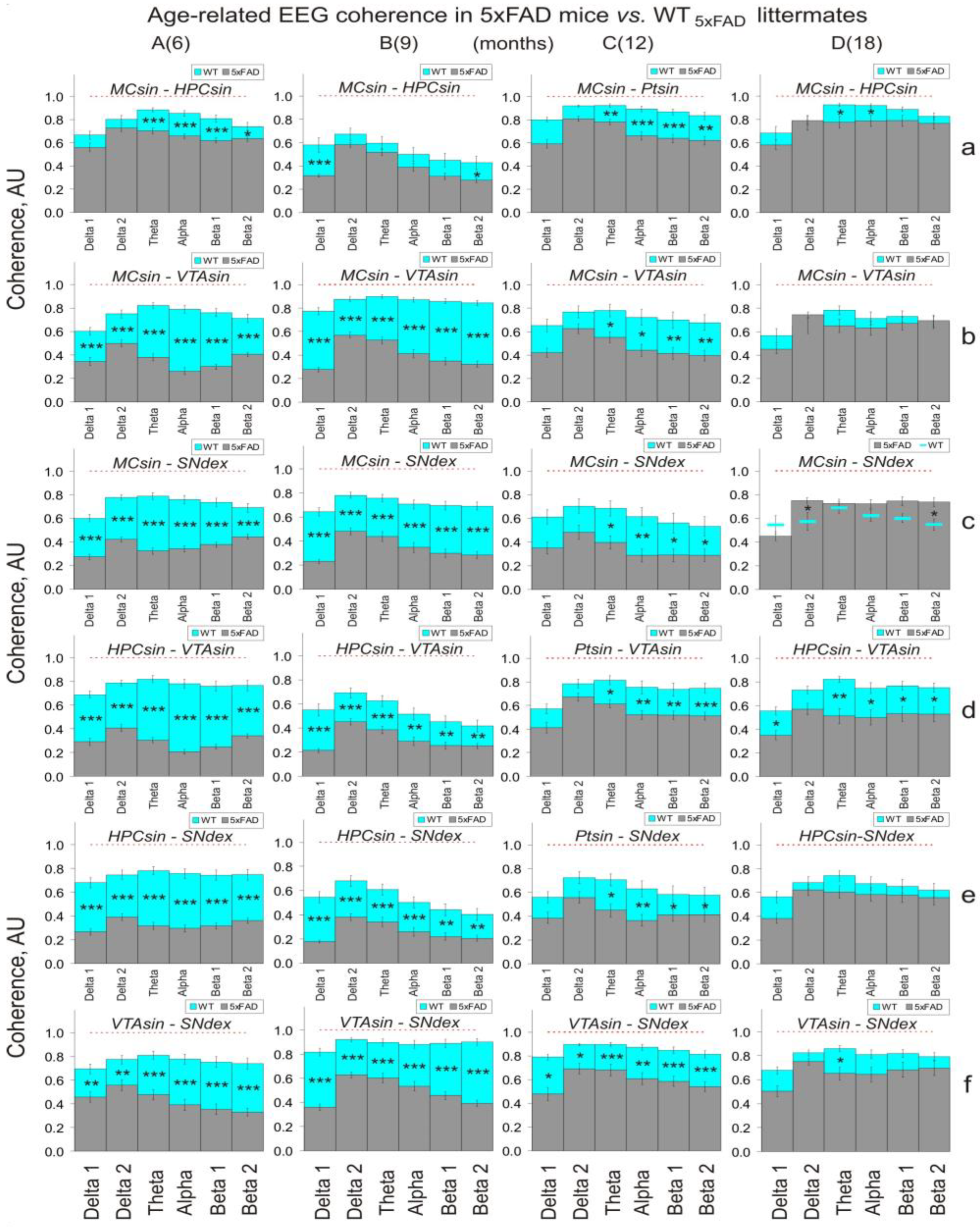
3.1. EEG Coherence in 5xFAD Mice of Different Ages
3.2. EEG Coherence in FUS Mice of Two- and Five-Month Ages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
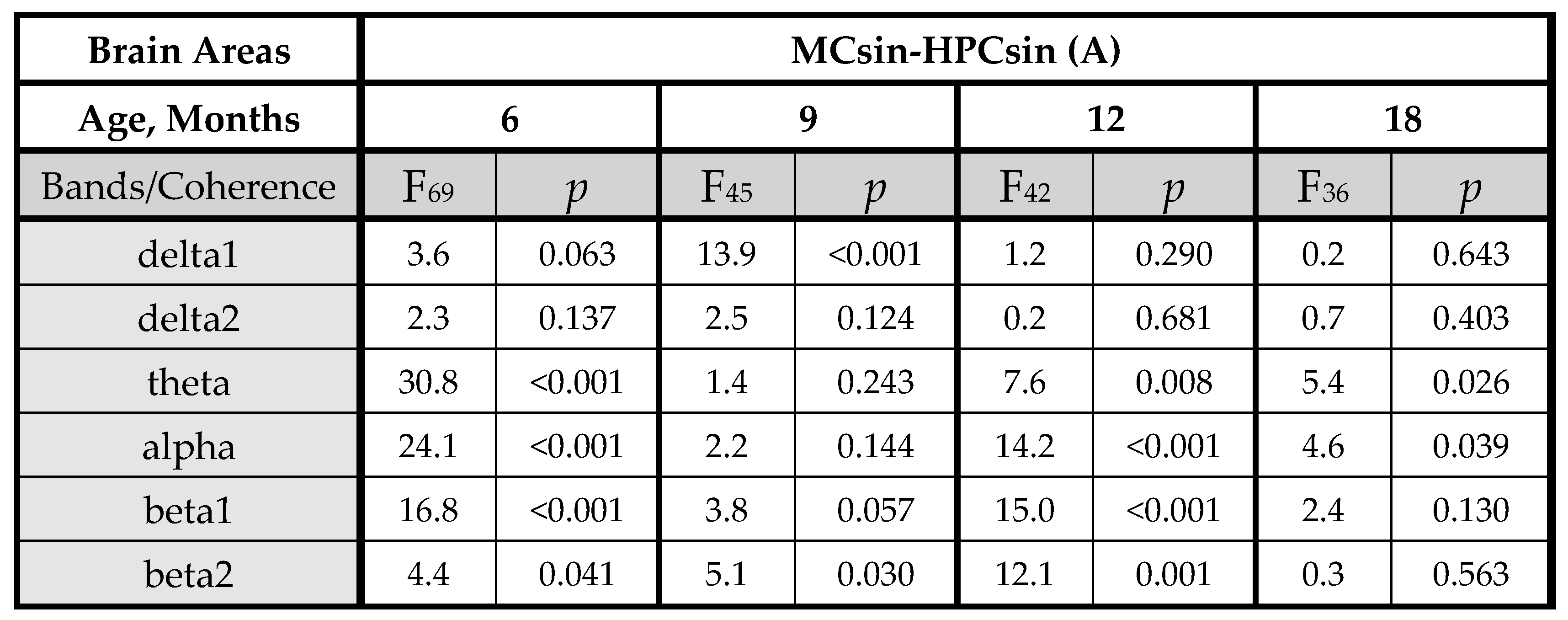
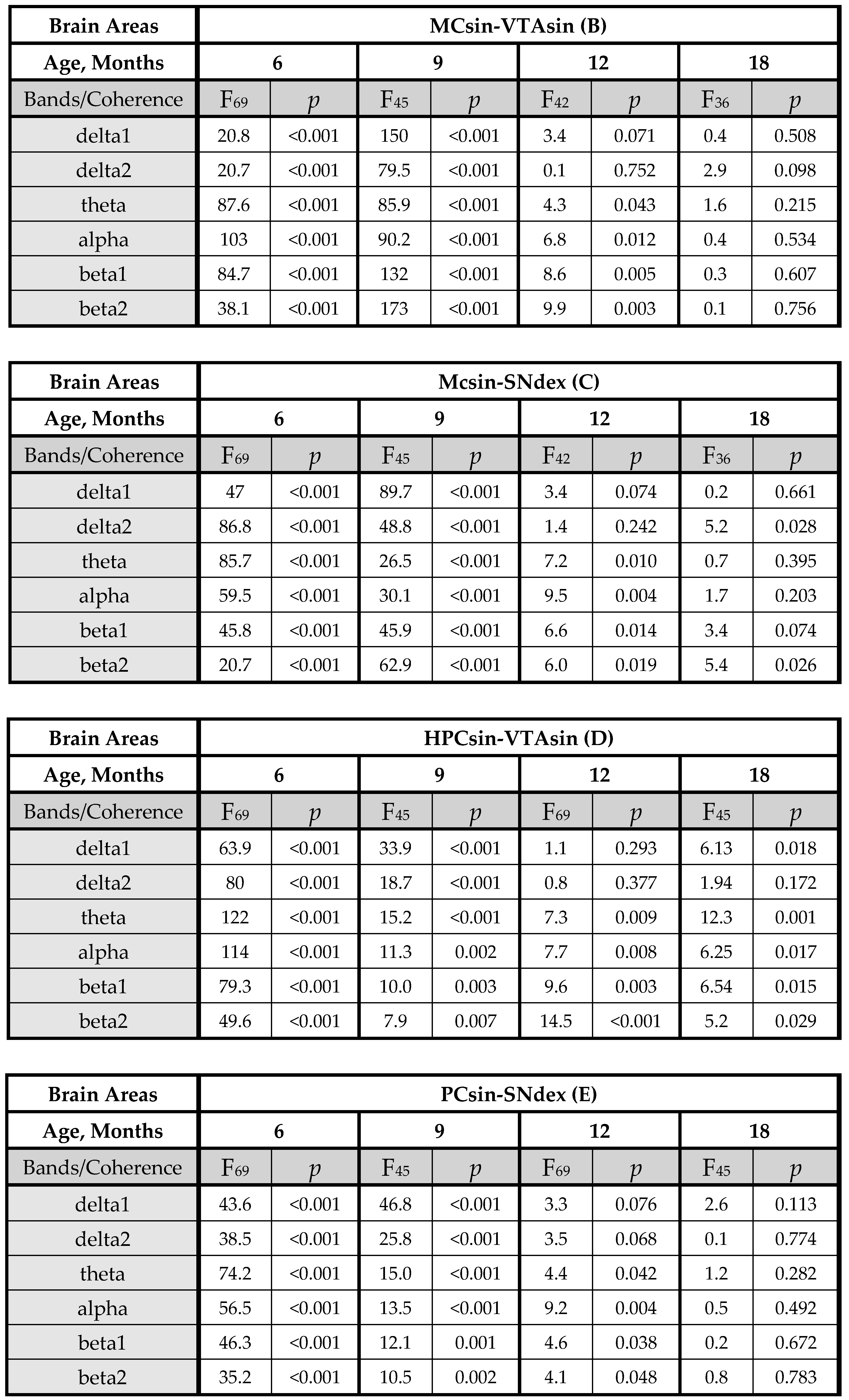
Appendix A



References
- Peng, S.; Zeng, L.; Haure-Mirande, J.V.; Wang, M.; Huffman, D.M.; Haroutunian, V.; Ehrlich, M.E.; Zhang, B.; Tu, Z. Transcriptomic Changes Highly Similar to Alzheimer’s Disease Are Observed in a Subpopulation of Individuals during Normal Brain Aging. Front. Aging Neurosci. 2021, 13, 711524. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, B.; Yao, H.; Zhan, Y.; Zhang, Z.; Cui, Y.; Xu, K.; Ma, J.; Wang, L.; An, N.; et al. Aberrant intra- and inter-network connectivity architectures in Alzheimer’s disease and mild cognitive impairment. Sci. Rep. 2015, 5, 14824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Gao, Y.; Potter, T.; Benoit, J.; Shen, J.; Schulz, P.E.; Zhang, Y.; The Alzheimer’s Disease Neuroimaging Initiative. Interactions between Aging and Alzheimer’s Disease on Structural Brain Networks. Front. Aging Neurosci. 2021, 13, 639795. [Google Scholar] [CrossRef]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Percio, C.; Dubois, B.; Escudero, J.; Fernández, A.; Frisoni, G.; et al. What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiol. Aging 2020, 85, 58–73. [Google Scholar] [CrossRef]
- Watanabe, H.; Bagarinao, E.; Maesawa, S.; Hara, K.; Kawabata, K.; Ogura, A.; Ohdake, R.; Shima, S.; Mizutani, Y.; Ueda, A.; et al. Characteristics of Neural Network Changes in Normal Aging and Early Dementia. Front. Aging Neurosci. 2021, 13, 747359. [Google Scholar] [CrossRef]
- Aron, L.; Zullo, J.; Yankner, B.A. The adaptive aging brain. Curr. Opin. Neurobiol. 2021, 72, 91–100. [Google Scholar] [CrossRef]
- Tsai, L.H.; Madabhushi, R. A protective factor for the ageing brain. Nature 2014, 507, 439–440. [Google Scholar] [CrossRef]
- Nimmrich, V.; Draguhn, A.; Axmacher, N. Neuronal Network Oscillations in Neurodegenerative Diseases. Neuromol. Med. 2015, 17, 270–284. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef]
- Vorobyov, V.; Bakharev, B.; Medvinskaya, N.; Nesterova, I.; Samokhin, A.; Deev, A.; Tatarnikova, O.; Ustyugov, A.A.; Sengpiel, F.; Bobkova, N. Loss of Midbrain Dopamine Neurons and Altered Apomorphine EEG Effects in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 70, 241–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorobyov, V.; Deev, A.; Oganesyan, Z.; Sengpiel, F.; Ustyugov, A.A. Baseline Electroencephalogram and Its Evolution after Activation of Dopaminergic System by Apomorphine in Middle-Aged 5XFAD Transgenic Mice, a Model of Alzheimer’s Disease. Dynamics 2022, 2, 356–366. [Google Scholar] [CrossRef]
- Vorobyov, V.; Deev, A.; Sengpiel, F.; Nebogatikov, V.; Ustyugov, A.A. Cortical and Striatal Electroencephalograms and Apomorphine Effects in the FUS Mouse Model of Amyotrophic Lateral Sclerosis. J. Alzheimer’s Dis. 2021, 81, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Cook, I.A.; Leuchter, A.F. Synaptic dysfunction in Alzheimer’s disease: Clinical assessment using quantitative EEG. Behav. Brain Res. 1996, 78, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sankari, Z.; Adeli, H.; Adeli, A. Intrahemispheric, interhemispheric, and distal EEG coherence in Alzheimer’s disease. Clin. Neurophysiol. 2011, 122, 897–906. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Yu, H.; Wei, X.; Yang, C.; Deng, B. Power spectral density and coherence analysis of Alzheimer’s EEG. Cogn. Neurodyn. 2015, 9, 291–304. [Google Scholar] [CrossRef]
- Abazid, M.; Houmani, N.; Boudy, J.; Dorizzi, B.; Mariani, J.; Kinugawa, K. Comparative Study of Functional Connectivity Measures for Brain Network Analysis in the Context of AD Detection with EEG. Entropy 2021, 23, 1553. [Google Scholar] [CrossRef]
- Brunet, A.; Stuart-Lopez, G.; Burg, T.; Scekic-Zahirovic, J.; Rouaux, C. Cortical Circuit Dysfunction as a Potential Driver of Amyotrophic Lateral Sclerosis. Front. Neurosci. 2020, 14, 363. [Google Scholar] [CrossRef]
- Kim, J.; Hughes, E.G.; Shetty, A.S.; Arlotta, P.; Goff, L.A.; Bergles, D.E.; Brown, S.P. Changes in the Excitability of Neocortical Neurons in a Mouse Model of Amyotrophic Lateral Sclerosis Are Not Specific to Corticospinal Neurons and Are Modulated by Advancing Disease. J. Neurosci. 2017, 37, 9037–9053. [Google Scholar] [CrossRef] [Green Version]
- Abidi, M.; de Marco, G.; Couillandre, A.; Feron, M.; Mseddi, E.; Termoz, N.; Querin, G.; Pradat, P.F.; Bede, P. Adaptive functional reorganization in amyotrophic lateral sclerosis: Coexisting degenerative and compensatory changes. Eur. J. Neurol. 2020, 27, 121–128. [Google Scholar] [CrossRef]
- Bede, P.; Bogdahn, U.; Lope, J.; Chang, K.M.; Xirou, S.; Christidi, F. Degenerative and regenerative processes in amyotrophic lateral sclerosis: Motor reserve, adaptation and putative compensatory changes. Neural Regen. Res. 2021, 16, 1208–1209. [Google Scholar] [CrossRef] [PubMed]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, S.; Sato, S.; Fukuda, T.; Tada, N.; Hattori, N. Aging-related motor function and dopaminergic neuronal loss in C57BL/6 mice. Mol. Brain 2020, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Karandreas, N.; Papadopoulou, M.; Kokotis, P.; Papapostolou, A.; Tsivgoulis, G.; Zambelis, T. Impaired interhemispheric inhibition in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2007, 8, 112–118. [Google Scholar] [CrossRef]
- Greene, D.J.; Laumann, T.O.; Dubis, J.W.; Ihnen, S.K.; Neta, M.; Power, J.D.; Pruett, J.R., Jr.; Black, K.J.; Schlaggar, B.L. Developmental changes in the organization of functional connections between the basal ganglia and cerebral cortex. J. Neurosci. 2014, 34, 5842–5854. [Google Scholar] [CrossRef] [Green Version]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Hogan, M.J.; Swanwick, G.R.; Kaiser, J.; Rowan, M.; Lawlor, B. Memory-related EEG power and coherence reductions in mild Alzheimer’s disease. Int. J. Psychophysiol. 2003, 49, 147–163. [Google Scholar] [CrossRef]
- Stevens, A.; Kircher, T.; Nickola, M.; Bartels, M.; Rosellen, N.; Wormstall, H. Dynamic regulation of EEG power and coherence is lost early and globally in probable DAT. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 199–204. [Google Scholar] [CrossRef]
- Ahnaou, A.; Moechars, D.; Raeymaekers, L.; Biermans, R.; Manyakov, N.V.; Bottelbergs, A.; Wintmolders, C.; Van Kolen, K.; Van De Casteele, T.; Kemp, J.A.; et al. Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci. Rep. 2017, 7, 14189. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, M.J.; Breakspear, M.; Sachdev, P. Complex mental activity and the aging brain: Molecular, cellular and cortical network mechanisms. Brain Res. Rev. 2007, 56, 198–213. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Uhlhaas, P.; Singer, W. Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron 2006, 52, 155–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palop, J.J.; Mucke, L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Womelsdorf, T.; Schoffelen, J.M.; Oostenveld, R.; Singer, W.; Desimone, R.; Engel, A.K.; Fries, P. Modulation of neuronal interactions through neuronal synchronization. Science 2007, 316, 1609–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, T.; Prichep, L.; Dierks, T.; Hubl, D.; Wahlund, L.O.; John, E.R.; Jelic, V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2005, 26, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Aron, L.; Zullo, J.; Pan, Y.; Kim, H.; Chen, Y.; Yang, T.H.; Kim, H.M.; Drake, D.; Liu, X.S.; et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature 2014, 507, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Guo, L.; Lu, L.; Sun, H.; Shao, M.; Beck, S.J.; Li, L.; Ramachandran, J.; Du, Y.; Du, H. Synaptosomal Mitochondrial Dysfunction in 5xFAD Mouse Model of Alzheimer’s Disease. PLoS ONE 2016, 11, e0150441. [Google Scholar] [CrossRef] [Green Version]
- Perier, C.; Bender, A.; García-Arumí, E.; Melià, M.J.; Bové, J.; Laub, C.; Klopstock, T.; Elstner, M.; Mounsey, R.B.; Teismann, P.; et al. Accumulation of mitochondrial DNA deletions within dopaminergic neurons triggers neuroprotective mechanisms. Brain 2013, 136 Pt 8, 2369–2378. [Google Scholar] [CrossRef] [Green Version]
- Rosen, Z.B.; Cheung, S.; Siegelbaum, S.A. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat. Neurosci. 2015, 18, 1763–1771. [Google Scholar] [CrossRef]
- Fertan, E.; Brown, R.E. Age-related deficits in working memory in 5xFAD mice in the Hebb-Williams maze. Behav. Brain Res. 2022, 424, 113806. [Google Scholar] [CrossRef]
- Shaerzadeh, F.; Phan, L.; Miller, D.; Dacquel, M.; Hachmeister, W.; Hansen, C.; Bechtle, A.; Tu, D.; Martcheva, M.; Foster, T.C.; et al. Microglia senescence occurs in both substantia nigra and ventral tegmental area. Glia 2020, 68, 2228–2245. [Google Scholar] [CrossRef]
- Nekrasov, P.V.; Vorobyov, V.V. Dopaminergic mediation in the brain aging and neurodegenerative diseases: A role of senescent cells. Neural Regen. Res. 2018, 13, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Verstraete, E.; de Reus, M.A.; Veldink, J.H.; van den Berg, L.H.; van den Heuvel, M.P. Correlation between structural and functional connectivity impairment in amyotrophic lateral sclerosis. Hum. Brain Mapp. 2014, 35, 4386–4395. [Google Scholar] [CrossRef] [Green Version]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Thonhoff, J.R.; Simpson, E.P.; Appel, S.H. Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr. Opin. Neurol. 2018, 31, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.J.; Restrepo, C.R.; Khan, T.; Dominique, M.A.; Fang, T.C.; Canter, R.G.; Roberts, C.J.; Miller, K.R.; Ransohoff, R.M.; Trojanowski, J.Q.; et al. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat. Neurosci. 2018, 21, 329–340. [Google Scholar] [CrossRef]
- Bendat, J.S.; Piersol, A.G. Random Data; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://web.archive.org/web/20070831220654/http://www.codegear.com/products/cppbuilder (accessed on 8 April 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyov, V.; Deev, A.; Chaprov, K.; Ustyugov, A.A.; Lysikova, E. Age-Related Modifications of Electroencephalogram Coherence in Mice Models of Alzheimer’s Disease and Amyotrophic Lateral Sclerosis. Biomedicines 2023, 11, 1151. https://doi.org/10.3390/biomedicines11041151
Vorobyov V, Deev A, Chaprov K, Ustyugov AA, Lysikova E. Age-Related Modifications of Electroencephalogram Coherence in Mice Models of Alzheimer’s Disease and Amyotrophic Lateral Sclerosis. Biomedicines. 2023; 11(4):1151. https://doi.org/10.3390/biomedicines11041151
Chicago/Turabian StyleVorobyov, Vasily, Alexander Deev, Kirill Chaprov, Aleksey A. Ustyugov, and Ekaterina Lysikova. 2023. "Age-Related Modifications of Electroencephalogram Coherence in Mice Models of Alzheimer’s Disease and Amyotrophic Lateral Sclerosis" Biomedicines 11, no. 4: 1151. https://doi.org/10.3390/biomedicines11041151




