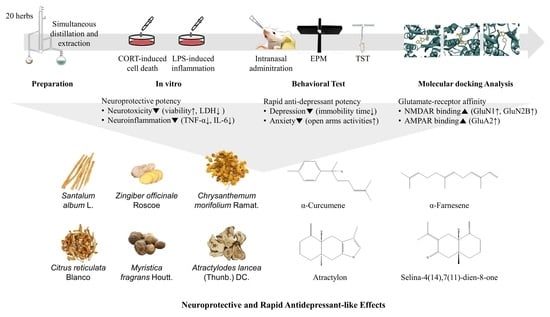
Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Essential Oils
2.2. Animal Experiments and Treatments
2.3. Tail Suspension Test (TST)
2.4. Elevated Plus Maze Test (EPM)
2.5. Cell Culture and Treatments
2.6. Water-Soluble Tetrazolium Salt Assay (WST)
2.7. Lactate Dehydrogenase Assay (LDH)
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results
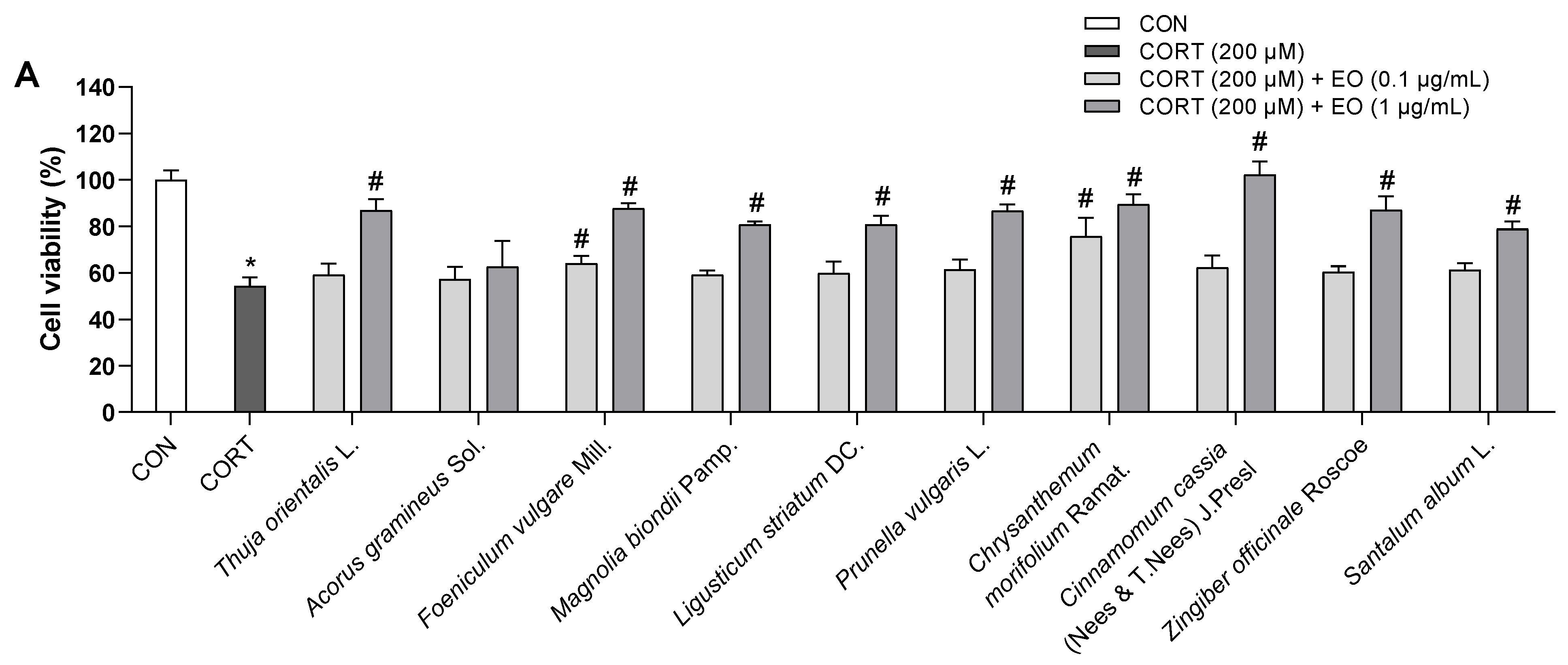
3.1. Effects of Essential Oils on CORT-Induced Neurotoxicity in PC12 Cells
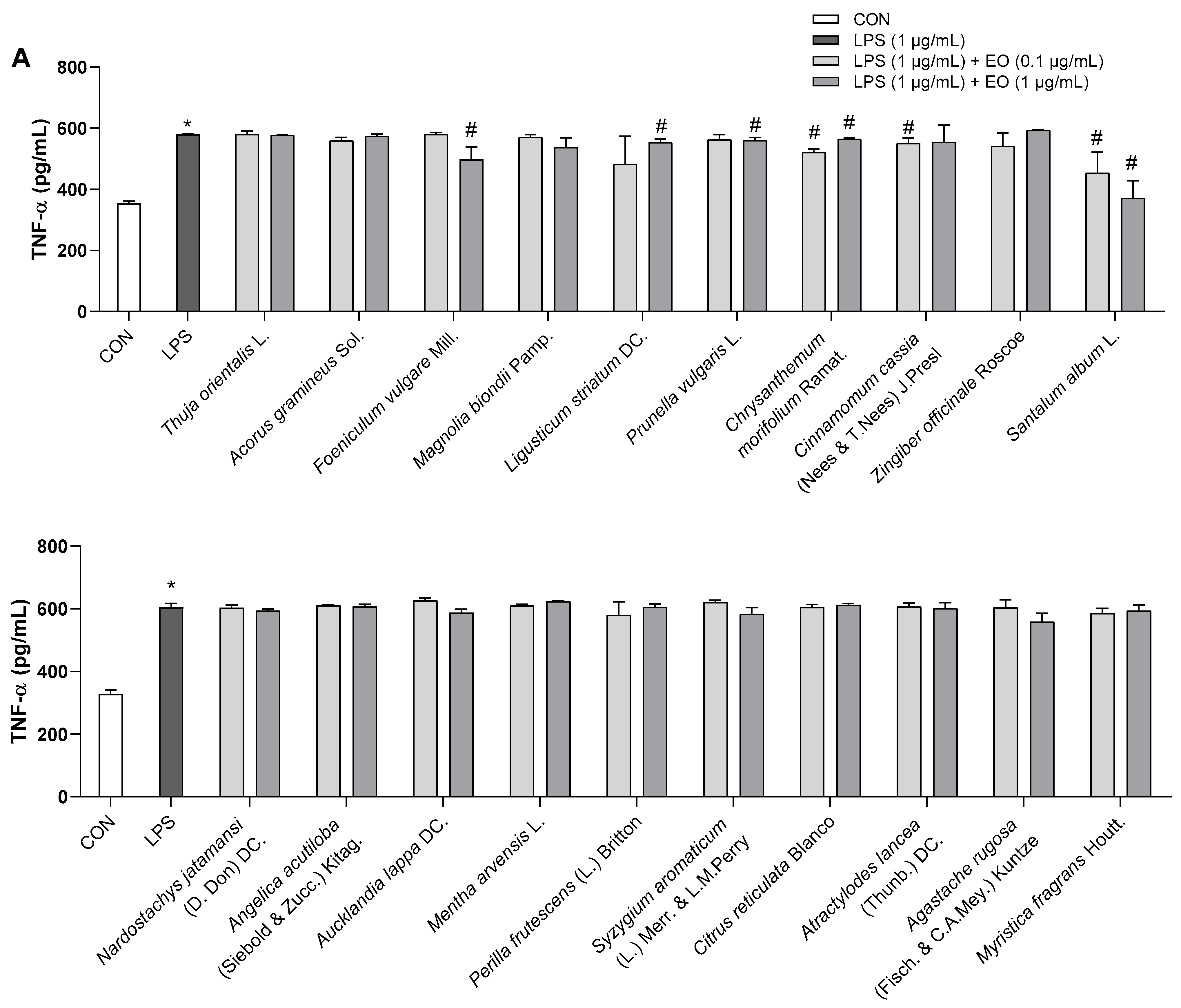
3.2. Effects of Essential Oils on LPS-Induced Neuroinflammation in BV2 Cells
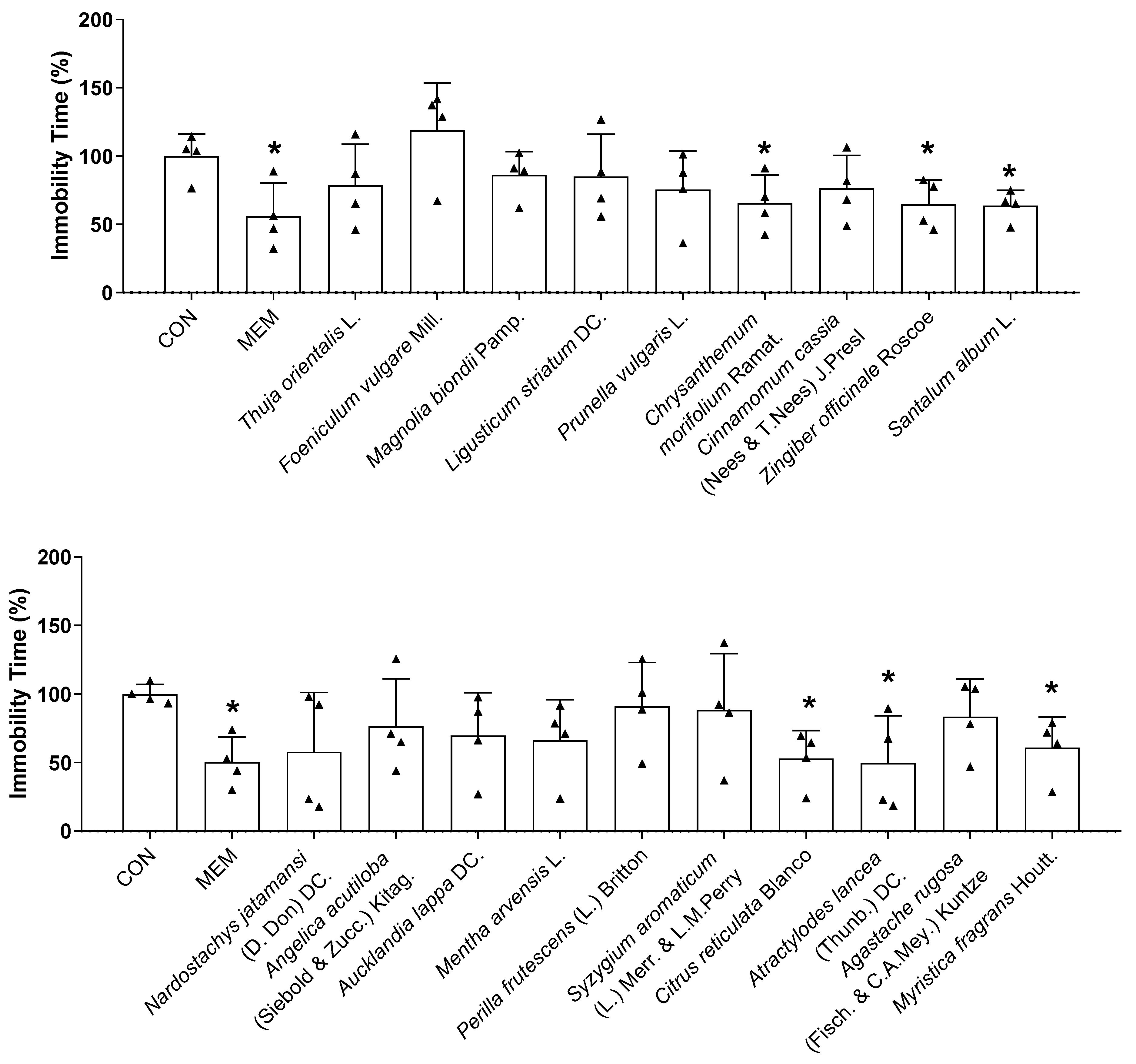
3.3. Rapid-Acting Effect of Essential Oils on Behavior Changes of Mice in the TST and EPM
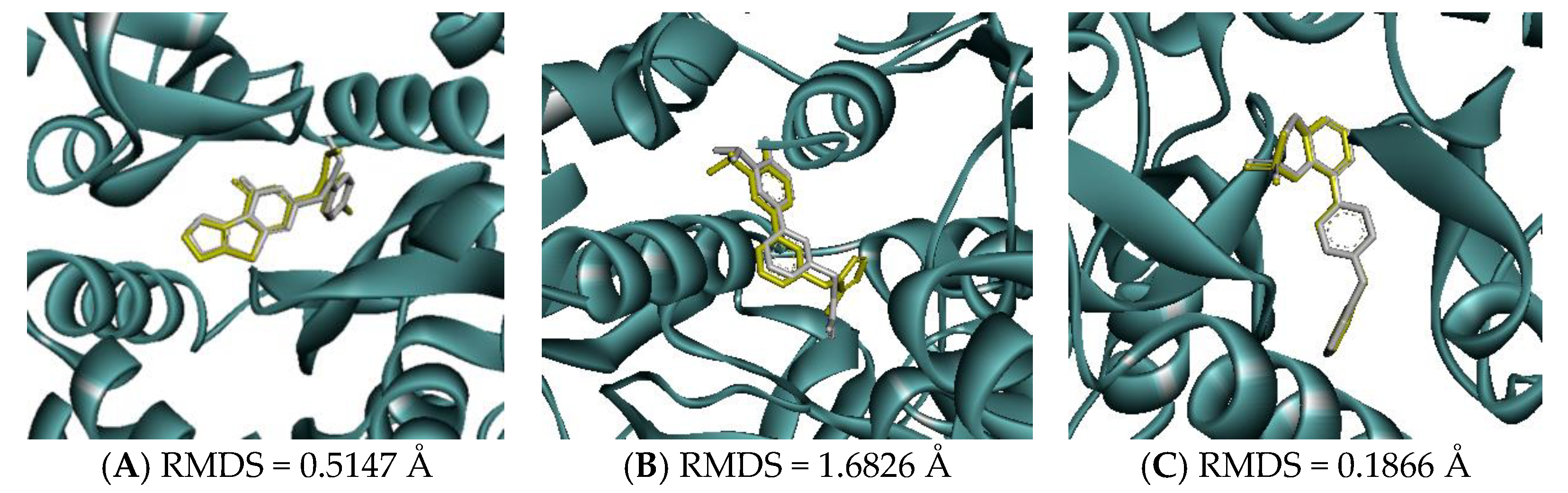
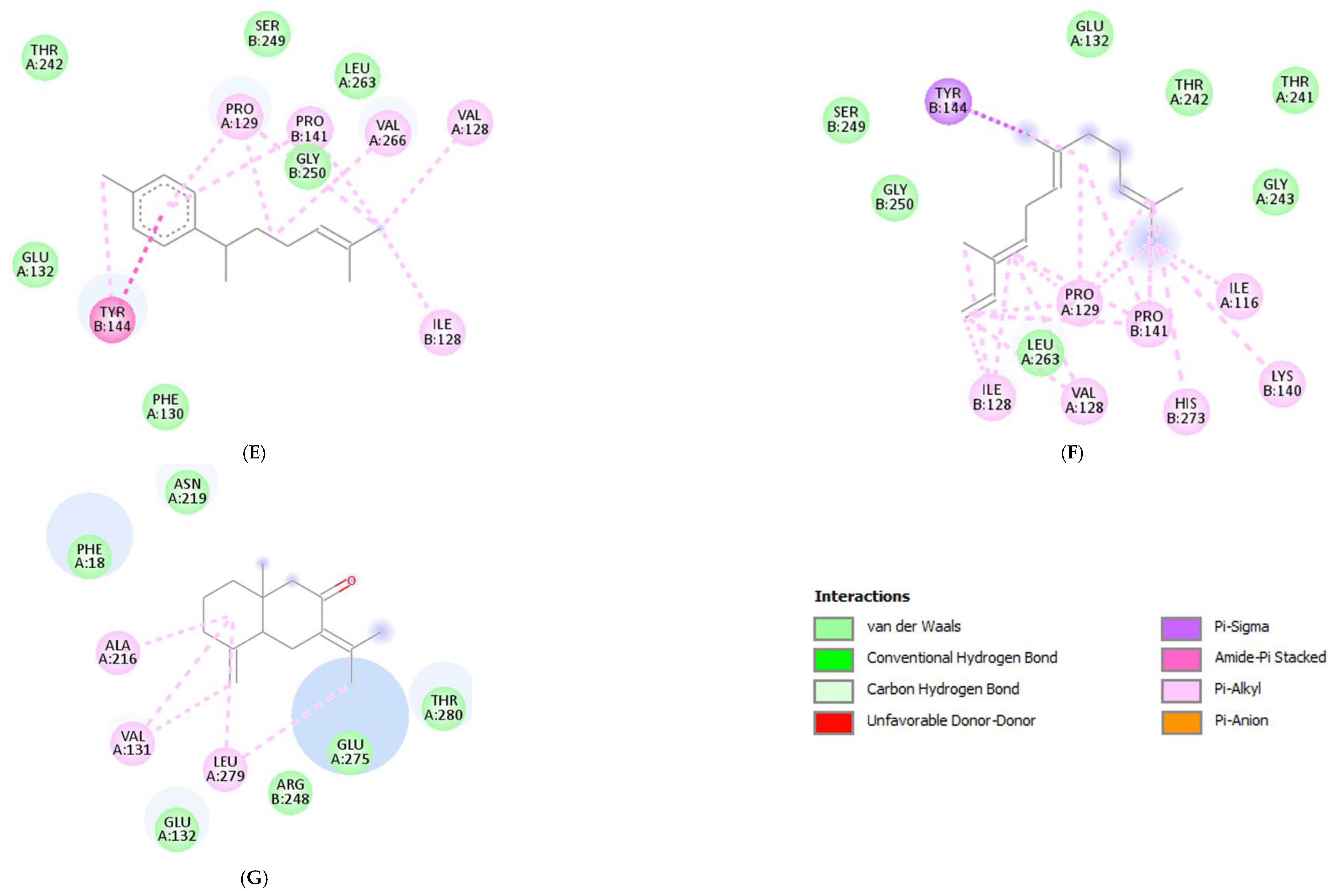
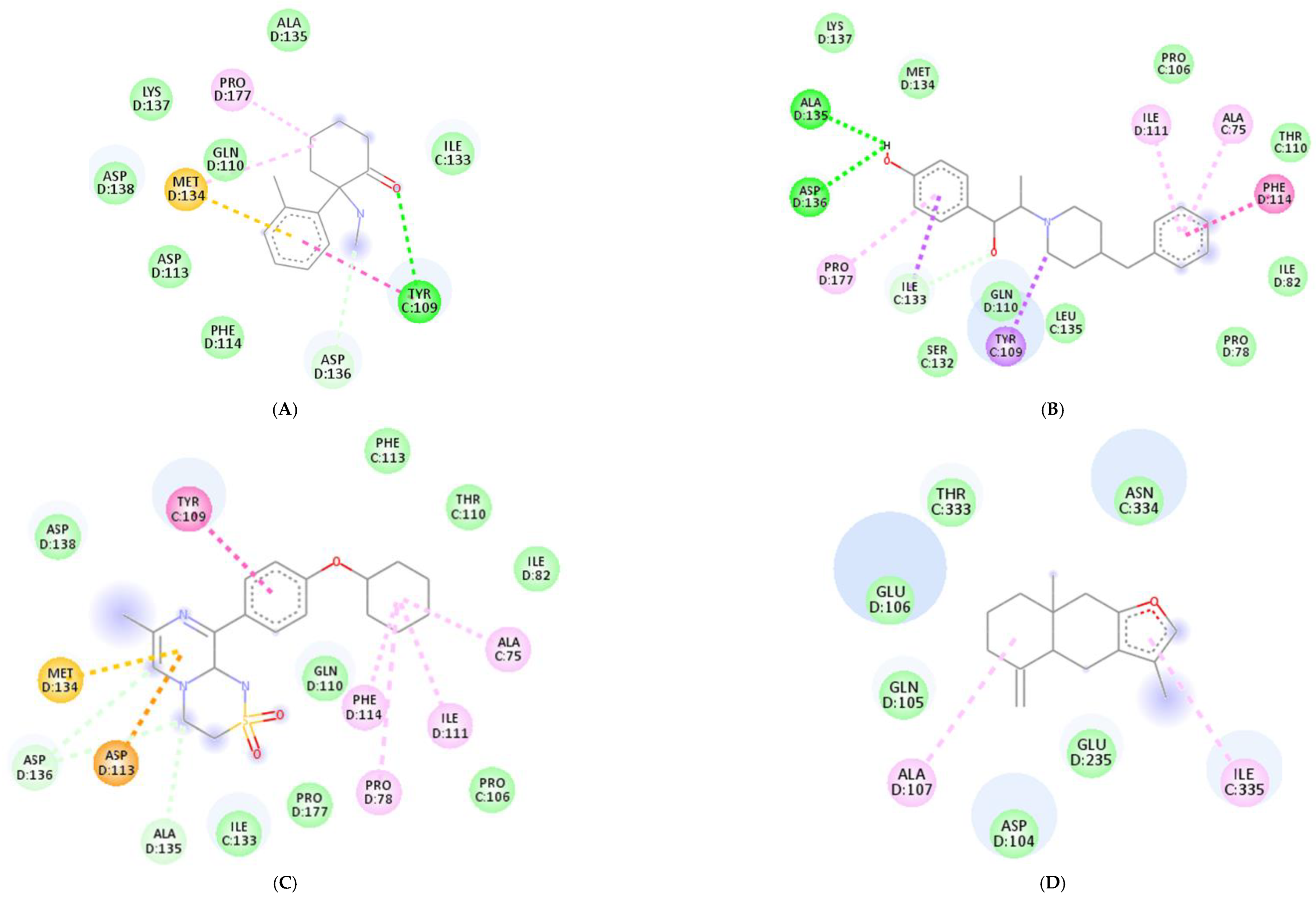
3.4. Molecular Docking Analysis of Essential Oil Main Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chand, S.P.; Arif, H. Depression. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430847/ (accessed on 7 April 2023).
- World Health Organization. Depression; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 7 April 2023).
- Peng, S.; Zhou, Y.; Lu, M.; Wang, Q. Review of Herbal Medicines for the Treatment of Depression. Nat. Prod. Commun. 2022, 17, 1934578X221139082. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; Lv, M.; Xie, M.; Wang, K.; Yang, M.; Lv, C.; Li, X. Anti-depression effect and mechanism of Suanzaoren Decoction on mice with depression. IOP Conf. Ser. Earth Environ. Sci. 2021, 714, 022065. [Google Scholar] [CrossRef]
- Borgogna, N.C.; Aita, S.L. Is the serotonin hypothesis dead? If so, how will clinical psychology respond? Front. Psychol. 2022, 13, 1027375. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Fiksdal, A.; Hanlin, L.; Kuras, Y.; Gianferante, D.; Chen, X.; Thoma, M.V.; Rohleder, N. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology 2019, 102, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Pariante, C.M.; Lightman, S.L. The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 2008, 31, 464–468. [Google Scholar] [CrossRef]
- Bendezú, J.J.; Calhoun, C.D.; Vinograd, M.; Patterson, M.W.; Rudolph, K.D.; Giletta, M.; Hastings, P.; Nock, M.K.; Slavich, G.M.; Prinstein, M.J. Exploring joint HPA-inflammatory stress response profiles in adolescent girls: Implications for developmental models of neuroendocrine dysregulation. Dev. Psychobiol. 2022, 64, e22247. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Ishige, A.; Aburada, M.; Tabira, T. Chronic stress attenuates glucocorticoid negative feedback: Involvement of the prefrontal cortex and hippocampus. Neuroscience 2003, 119, 887–897. [Google Scholar] [CrossRef]
- Seguin, J.A.; Brennan, J.; Mangano, E.; Hayley, S. Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr. Dis. Treat. 2009, 5, 5–14. [Google Scholar] [PubMed]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60 (Suppl. S4), 4–13. [Google Scholar] [PubMed]
- Wang, S.M.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Baldwin, D.S. Monoamine Oxidase Inhibitors (MAOIs) in Psychiatric Practice: How to Use them Safely and Effectively. CNS Drugs 2021, 35, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G.; Linnros, S.E. Delayed Antidepressant Efficacy and the Desensitization Hypothesis. ACS Chem. Neurosci. 2019, 10, 3048–3052. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Salvadore, G.; Luckenbaugh, D.A.; Manji, H.K.; Zarate, C.A., Jr. Rapid onset of antidepressant action: A new paradigm in the research and treatment of major depressive disorder. J. Clin. Psychiatry 2008, 69, 946–958. [Google Scholar] [CrossRef]
- Soares, G.A.B.E.; Bhattacharya, T.; Chakrabarti, T.; Tagde, P.; Cavalu, S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants 2021, 11, 21. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y.; Chi, P.; Liu, H.; Jing, Z.; Cao, H.; Du, Y.; Zhao, Y.; Qin, X.; Zhang, W.; et al. Essential oils: Chemical constituents, potential neuropharmacological effects and aromatherapy—A review. Pharmacol. Res.—Mod. Chin. Med. 2023, 6, 100210. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential Oils. In Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar] [CrossRef]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Yang, Z.-Y.; Fan, G.; Ren, J.-N.; Yin, K.-J.; Pan, S.-Y. Antidepressant-like Effect of Citrus sinensis (L.) Osbeck Essential Oil and Its Main Component Limonene on Mice. J. Agric. Food Chem. 2019, 67, 13817–13828. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vidaña, D.I.; Po, K.K.; Fung, T.K.; Chow, J.K.; Lau, W.K.; So, P.K.; Lau, B.W.; Tsang, H.W. Lavender essential oil ameliorates depression-like behavior and increases neurogenesis and dendritic complexity in rats. Neurosci. Lett. 2019, 701, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Chioca, L.R.; Ferro, M.M.; Baretta, I.P.; Oliveira, S.M.; Silva, C.R.; Ferreira, J.; Losso, E.M.; Andreatini, R. Anxiolytic-like effect of lavender essential oil inhalation in mice: Participation of serotonergic but not GABAA/benzodiazepine neurotransmission. J. Ethnopharmacol. 2013, 147, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Han, Y.; Zhang, D.; Li, Z.; Jing, Y.; Hu, B.; Sun, S. Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics 2022, 14, 2070. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar] [PubMed]
- Qi, X.J.; Liu, X.Y.; Tang, L.M.; Li, P.F.; Qiu, F.; Yang, A.H. Anti-depressant effect of curcumin-loaded guanidine-chitosan thermo-sensitive hydrogel by nasal delivery. Pharm. Dev. Technol. 2020, 25, 316–325. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Li, K.; Gao, L.-N.; Zhang, Y.; Lin, K.-M.; Cui, Y.-L. Intranasal delivery of berberine via in situ thermoresponsive hydrogel with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 2020, 8, 2853–2865. [Google Scholar] [CrossRef]
- Qi, X.-J.; Xu, D.; Tian, M.-L.; Zhou, J.-F.; Wang, Q.-S.; Cui, Y.-L. Thermosensitive hydrogel designed for improving the antidepressant activities of genipin via intranasal delivery. Mater. Des. 2021, 206, 109816. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, H.; Xue, W.; Tao, W.; Chen, C.; Wu, R.; Ren, L.; Tang, J.; Wu, H.; Cai, B.; et al. Instant and Lasting Down-Regulation of NR1 Expression in the Hippocampus is Associated Temporally with Antidepressant Activity after Acute Yueju. Cell. Mol. Neurobiol. 2016, 36, 1189–1196. [Google Scholar] [CrossRef]
- Hall, B.J.; Ripley, B.; Ghosh, A. NR2B signaling regulates the development of synaptic AMPA receptor current. J. Neurosci. 2007, 27, 13446–13456. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ju, P.; Liu, H.; Wu, X.; Niu, Z.; Zhu, Y.; Zhang, C.; Fang, Y. Ifenprodil rapidly ameliorates depressive-like behaviors, activates mTOR signaling and modulates proinflammatory cytokines in the hippocampus of CUMS rats. Psychopharmacology 2020, 237, 1421–1433. [Google Scholar] [CrossRef]
- Li, J.-M.; Liu, L.-L.; Su, W.-J.; Wang, B.; Zhang, T.; Zhang, Y.; Jiang, C.-L. Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology 2019, 146, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Iijima, M.; Chaki, S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav. Brain Res. 2011, 224, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Suzuki, A.; Kunugi, A.; Tajima, Y.; Yamada, R.; Kimura, H. TAK-653, an AMPA receptor potentiator with minimal agonistic activity, produces an antidepressant-like effect with a favorable safety profile in rats. Pharmacol. Biochem. Behav. 2021, 211, 173289. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Hogg, S. A review of the validity and variability of the Elevated Plus-Maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 1996, 54, 21–30. [Google Scholar] [CrossRef]
- Joyce Gem, M.C.; Joanna, J.O.; Junie, B.B. In Sılıco Screenıng for Neuroreceptor Targets and Derıvatızatıon of Alkaloıds from Phaeanthus Ophthalmıcus. Pharmacophore 2022, 13, 27–43. [Google Scholar] [CrossRef]
- Kaniakova, M.; Korabecny, J.; Holubova, K.; Kleteckova, L.; Chvojkova, M.; Hakenova, K.; Prchal, L.; Novak, M.; Dolezal, R.; Hepnarova, V.; et al. 7-phenoxytacrine is a dually acting drug with neuroprotective efficacy in vivo. Biochem. Pharmacol. 2021, 186, 114460. [Google Scholar] [CrossRef]
- Wei, L.; Qi, X.; Yu, X.; Zheng, Y.; Luo, X.; Wei, Y.; Ni, P.; Zhao, L.; Wang, Q.; Ma, X.; et al. 3,4-Dihydrobenzo[e][1,2,3] oxathiazine 2,2-dioxide analogs act as potential AMPA receptor potentiators with antidepressant activity. Eur. J. Med. Chem. 2023, 251, 115252. [Google Scholar] [CrossRef] [PubMed]
- Sweilam, S.H.; Alqarni, M.H.; Youssef, F.S. Antimicrobial Alkaloids from Marine-Derived Fungi as Drug Leads versus COVID-19 Infection: A Computational Approach to Explore their Anti-COVID-19 Activity and ADMET Properties. Evid. Based Complement. Altern. Med. 2022, 2022, 5403757. [Google Scholar] [CrossRef]
- Ramadhan, D.S.F.; Siharis, F.; Abdurrahman, S.; Isrul, M.; Fakih, T.M. In silico analysis of marine natural product from sponge (Clathria Sp.) for their activity as inhibitor of SARS-CoV-2 Main Protease. J. Biomol. Struct. Dyn. 2022, 40, 11526–11532. [Google Scholar] [CrossRef]
- Wu, W. GC-MS analysis of chemical components in essential oil from Flos magnoliae. Zhong Yao Cai 2000, 23, 538–541. [Google Scholar] [PubMed]
- Hu, M.; Bai, M.; Ye, W.; Wang, Y.; Wu, H. Variations in Volatile Oil Yield and Composition of “Xin-yi” (Magnolia biondii Pamp. Flower Buds) at Different Growth Stages. J. Oleo Sci. 2018, 67, 779–787. [Google Scholar] [CrossRef]
- Zeng, Z.; Xie, R.; Zhang, T.; Zhang, H.; Chen, J.Y. Analysis of volatile compositions of Magnolia biondii pamp by steam distillation and headspace solid phase micro-extraction. J. Oleo Sci. 2011, 60, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kuang, C.L.; Lv, D.; Shen, G.H.; Li, S.S.; Luo, Q.Y.; Zhang, Z.Q. Chemical composition and antimicrobial activities of volatile oil extracted from Chrysanthemum morifolium Ramat. J. Food Sci. Technol. 2018, 55, 2786–2794. [Google Scholar] [CrossRef]
- Peng, A.; Lin, L.; Zhao, M. Screening of key flavonoids and monoterpenoids for xanthine oxidase inhibitory activity-oriented quality control of Chrysanthemum morifolium Ramat. ‘Boju’ based on spectrum-effect relationship coupled with UPLC-TOF-MS and HS-SPME-GC/MS. Food Res. Int. 2020, 137, 109448. [Google Scholar] [CrossRef]
- Li, X.; Ao, M.; Zhang, C.; Fan, S.; Chen, Z.; Yu, L. Zingiberis Rhizoma Recens: A Review of Its Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Altern. 2021, 2021, 6668990. [Google Scholar] [CrossRef]
- Yu, D.-X.; Guo, S.; Wang, J.-M.; Yan, H.; Zhang, Z.-Y.; Yang, J.; Duan, J.-A. Comparison of Different Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-GC-MS Coupled with Fast GC E-Nose. Foods 2022, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandotra, P.; Ram, G.; Anand, R.; Gupta, A.P.; Husain, K.; Bedi, Y.S.; Mallavarapu, G.R. Composition of a monoterpenoid-rich essential oil from the rhizome of Zingiber officinale from north western Himalayas. Nat. Prod. Commun. 2011, 6, 93–96. [Google Scholar] [CrossRef]
- de Groot, A.C.; Schmidt, E. Essential Oils, Part VI: Sandalwood Oil, Ylang-Ylang Oil, and Jasmine Absolute. Dermatitis 2017, 28, 14–21. [Google Scholar] [CrossRef]
- Braun, N.A.; Meier, M.; Pickenhagen, W. Isolation and Chiral GC Analysis of β-Bisabolols—Trace Constituents from the Essential Oil of Santalum album L. (Santalaceae). J. Essent. Oil Res. 2003, 15, 63–65. [Google Scholar] [CrossRef]
- Mohankumar, A.; Kalaiselvi, D.; Levenson, C.; Shanmugam, G.; Thiruppathi, G.; Nivitha, S.; Sundararaj, P. Antioxidant and stress modulatory efficacy of essential oil extracted from plantation-grown Santalum album L. Ind. Crop. Prod. 2019, 140, 111623. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Elhady, S.S.; Bannan, D.F.; Malatani, R.T.; Gad, H.A. Citrus reticulata Leaves Essential Oil as an Antiaging Agent: A Comparative Study between Different Cultivars and Correlation with Their Chemical Compositions. Plants 2022, 11, 3335. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Guo, L.; Dou, L.-L.; Zhou, C.-L.; Xu, F.-G.; Zheng, G.-D.; Li, P.; Liu, E.H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ by gas chromatograph-mass spectrometry based metabolomics approach. Food Chem. 2016, 212, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Chutia, M.; Deka Bhuyan, P.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT—Food Sci. Technol. 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Lota, M.-L.; de Rocca Serra, D.; Tomi, F.; Joseph, C. Chemical variability of peel and leaf essential oils of mandarins from Citrus reticulata Blanco. Biochem. Syst. Ecol. 2000, 28, 61–78. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhao, Z.Y.; Chang, L.K.; Cao, Y.; Wang, S.; Kang, C.Z.; Wang, H.Y.; Zhou, L.; Huang, L.Q.; Guo, L.P. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 2021, 266, 113415. [Google Scholar] [CrossRef]
- Xu, R.; Lu, J.; Wu, J.; Yu, D.; Chu, S.; Guan, F.; Liu, W.; Hu, J.; Peng, H.; Zha, L. Comparative analysis in different organs and tissue-specific metabolite profiling of Atractylodes lancea from four regions by GC-MS and laser microdissection. J. Sep. Sci. 2022, 45, 1067–1079. [Google Scholar] [CrossRef]
- Lei, H.; Yue, J.; Yin, X.Y.; Fan, W.; Tan, S.H.; Qin, L.; Zhao, Y.N.; Bai, J.H. HS-SPME coupled with GC-MS for elucidating differences between the volatile components in wild and cultivated Atractylodes chinensis. Phytochem. Anal. 2023, 34, 317–328. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Simal-Gandara, J.; Murugan, M.; Dhanya, M.K.; Pandian, A. Nutmeg (Myristica fragrans Houtt.) essential oil: A review on its composition, biological, and pharmacological activities. Phytother. Res. 2022, 36, 2839–2851. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Ekundayo, O. Chemical Composition of Essential Oil of Myristica Fragrans Houtt (Nutmeg) From Nigeria. J. Essent. Oil Bear. Plants 2003, 6, 21–26. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.; Hocayen, P.D.A.S.; Andrade, L.N.; Andreatini, R. A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models. Molecules 2015, 20, 18620–18660. [Google Scholar] [CrossRef]
- Almeida, R.C.; Souza, D.G.; Soletti, R.C.; López, M.G.; Rodrigues, A.L.; Gabilan, N.H. Involvement of PKA, MAPK/ERK and CaMKII, but not PKC in the acute antidepressant-like effect of memantine in mice. Neurosci. Lett. 2006, 395, 93–97. [Google Scholar] [CrossRef] [PubMed]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, Cd003154. [Google Scholar] [CrossRef]
- Pascual, O.; Ben Achour, S.; Rostaing, P.; Triller, A.; Bessis, A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2012, 109, E197–E205. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Si, Q.S.; Kataoka, K. Lipopolysaccharide-induced microglial activation in culture: Temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci. Res. 1999, 35, 95–100. [Google Scholar] [CrossRef]
- Dunn, A.J.; Wang, J.; Ando, T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv. Exp. Med. Biol. 1999, 461, 117–127. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, L.; Guo, X.D.; Cao, L.L.; Xue, T.F.; Zhao, X.J.; Yang, D.D.; Yang, J.; Ji, J.; Huang, J.Y.; et al. Rosiglitazone Exerts an Anti-depressive Effect in Unpredictable Chronic Mild-Stress-Induced Depressive Mice by Maintaining Essential Neuron Autophagy and Inhibiting Excessive Astrocytic Apoptosis. Front. Mol. Neurosci. 2017, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Tang, Y.; Du, P.; Shen, Z.; Yang, J.; Cai, M.; Zhang, Y.; Li, H.; Shen, H. GPR39 protects against corticosterone-induced neuronal injury in hippocampal cells through the CREB-BDNF signaling pathway. J. Affect. Disord. 2020, 272, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xu, X.; Chen, X.; Qi, W.; Lu, J.; Yan, X.; Zhao, D.; Cong, D.; Li, X.; Sun, L. Protective effect of pig brain polypeptides against corticosterone-induced oxidative stress, inflammatory response, and apoptosis in PC12 cells. Biomed. Pharmacother. 2019, 115, 108890. [Google Scholar] [CrossRef] [PubMed]
- Gruver-Yates, A.L.; Cidlowski, J.A. Tissue-specific actions of glucocorticoids on apoptosis: A double-edged sword. Cells 2013, 2, 202–223. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Cao, L.L.; Liang, Y.Y.; Wang, P. The Molecular Mechanism of Chronic High-Dose Corticosterone-Induced Aggravation of Cognitive Impairment in APP/PS1 Transgenic Mice. Front. Mol. Neurosci. 2020, 13, 613421. [Google Scholar] [CrossRef]
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Gradin, V.B.; Pomi, A. The role of hippocampal atrophy in depression: A neurocomputational approach. J. Biol. Phys. 2008, 34, 107–120. [Google Scholar] [CrossRef]
- Hao, Y.; Shabanpoor, A.; Metz, G.A. Stress and corticosterone alter synaptic plasticity in a rat model of Parkinson’s disease. Neurosci. Lett. 2017, 651, 79–87. [Google Scholar] [CrossRef]
- Xu, L.; Holscher, C.; Anwyl, R.; Rowan, M.J. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc. Natl. Acad. Sci. USA 1998, 95, 3204–3208. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef]
- Park, Y.-k.; Chung, Y.S.; Kim, Y.S.; Kwon, O.Y.; Joh, T.H. Inhibition of gene expression and production of iNOS and TNF-α in LPS-stimulated microglia by methanol extract of Phellodendri cortex. Int. Immunopharmacol. 2007, 7, 955–962. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Oh, E.Y.; Kim, G.Y.; Choi, B.T.; Kim, C.; Choi, Y.H. Ethanol extract of Cnidium officinale exhibits anti-inflammatory effects in BV2 microglial cells by suppressing NF-κB nuclear translocation and the activation of the PI3K/Akt signaling pathway. Int. J. Mol. Med. 2013, 32, 876–882. [Google Scholar] [CrossRef]
- Wu, F.; Li, H.; Zhao, L.; Li, X.; You, J.; Jiang, Q.; Li, S.; Jin, L.; Xu, Y. Protective effects of aqueous extract from Acanthopanax senticosus against corticosterone-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2013, 148, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jin, W.; Cui, Y.; Ao, M.; Liu, H.; Xu, H.; Yu, L. Protective effects of macamides from Lepidium meyenii Walp. against corticosterone-induced neurotoxicity in PC12 cells. RSC Adv. 2019, 9, 23096–23108. [Google Scholar] [CrossRef]
- Dhingra, D.; Sharma, A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J. Med. Food 2006, 9, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Kobayashi, D.; Kawashiri, T.; Kubota, T.; Kawano, K.; Yamamuro, Y.; Miyagi, A.; Deguchi, Y.; Chijimatsu, T.; Shimazoe, T. Mechanisms and Safety of Antidepressant-Like Effect of Nutmeg in Mice. Biol. Pharm. Bull. 2022, 45, 738–742. [Google Scholar] [CrossRef]
- Muris, P.; Merckelbach, H.; Schmidt, H.; Gadet, B.; Bogie, N. Anxiety and depression as correlates of self-reported behavioural inhibition in normal adolescents. Behav. Res. Ther. 2001, 39, 1051–1061. [Google Scholar] [CrossRef]
- Tiller, J.W. Depression and anxiety. Med. J. Aust. 2013, 199, S28–S31. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Sanacora, G.; Schatzberg, A.F. Ketamine: Promising Path or False Prophecy in the Development of Novel Therapeutics for Mood Disorders? Neuropsychopharmacology 2015, 40, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Shahwan, M.; Khan, M.S.; Husain, F.M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rehman, M.T.; Hassan, M.I.; Islam, A. Elucidating the Interaction of Human Ferritin with Quercetin and Naringenin: Implication of Natural Products in Neurodegenerative Diseases: Molecular Docking and Dynamics Simulation Insight. ACS Omega 2021, 6, 7922–7930. [Google Scholar] [CrossRef]
- Khan, A.; Mohammad, T.; Shamsi, A.; Hussain, A.; Alajmi, M.F.; Husain, S.A.; Iqbal, M.A.; Hassan, M.I. Identification of plant-based hexokinase 2 inhibitors: Combined molecular docking and dynamics simulation studies. J. Biomol. Struct. Dyn. 2022, 40, 10319–10331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Z.; Gao, Y.; Wu, Y.; Li, Z.; Liu, H.; Zhang, C. Bidirectional crosstalk between stress-induced gastric ulcer and depression under chronic stress. PLoS ONE 2012, 7, e51148. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, X.; Tan, D.; Jiang, Z. Atractylon treatment prevents sleep-disordered breathing-induced cognitive dysfunction by suppression of chronic intermittent hypoxia-induced M1 microglial activation. Biosci. Rep. 2020, 40, BSR20192800. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and Antinociceptive Constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant Activity of Water Extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT—Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, S.; Liu, Y.; Daniyal, M.; Jian, Y.; Peng, C.; Shen, J.; Liu, S.; Wang, W. The flower head of Chrysanthemum morifolium Ramat. (Juhua): A paradigm of flowers serving as Chinese dietary herbal medicine. J. Ethnopharmacol. 2020, 261, 113043. [Google Scholar] [CrossRef]
- Ibi, M.; Yabe-Nishimura, C. Chapter 1—The role of reactive oxygen species in the pathogenic pathways of depression. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Martin, C.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 3–16. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, N.; Xu, R.; Cao, Y.; Zhang, Y.; Liu, Z.; Zheng, X.; Feng, W. A metabolomic study on the anti-depressive effects of two active components from Chrysanthemum morifolium. Artif. Cells Nanomed. Biotechnol. 2020, 48, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Klein, R.A.; Quinn, M.T. Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils. Pharmaceuticals 2022, 15, 642. [Google Scholar] [CrossRef]
- Ha, M.T.; Vu, N.K.; Tran, T.H.; Kim, J.A.; Woo, M.H.; Min, B.S. Phytochemical and pharmacological properties of Myristica fragrans Houtt.: An updated review. Arch. Pharmacal Res. 2020, 43, 1067–1092. [Google Scholar] [CrossRef]
- Workman, E.R.; Niere, F.; Raab-Graham, K.F. mTORC1-dependent protein synthesis underlying rapid antidepressant effect requires GABABR signaling. Neuropharmacology 2013, 73, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Mendez-David, I.; David, D.J.; Darcet, F.; Wu, M.V.; Kerdine-Römer, S.; Gardier, A.M.; Hen, R. Rapid Anxiolytic Effects of a 5-HT4 Receptor Agonist Are Mediated by a Neurogenesis-Independent Mechanism. Neuropsychopharmacology 2014, 39, 1366–1378. [Google Scholar] [CrossRef]
- Faye, C.; Hen, R.; Guiard, B.P.; Denny, C.A.; Gardier, A.M.; Mendez-David, I.; David, D.J. Rapid Anxiolytic Effects of RS67333, a Serotonin Type 4 Receptor Agonist, and Diazepam, a Benzodiazepine, Are Mediated by Projections from the Prefrontal Cortex to the Dorsal Raphe Nucleus. Biol. Psychiatry 2020, 87, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Kasuya, H.; Maeda, K.; Koike, K. Daily inhalation of α-pinene in mice: Effects on behavior and organ accumulation. Phytother. Res. 2014, 28, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Shringarpure, M.; Gharat, S.; Momin, M.; Omri, A. Management of epileptic disorders using nanotechnology-based strategies for nose-to-brain drug delivery. Expert Opin. Drug Deliv. 2021, 18, 169–185. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. A Review and Update of Mechanisms of Estrogen in the Hippocampus and Amygdala for Anxiety and Depression Behavior. Neuropsychopharmacology 2006, 31, 1097–1111. [Google Scholar] [CrossRef]
- Meltzer, E.O. Formulation considerations of intranasal corticosteroids for the treatment of allergic rhinitis. Ann. Allergy Asthma Immunol. 2007, 98, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Caimmi, D.; Neukirch, C.; Louis, R.; Malard, O.; Thabut, G.; Demoly, P. Effect of the Use of Intranasal Spray of Essential Oils in Patients with Perennial Allergic Rhinitis: A Prospective Study. Int. Arch. Allergy Immunol. 2021, 182, 182–189. [Google Scholar] [CrossRef]
- Vale, T.G.; Matos, F.J.A.; de Lima, T.C.M.; Viana, G.S.B. Behavioral effects of essential oils from Lippia alba (Mill.) N.E. Brown chemotypes. J. Ethnopharmacol. 1999, 67, 127–133. [Google Scholar] [CrossRef]
- Guo, J.; Duan, J.A.; Tang, Y.; Jia, N.; Li, X.; Zhang, J. Fast onset of action and the analgesic and sedative efficacy of essential oil from Rhizoma Chuanxiong after nasal administration. Pharmazie 2010, 65, 296–299. [Google Scholar] [PubMed]
- Guo, J.; Duan, J.A.; Tang, Y.; Li, Y. Sedative and anticonvulsant activities of styrax after oral and intranasal administration in mice. Pharm. Biol. 2011, 49, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Can, Ö.D.; Demir Özkay, Ü.; Kıyan, H.T.; Demirci, B. Psychopharmacological profile of Chamomile (Matricaria recutita L.) essential oil in mice. Phytomedicine 2012, 19, 306–310. [Google Scholar] [CrossRef] [PubMed]






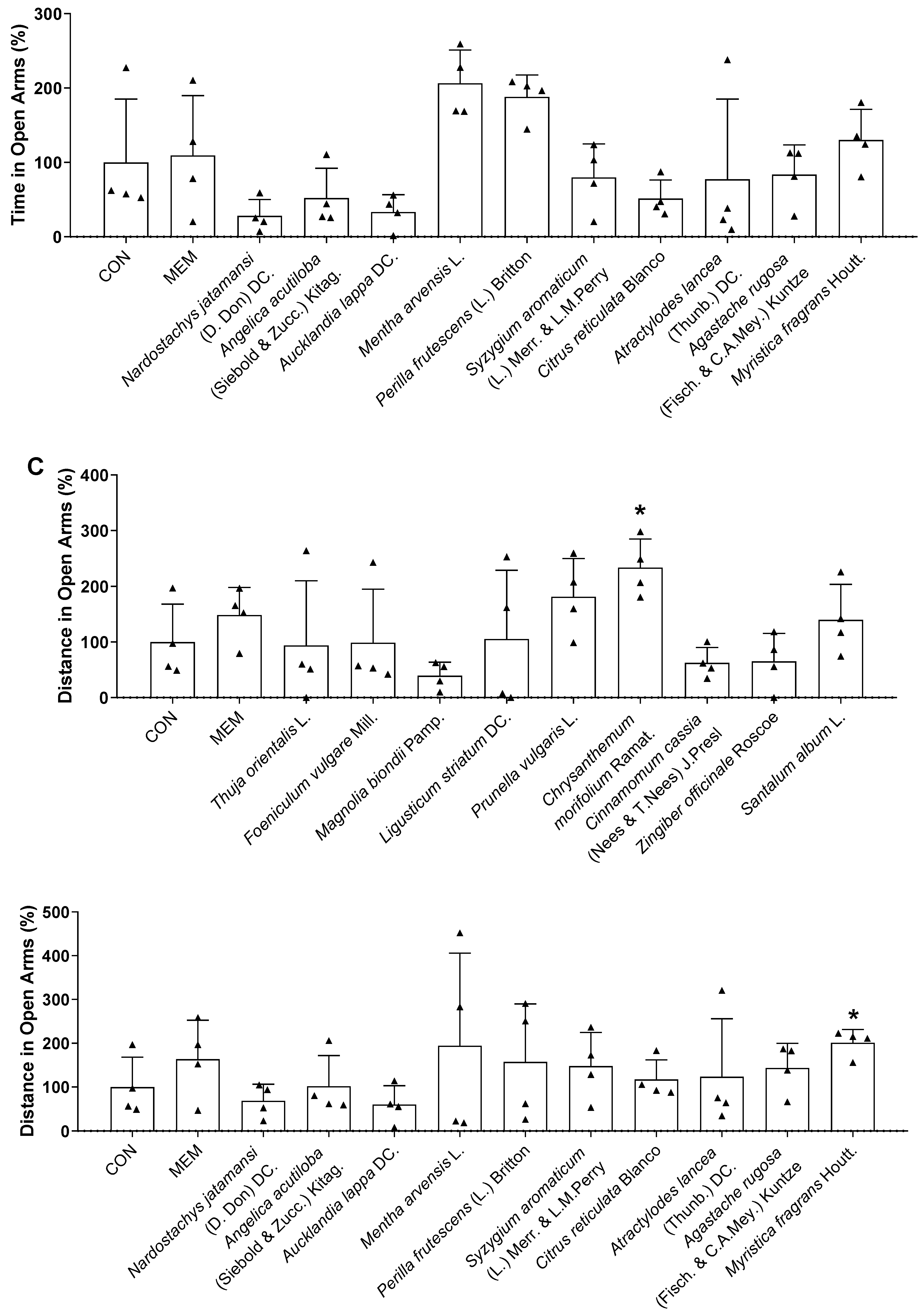

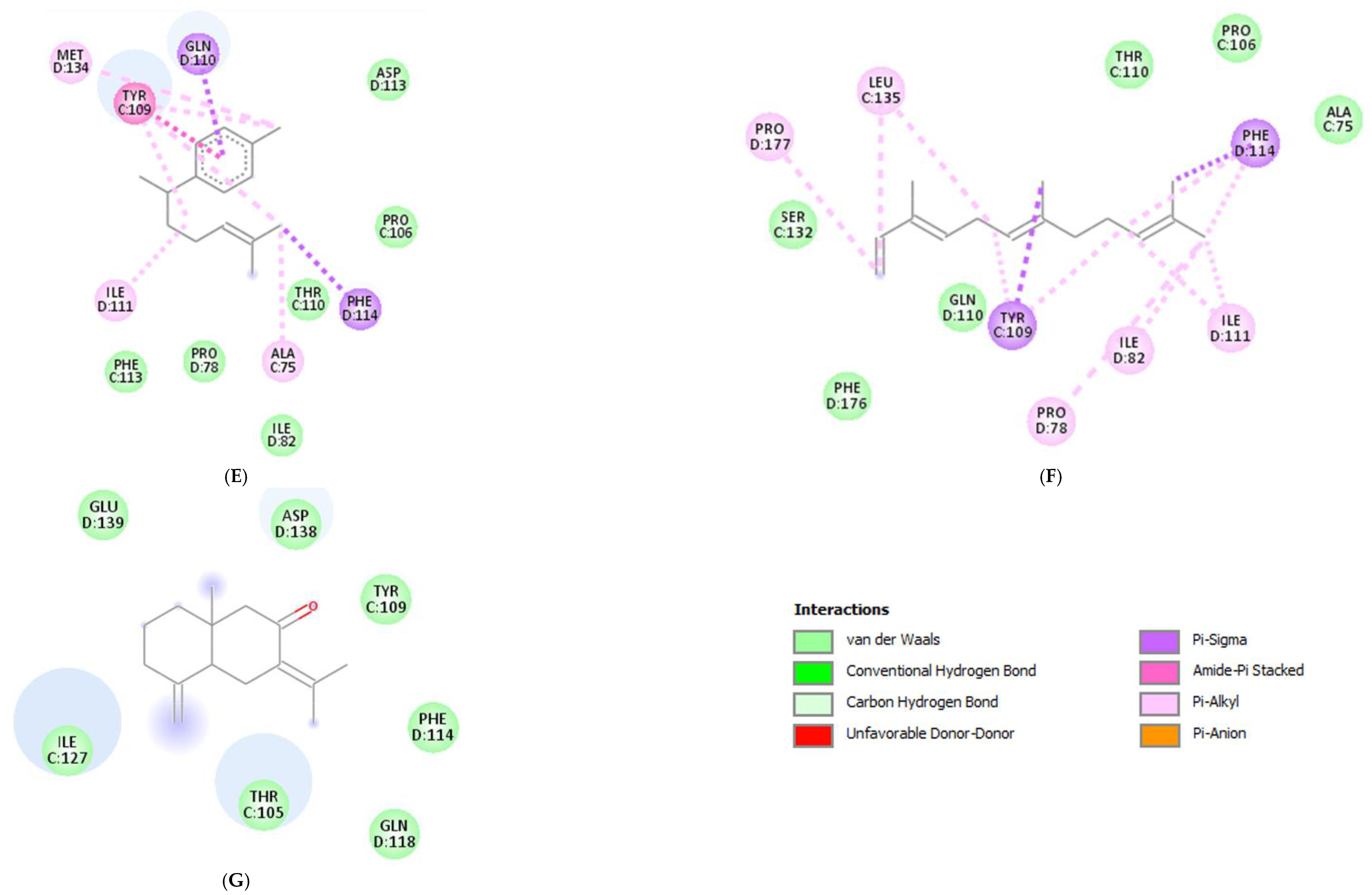
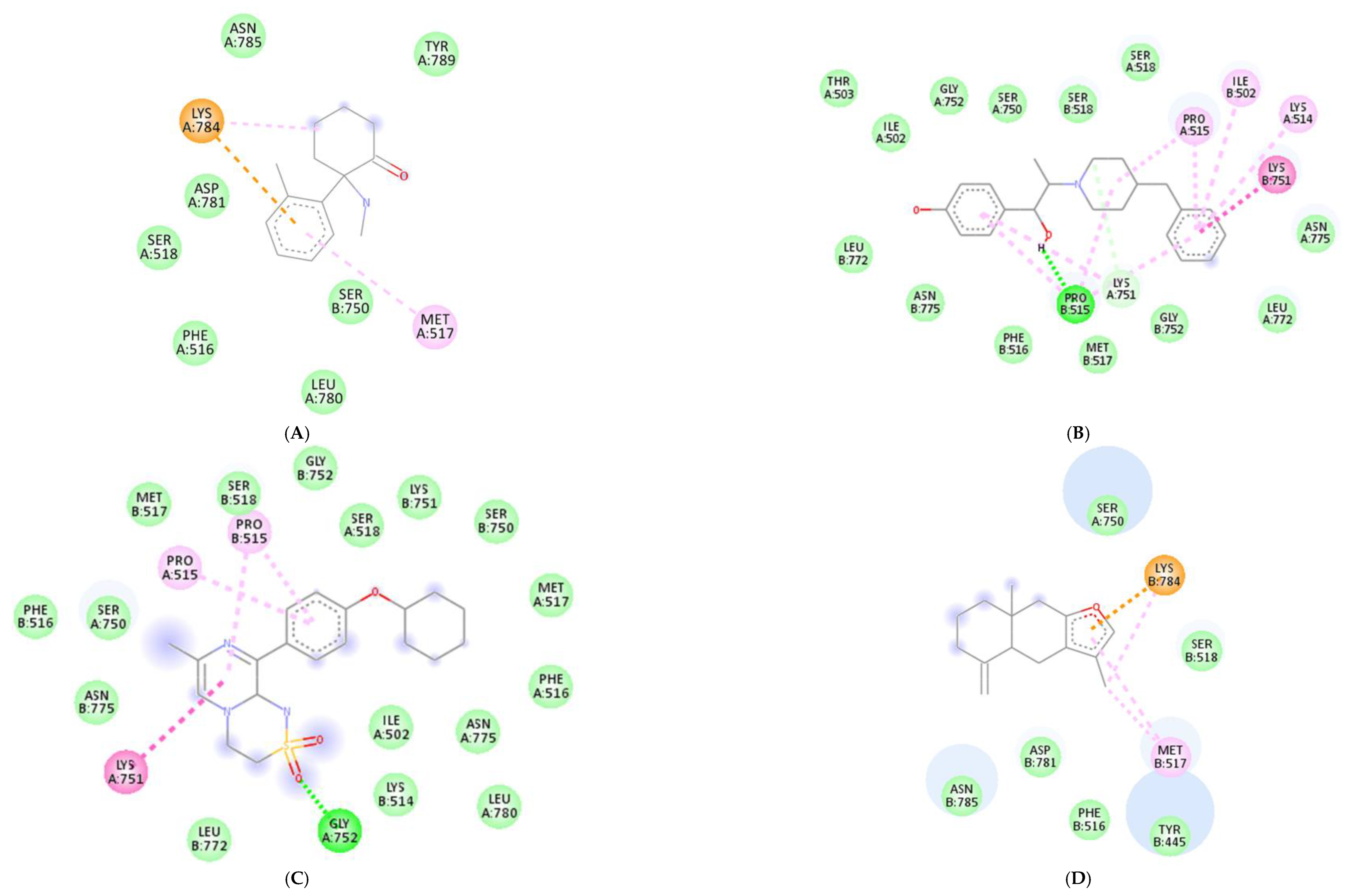

| No. | Essential Oil | Main Compounds | Ref. | ||||
|---|---|---|---|---|---|---|---|
| 1 | Magnolia biondii Pamp. | Camphor | trans-Caryophyllene | 1,8-Cineole | α-Pinene | β-Pinene | [44,45,46] |
| 2 | Chrysanthemum morifolium Ramat. | α-Curcumene | α-Farnesene | n-Heptadecane | Linoleic Acid | Nonadecane | [47,48] |
| 3 | Zingiber officinale Roscoe | Camphene | 1,8-Cineole | β-Myrcene | β-Phellandrene | α-Pinene | [49,50,51] |
| 4 | Santalum album L. | (Z)-trans-α-Bergamotol | (Z)-Lanceol | (E)-Nuciferol | (Z)-α-Santalol | (E)-β-Santalol | [52,53,54] |
| 5 | Citrus reticulata Blanco | Limonene | Linalool | β-Myrcene | α-Pinene | γ-Terpinene | [55,56,57,58] |
| 6 | Atractylodes lancea (Thunb.) DC. | Atractylodin | Atractylon | β-Eudesmol | Elemol | Selina-4(14),7(11)-dien-8-one | [59,60,61] |
| 7 | Myristica fragrans Houtt. | Limonene | β-Myrcene | α-Phellandrene | α-Pinene | β-Pinene | [62,63,64] |
| No. | Compound | Pubchem CID |
|---|---|---|
| Reference compounds | ||
| 1 | Ketamine | 3821 |
| 2 | Ifenprodil | 3689 |
| 3 | TAK-653 | 56655833 |
| Herbal compounds | ||
| 1 | Atractylodin | 5321047 |
| 2 | Atractylon | 3080635 |
| 3 | (Z)-trans-α-Bergamotol | 5368743 |
| 4 | Camphene | 6616 |
| 5 | Camphor | 2537 |
| 6 | trans-Caryophyllene | 5281515 |
| 7 | 1,8-Cineole | 2758 |
| 8 | α-Curcumene | 92139 |
| 9 | Elemol | 92138 |
| 10 | β-Eudesmol | 91457 |
| 11 | α-Farnesene | 5281516 |
| 12 | n-Heptadecane | 12398 |
| 13 | (Z)-Lanceol | 15560069 |
| 14 | Limonene | 22311 |
| 15 | Linalool | 6549 |
| 16 | Linoleic Acid | 5280450 |
| 17 | β-Myrcene | 31253 |
| 18 | Nonadecane | 12401 |
| 19 | (E)-Nuciferol | 6429177 |
| 20 | α-Phellandrene | 7460 |
| 21 | β-Phellandrene | 11142 |
| 22 | α-Pinene | 6654 |
| 23 | β-Pinene | 14896 |
| 24 | (Z)-α-Santalol | 11085337 |
| 25 | (E)-β-Santalol | 11031396 |
| 26 | Selina-4(14),7(11)-dien-8-one | 13986099 |
| 27 | γ-Terpinene | 7461 |
| No. | Ligand | Binding Energy (kcal/mol) | ||
|---|---|---|---|---|
| GluN1 | GluN2B | GluA2 | ||
| Native ligand | −10.4 | −10.7 | −9.9 | |
| 1 | Ketamine | −8.2 | −5.9 | −4.9 |
| 2 | Ifenprodil | −9.6 | −9.7 | −9.2 |
| 3 | TAK-653 | −8.6 | −8.6 | −9.2 |
| 4 | Atractylodin | −7.2 | −7.2 | −6.0 |
| 5 | Atractylon | −8.7 | −8.8 | −6.1 |
| 6 | (Z)-trans-α-Bergamotol | −7.9 | −7.0 | −5.8 |
| 7 | Camphene | −5.8 | −5.3 | −4.7 |
| 8 | Camphor | −5.9 | −4.6 | −4.3 |
| 9 | trans-Caryophyllene | −7.0 | −6.4 | −5.6 |
| 10 | 1,8-Cineole | −6.0 | −5.6 | −4.5 |
| 11 | α-Curcumene | −8.8 | −9.3 | −7.0 |
| 12 | Elemol | −7.2 | −7.8 | −6.4 |
| 13 | β-Eudesmol | −7.0 | −6.0 | −6.2 |
| 14 | α-Farnesene | −8.2 | −8.4 | −6.7 |
| 15 | n-Heptadecane | −6.3 | −6.6 | −5.8 |
| 16 | (Z)-Lanceol | −7.8 | −8.1 | −6.9 |
| 17 | Limonene | −6.6 | −7.5 | −6.0 |
| 18 | Linalool | −6.4 | −6.7 | −5.7 |
| 19 | Linoleic Acid | −7.6 | −7.2 | −6.6 |
| 20 | β-Myrcene | −6.4 | −6.9 | −5.0 |
| 21 | Nonadecane | −6.8 | −8.1 | −6.9 |
| 22 | (E)-Nuciferol | −8.1 | −8.4 | −6.9 |
| 23 | α-Phellandrene | −6.7 | −7.4 | −6.1 |
| 24 | β-Phellandrene | −6.4 | −7.5 | −6.1 |
| 25 | α-Pinene | −6.1 | −5.7 | −4.6 |
| 26 | β-Pinene | −6.1 | −6.2 | −4.4 |
| 27 | (Z)-α-Santalol | −7.4 | −6.4 | −5.7 |
| 28 | (E)-β-Santalol | −7.2 | −5.5 | −6.0 |
| 29 | Selina-4(14),7(11)-dien-8-one | −8.8 | −6.8 | −7.6 |
| 30 | γ-Terpinene | −7.0 | −7.4 | −5.9 |
| No. | Herb | In Vitro | In Vivo | |||||
|---|---|---|---|---|---|---|---|---|
| Neuroprotective Dose (µg/mL) | Anti-Neuroinflammatory Dose (µg/mL) | Rapid Anti-Depressant Dose (mg/kg) | Rapid Anti-Anxiety Dose (mg/kg) | |||||
| TNF-α | IL-6 | |||||||
| 1 | Thuja orientalis L. | 1 | - | - | - | - | ||
| 2 | Acorus gramineus Sol. | - | - | - | - | - | ||
| 3 | Foeniculum vulgare Mill. | 0.1, 1 | 1 | - | - | - | ||
| 4 | Magnolia biondii Pamp. | 1 | - | - | - | - | ||
| 5 | Ligusticum striatum DC. | 1 | 1 | 1 | - | - | ||
| 6 | Prunella vulgaris L. | 1 | 1 | - | - | - | ||
| 7 | Chrysanthemum morifolium Ramat. | 0.1, 1 | 0.1, 1 | - | 25 | 25 | ||
| 8 | Cinnamomum cassia (Nees & T.Nees) J.Presl | 1 | 0.1 | 0.1, 1 | - | - | ||
| 9 | Zingiber officinale Roscoe | 1 | - | - | 25 | - | ||
| 10 | Santalum album L. | 1 | 0.1, 1 | 1 | 25 | - | ||
| 11 | Nardostachys jatamansi (D. Don) DC. | 1 | - | - | - | - | ||
| 12 | Angelica acutiloba (Siebold & Zucc.) Kitag. | 0.1, 1 | - | - | - | - | ||
| 13 | Aucklandia lappa DC. | 0.1, 1 | - | 1 | - | - | ||
| 14 | Mentha arvensis L. | 1 | - | 1 | - | - | ||
| 15 | Perilla frutescens (L.) Britton | 1 | - | 0.1, 1 | - | - | ||
| 16 | Syzygium aromaticum (L.) Merr. & L.M.Perry | 0.1, 1 | - | 0.1, 1 | - | - | ||
| 17 | Citrus reticulata Blanco | 0.1, 1 | - | 0.1, 1 | 25 | - | ||
| 18 | Atractylodes lancea (Thunb.) DC. | 0.1, 1 | - | 0.1, 1 | 25 | - | ||
| 19 | Agastache rugosa (Fisch. & C.A.Mey.) Kuntze. | 1 | - | - | - | - | ||
| 20 | Myristica fragrans Houtt. | 0.1, 1 | - | 0.1, 1 | 25 | 25 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, K.N.; Nguyen, N.P.K.; Nguyen, L.T.H.; Shin, H.-M.; Yang, I.-J. Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils. Biomedicines 2023, 11, 1248. https://doi.org/10.3390/biomedicines11051248
Tran KN, Nguyen NPK, Nguyen LTH, Shin H-M, Yang I-J. Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils. Biomedicines. 2023; 11(5):1248. https://doi.org/10.3390/biomedicines11051248
Chicago/Turabian StyleTran, Khoa Nguyen, Nhi Phuc Khanh Nguyen, Ly Thi Huong Nguyen, Heung-Mook Shin, and In-Jun Yang. 2023. "Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils" Biomedicines 11, no. 5: 1248. https://doi.org/10.3390/biomedicines11051248