Maquiberry Cystatins: Recombinant Expression, Characterization, and Use to Protect Tooth Dentin and Enamel
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA Extraction
2.2. Preprocessing Reads, De Novo Assembly and Completeness Assembly
2.3. Screening for Maquicystatins
2.4. Protein Sequence Analysis
2.5. Expression and Purification of Recombinant Cystatins
2.6. Enzyme Inhibition Assays
2.7. Definition of MaquiCPI-3 Concentrations for the Prevention of Enamel and Dentin Erosion In Vitro
2.7.1. Preparation of Bovine Enamel and Dentin Samples
2.7.2. Human Saliva Acquisition
2.7.3. Treatment Groups
2.7.4. Treatment, Acquired Pellicle Formation and Erosive Process
2.7.5. Percentage of Surface Microhardness Change (%SMC)
2.7.6. Statistical Analysis
3. Results
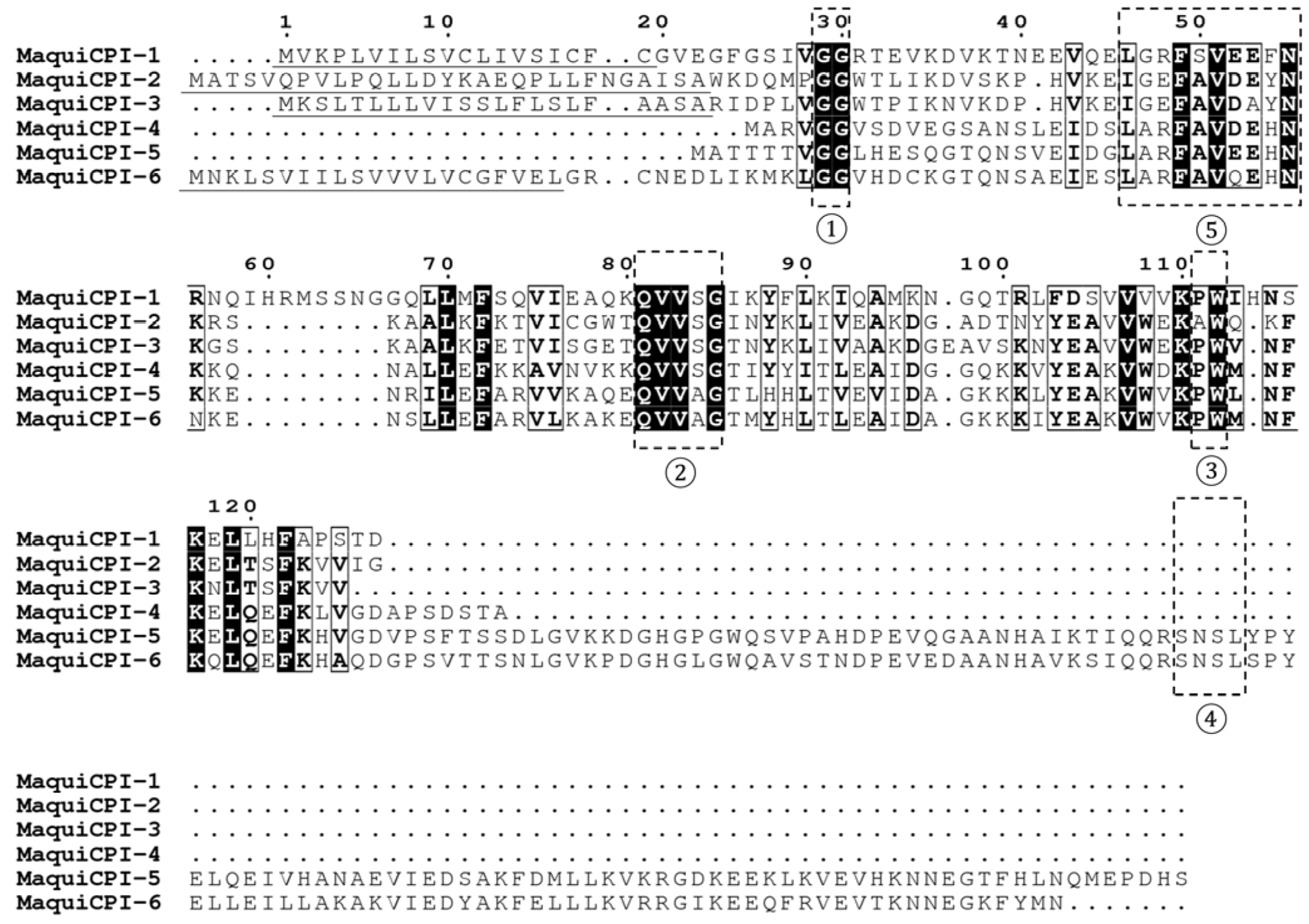
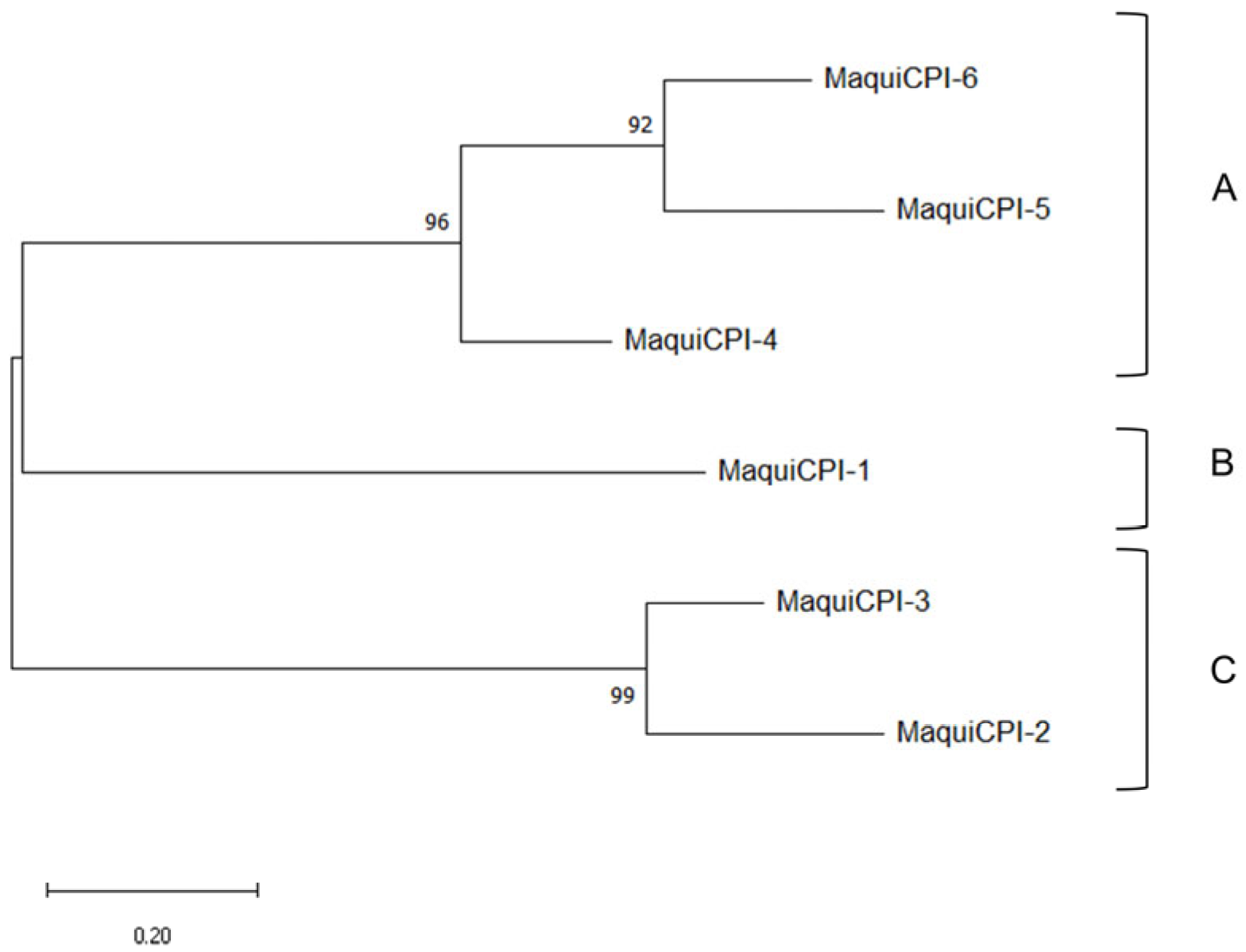
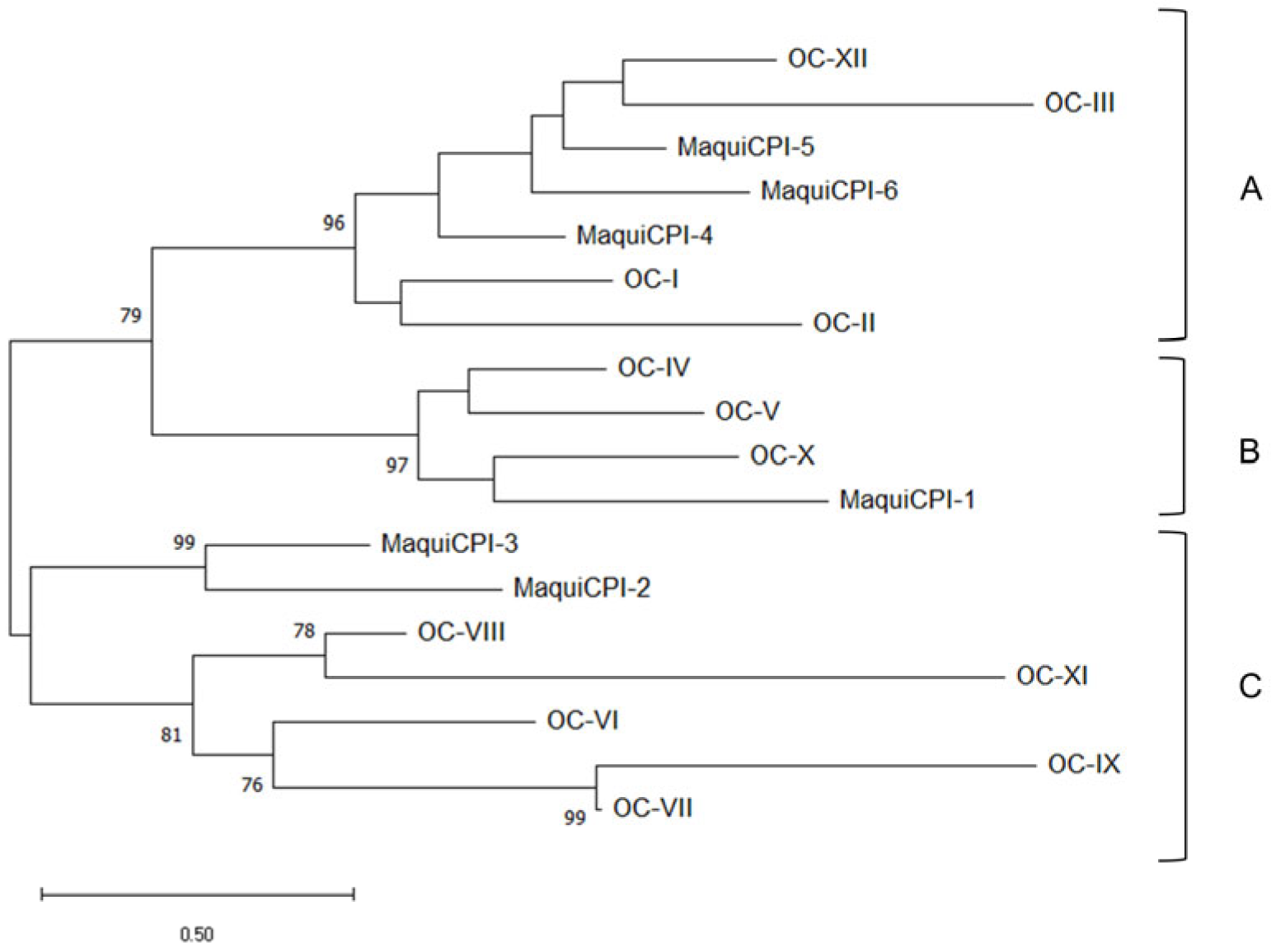
3.1. In Silico Analysis of Cystatin Sequences
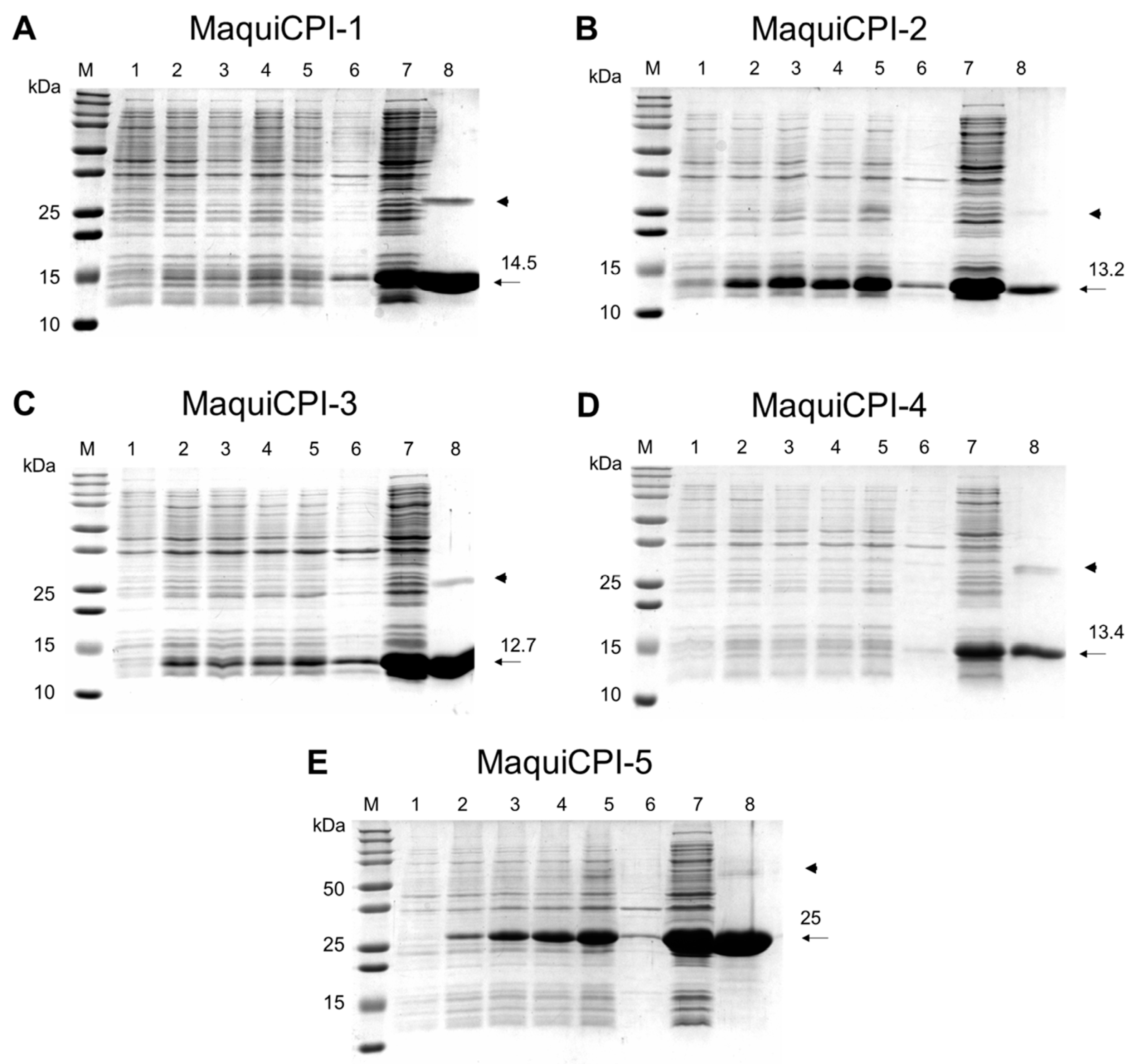
3.2. Protein Expression Purification
3.3. Inhibitory Activity Assay
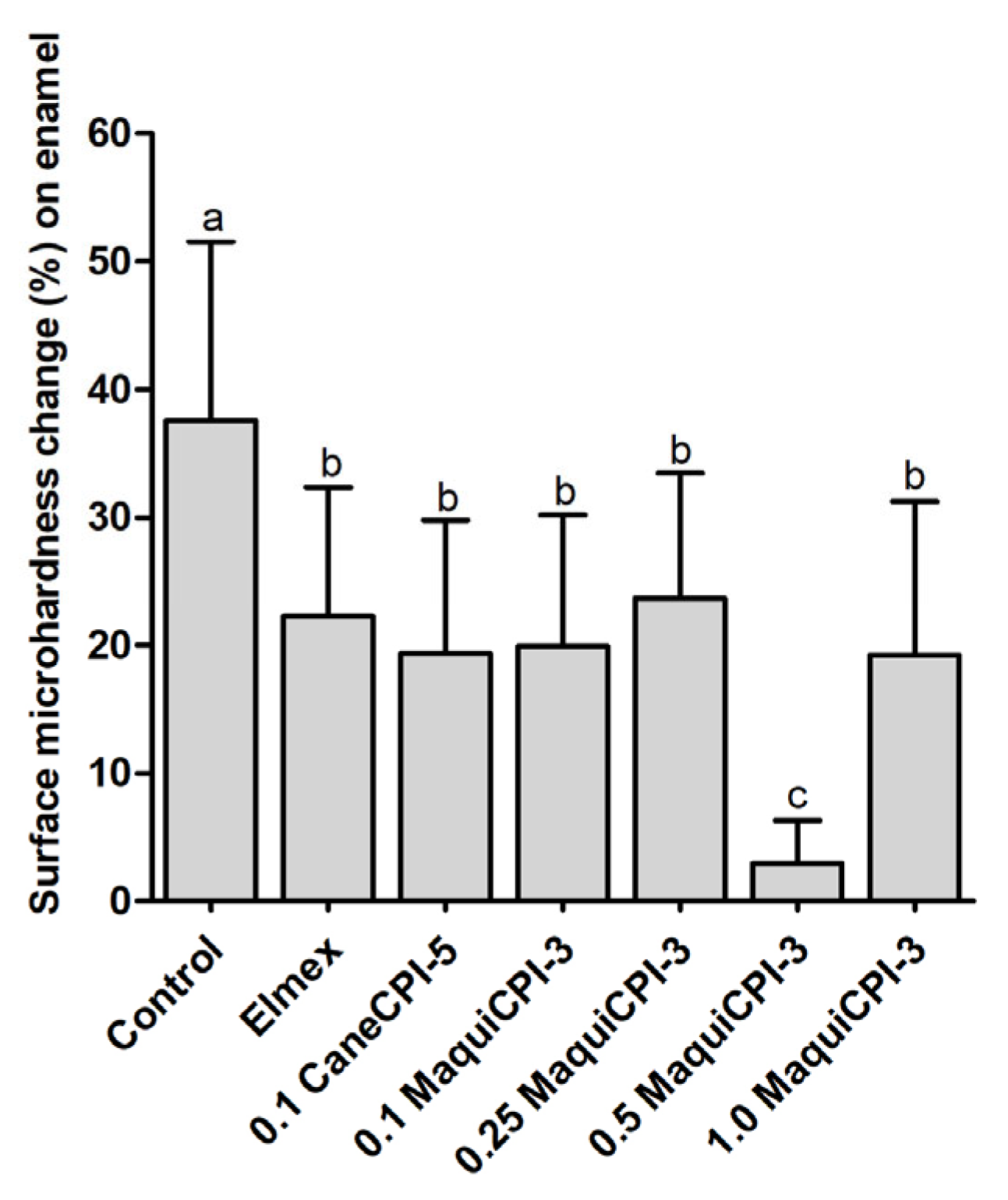
3.4. Percentage of Surface Microhardness Change on Enamel
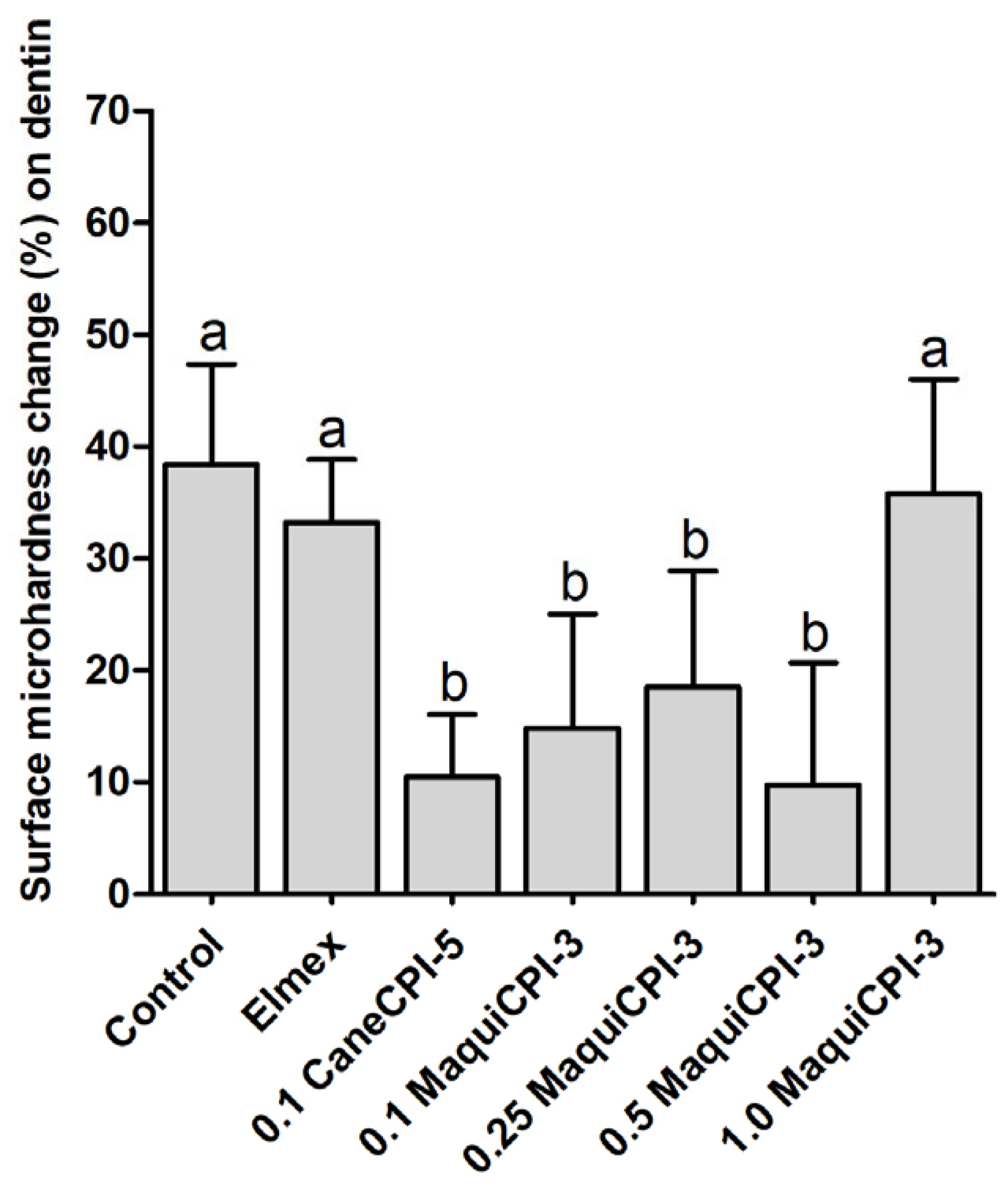
3.5. Percentage of Surface Microhardness Change on Dentin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, A.J.; Rawlings, N.D.; Davies, M.E.; Machleidt, W.; Salvesen, G.; Turk, V. Cysteine Protease Inhibitors of the Cystatin Superfamily. In Proteinase Inhibitors; Barret, A.J., Salvesen, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 529–569. [Google Scholar]
- Barrett, A.J. The Cystatins: A New Class of Peptidase Inhibitors. Trends Biochem. Sci. 1987, 12, 193–196. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS Database of Proteolytic Enzymes, Their Substrates and Inhibitors in 2017 and a Comparison with Peptidases in the PANTHER Database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, M.; Ritonja, A.; Brown, M.A.; Grubb, A.; Machleidt, W.; Barrett, A.J. Identification of the Probable Inhibitory Reactive Sites of the Cysteine Proteinase Inhibitors Human Cystatin C and Chicken Cystatin. J. Biol. Chem. 1987, 262, 9688–9694. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J. Evolution of Proteins of the Cystatin Superfamily. J. Mol. Evol. 1990, 30, 60–71. [Google Scholar] [CrossRef]
- Turk, V.; Bode, W. The Cystatins: Protein Inhibitors of Cysteine Proteinases. FEBS Lett. 1991, 285, 213–219. [Google Scholar] [CrossRef]
- Margis, R.; Reis, E.M.; Villeret, V. Structural and Phylogenetic Relationships among Plant and Animal Cystatins. Arch. Biochem. Biophys. 1998, 359, 24–30. [Google Scholar] [CrossRef]
- Martinez, M.; Diaz-Mendoza, M.; Carrillo, L.; Diaz, I. Carboxy Terminal Extended Phytocystatins Are Bifunctional Inhibitors of Papain and Legumain Cysteine Proteinases. FEBS Lett. 2007, 581, 2914–2918. [Google Scholar] [CrossRef]
- Hwang, J.E.; Hong, J.K.; Je, J.H.; Lee, K.O.; Kim, D.Y.; Lee, S.Y.; Lim, C.O. Regulation of Seed Germination and Seedling Growth by an Arabidopsis Phytocystatin Isoform, AtCYS6. Plant Cell Rep. 2009, 28, 1623–1632. [Google Scholar] [CrossRef]
- Díaz-Mendoza, M.; Velasco-Arroyo, B.; González-Melendi, P.; Martínez, M.; Díaz, I. C1A Cysteine Protease–Cystatin Interactions in Leaf Senescence. J. Exp. Bot. 2014, 65, 3825–3833. [Google Scholar] [CrossRef]
- Tan, Y.; Li, M.; Ma, F. Overexpression of MpCYS2, a Phytocystatin Gene from Malus prunifolia (Willd.) Borkh., Confers Drought Tolerance and Protects against Oxidative Stress in Arabidopsis. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 123, 15–27. [Google Scholar] [CrossRef]
- Van der Vyver, C.; Schneidereit, J.; Driscoll, S.; Turner, J.; Kunert, K.; Foyer, C.H. Oryzacystatin I Expression in Transformed Tobacco Produces a Conditional Growth Phenotype and Enhances Chilling Tolerance. Plant Biotechnol. J. 2003, 1, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wei, X.; Wang, P.; Sun, X.; Li, M.; Ma, F. A Phytocystatin Gene from Malus prunifolia (Willd.) Borkh., MpCYS5, Confers Salt Stress Tolerance and Functions in Endoplasmic Reticulum Stress Response in Arabidopsis. Plant Mol. Biol. Rep. 2016, 34, 62–75. [Google Scholar] [CrossRef]
- Martinez, M.; Santamaria, M.E.; Diaz-Mendoza, M.; Arnaiz, A.; Carrillo, L.; Ortego, F.; Diaz, I. Phytocystatins: Defense Proteins against Phytophagous Insects and Acari. Int. J. Mol. Sci. 2016, 17, 1747. [Google Scholar] [CrossRef] [PubMed]
- Soares-Costa, A.; Beltramini, L.M.; Thiemann, O.H.; Henrique-Silva, F. A Sugarcane Cystatin: Recombinant Expression, Purification, and Antifungal Activity. Biochem. Biophys. Res. Commun. 2002, 296, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-L.; Yang, A.-H.; Chen, J.-T.; Yeh, K.-W.; Chan, M.-T. Heterologous Expression of Taro Cystatin Protects Transgenic Tomato against Meloidogyne Incognita Infection by Means of Interfering Sex Determination and Suppressing Gall Formation. Plant Cell Rep. 2010, 29, 231–238. [Google Scholar] [CrossRef]
- Benchabane, M.; Schlüter, U.; Vorster, J.; Goulet, M.-C.; Michaud, D. Plant Cystatins. Biochimie 2010, 92, 1657–1666. [Google Scholar] [CrossRef]
- Schneider, V.K.; da Silva Ferrara, T.F.; Rocha, S.V.; Santos-Júnior, C.D.; Neo-Justino, D.M.; da Cunha, A.F.; de Oliveira da Silva, J.P.M.; dos Santos Tersariol, I.L.; Carmona, A.K.; Henrique-Silva, F.; et al. Recombinant Expression, Characterization and Phylogenetic Studies of Novels Cystatins-like Proteins of Sweet Orange (Citrus sinensis) and Clementine (Citrus clementina). Int. J. Biol. Macromol. 2020, 152, 546–553. [Google Scholar] [CrossRef]
- Shibao, P.Y.T.; Santos-Júnior, C.D.; Santiago, A.C.; Mohan, C.; Miguel, M.C.; Toyama, D.; Vieira, M.A.S.; Narayanan, S.; Figueira, A.; Carmona, A.K.; et al. Sugarcane Cystatins: From Discovery to Biotechnological Applications. Int. J. Biol. Macromol. 2021, 167, 676–686. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Magliarelli, H.F.; Pereira, F.V.; Gianotti, A.; Soares-Costa, A.; Henrique-Silva, F.; Wakamatsu, A.; Soares, I.C.; Nonogaki, S.; Travassos, L.R.; et al. Sugarcane Cystatin CaneCPI-4 Inhibits Melanoma Growth by Angiogenesis Disruption. J. Cancer Sci. Ther. 2011, 3, 161–167. [Google Scholar] [CrossRef]
- Ferreira, B.A.; Toyama, D.; Henrique-Silva, F.; de Assis Araújo, F. Recombinant Sugarcane Cystatin CaneCPI-5 down Regulates Inflammation and Promotes Angiogenesis and Collagen Deposition in a Mouse Subcutaneous Sponge Model. Int. Immunopharmacol. 2021, 96, 107801. [Google Scholar] [CrossRef]
- Leguizamón, N.D.P.; Rodrigues, E.M.; de Campos, M.L.; Nogueira, A.V.B.; Viola, K.S.; Schneider, V.K.; Neo-Justino, D.M.; Tanomaru-Filho, M.; Zambuzzi, W.F.; Henrique-Silva, F.; et al. In Vivo and in Vitro Anti-Inflammatory and pro-Osteogenic Effects of Citrus Cystatin CsinCPI-2. Cytokine 2019, 123, 154760. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.M.S.; El Chamy Maluf, S.; Azevedo, M.F.; Paschoalin, T.; Budu, A.; Bagnaresi, P.; Henrique-Silva, F.; Soares-Costa, A.; Gazarini, M.L.; Carmona, A.K. Inhibition of Plasmodium falciparum Cysteine Proteases by the Sugarcane Cystatin CaneCPI-4. Parasitol. Int. 2018, 67, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Melo, I.R.S.; Dias, L.P.; Araújo, N.M.S.; Vasconcelos, I.M.; Martins, T.F.; de Morais, G.A.; Gonçalves, J.F.C.; Nagano, C.S.; Carneiro, R.F.; Oliveira, J.T.A. ClCPI, a Cysteine Protease Inhibitor Purified from Cassia leiandra Seeds Has Antifungal Activity against Candida tropicalis by Inducing Disruption of the Cell Surface. Int. J. Biol. Macromol. 2019, 133, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.C.; Khan, Z.N.; Miguel, M.C.; Gironda, C.C.; Soares-Costa, A.; Pelá, V.T.; Leite, A.L.; Edwardson, J.M.; Buzalaf, M.A.R.; Henrique-Silva, F. A New Sugarcane Cystatin Strongly Binds to Dental Enamel and Reduces Erosion. J. Dent. Res. 2017, 96, 1051–1057. [Google Scholar] [CrossRef]
- Araujo, T.T.; Camiloti, G.D.; Valle, A.D.; Silva, N.D.G.; Souza, B.M.; de Souza Carvalhoa, T.; Câmara, J.V.F.; Shibao, P.Y.T.; Henrique-Silva, F.; Cruvinel, T.; et al. A Sugarcane Cystatin (CaneCPI-5) Alters Microcosm Biofilm Formation and Reduces Dental Caries. Biofouling 2021, 37, 109–116. [Google Scholar] [CrossRef]
- Pelá, V.T.; Lunardelli, J.G.Q.; Tokuhara, C.K.; Gironda, C.C.; Silva, N.D.G.; Carvalho, T.S.; Santiago, A.C.; Souza, B.M.; Moraes, S.M.; Henrique-Silva, F.; et al. Safety and In Situ Antierosive Effect of CaneCPI-5 on Dental Enamel. J. Dent. Res. 2021, 100, 1344–1350. [Google Scholar] [CrossRef]
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in Bioactive Components and Antioxidant Capacity of Maqui, Aristotelia chilensis [Mol] Stuntz, Berries during Drying. LWT-Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Romero-González, J.; Shun Ah-Hen, K.; Lemus-Mondaca, R.; Muñoz-Fariña, O. Total Phenolics, Anthocyanin Profile and Antioxidant Activity of Maqui, Aristotelia chilensis (Mol.) Stuntz, Berries Extract in Freeze-Dried Polysaccharides Microcapsules. Food Chem. 2020, 313, 126115. [Google Scholar] [CrossRef]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, Anthocyanin and Antioxidant Content in Different Parts of Maqui Fruits (Aristotelia chilensis) during Ripening and Conservation Treatments after Harvest. Ind. Crops Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Pavon, N.; Dominguez, M.; Alarcon, J.; Balbontin, C.; Kubo, I.; El-Hafidi, M.; Avila, J.G. The Chilean Superfruit Black-Berry Aristotelia chilensis (Elaeocarpaceae), Maqui as Mediator in Inflammation-Associated Disorders. Food Chem. Toxicol. 2017, 108, 438–450. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M.; Poblete, J.; Pasten, A.; Bilbao-Sainz, C.; Wood, D.; McHugh, T.; Delporte, C. Effects of Drying Processes on Composition, Microstructure and Health Aspects from Maqui Berries. J. Food Sci. Technol. 2020, 57, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.L.F.; Leighton, F. Juice and Phenolic Fractions of the Berry Aristotelia chilensis Inhibit LDL Oxidation in Vitro and Protect Human Endothelial Cells against Oxidative Stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed Analyses of Fresh and Dried Maqui (Aristotelia chilensis (Mol.) Stuntz) Berries and Juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and Cardioprotective Activities of Phenolic Extracts from Fruits of Chilean Blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In Vitro and in Vivo Anti-Diabetic Effects of Anthocyanins from Maqui Berry (Aristotelia Chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Alarcon, J.; Avila, J.G.; Nieto, A. Anti-Inflammatory Activity of Aristotelia chilensis Mol. (Stuntz) (Elaeocarpaceae). Boletín Latinoam. Caribe Plantas Med. Aromáticas 2010, 9, 127–135. [Google Scholar]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in Dental Applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Weber, M.-T.; Hannig, M.; Pötschke, S.; Höhne, F.; Hannig, C. Application of Plant Extracts for the Prevention of Dental Erosion: An in Situ/in Vitro Study. Caries Res. 2015, 49, 477–487. [Google Scholar] [CrossRef]
- Niemeyer, S.H.; Baumann, T.; Lussi, A.; Meyer-Lueckel, H.; Scaramucci, T.; Carvalho, T.S. Salivary Pellicle Modification with Polyphenol-Rich Teas and Natural Extracts to Improve Protection against Dental Erosion. J. Dent. 2021, 105, 103567. [Google Scholar] [CrossRef]
- Trentini, G.E.; Rojas, M.; Gajardo, D.; Alburquenque, D.; Villagra, E.; Gómez, A.; Arru, L.; Arencibia, A.D. Elicitation of Phenylpropanoids in Maqui (Aristotelia chilensis [Mol.] Stuntz) Plants Micropropagated in Photomixotrophic Temporary Immersion Bioreactors (TIBs). Plant Cell Tissue Organ Cult. (PCTOC) 2021, 146, 607–619. [Google Scholar] [CrossRef]
- Zhbannikov, I.Y.; Hunter, S.S.; Foster, J.A.; Settles, M.L. SeqyClean. In Proceedings of the 8th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics, Boston, MA, USA, 20–23 August 2017; ACM: New York, NY, USA, 2017; pp. 407–416. [Google Scholar]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A Survey of Best Practices for RNA-Seq Data Analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.D.; Brenner, S.E.; Dudoit, S. Biases in Illumina Transcriptome Sequencing Caused by Random Hexamer Priming. Nucleic Acids Res. 2010, 38, e131. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.T.; Howe, A.; Zhang, Q.; Pyrkosz, A.B.; Brom, T.H. A Reference-Free Algorithm for Computational Normalization of Shotgun Sequencing Data. arXiv 2012, arXiv:1203.4802. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. In Gene Prediction. Methods in Molecular Biology; Kollmar, M., Ed.; Humana: New York, NY, USA, 2019; pp. 227–245. [Google Scholar]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Bode, W.; Engh, R.; Musil, D.; Thiele, U.; Huber, R.; Karshikov, A.; Brzin, J.; Kos, J.; Turk, V. The 2.0 A X-Ray Crystal Structure of Chicken Egg White Cystatin and Its Possible Mode of Interaction with Cysteine Proteinases. EMBO J. 1988, 7, 2593–2599. [Google Scholar] [CrossRef]
- Hiller, K.; Grote, A.; Scheer, M.; Munch, R.; Jahn, D. PrediSi: Prediction of Signal Peptides and Their Cleavage Positions. Nucleic Acids Res. 2004, 32, W375–W379. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Whelan, S.; Goldman, N. A General Empirical Model of Protein Evolution Derived from Multiple Protein Families Using a Maximum-Likelihood Approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, A.; Rios, W.M.; Soares-Costa, A.; Nogaroto, V.; Carmona, A.K.; Oliva, M.L.V.; Andrade, S.S.; Henrique-Silva, F. Recombinant Expression, Purification, and Functional Analysis of Two Novel Cystatins from Sugarcane (Saccharum officinarum). Protein Expr. Purif. 2006, 47, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Salvesen, G.S. Finding, Purification and Characterization of Natural Protease Inhibitors. In Proteolytic Enzymes; Beynon, R.J., Bond, S., Eds.; Oxford: New York, NY, USA, 2001; p. 359. ISBN 0-19-963663-X. [Google Scholar]
- Melo, R.L.; Alves, L.C.; Del Nery, E.; Juliano, L.; Juliano, M.A. Synthesis and Hydrolysis by Cysteine and Serine Proteases of Short Internally Quenched Fluorogenic Peptides. Anal. Biochem. 2001, 293, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pelá, V.T.; Brito, L.; Taira, E.A.; Henrique-Silva, F.; Pieretti, J.C.; Seabra, A.B.; de Almeida Baldini Cardoso, C.; de Souza, E.P.; Groisman, S.; Rodrigues, M.C.; et al. Preventive Effect of Chitosan Gel Containing CaneCPI-5 against Enamel Erosive Wear in Situ. Clin. Oral Investig. 2022, 26, 6511–6519. [Google Scholar] [CrossRef]
- da Silva Mira, P.C.; Souza-Flamini, L.E.; da Costa Guedes, D.F.; Da Cruz-Filho, A.M. Evaluation of the Chelating Effect of Chitosan Solubilized in Different Acids. J. Conserv. Dent. 2017, 20, 297. [Google Scholar] [CrossRef]
- Bannai, H.; Tamada, Y.; Maruyama, O.; Nakai, K.; Miyano, S. Extensive Feature Detection of N-Terminal Protein Sorting Signals. Bioinformatics 2002, 18, 298–305. [Google Scholar] [CrossRef]
- Madureira, H.C.; Da Cunha, M.; Jacinto, T. Immunolocalization of a Defense-Related 87kDa Cystatin in Leaf Blade of Tomato Plants. Environ. Exp. Bot. 2006, 55, 201–208. [Google Scholar] [CrossRef]
- Prins, A.; van Heerden, P.D.R.; Olmos, E.; Kunert, K.J.; Foyer, C.H. Cysteine Proteinases Regulate Chloroplast Protein Content and Composition in Tobacco Leaves: A Model for Dynamic Interactions with Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) Vesicular Bodies. J. Exp. Bot. 2008, 59, 1935–1950. [Google Scholar] [CrossRef]
- Alomrani, S.; Kunert, K.J.; Foyer, C.H. Papain-like Cysteine Proteases Are Required for the Regulation of Photosynthetic Gene Expression and Acclimation to High Light Stress. J. Exp. Bot. 2021, 72, 3441–3454. [Google Scholar] [CrossRef]
- Frank, S.; Hollmann, J.; Mulisch, M.; Matros, A.; Carrión, C.C.; Mock, H.-P.; Hensel, G.; Krupinska, K. Barley Cysteine Protease PAP14 Plays a Role in Degradation of Chloroplast Proteins. J. Exp. Bot. 2019, 70, 6057–6069. [Google Scholar] [CrossRef] [PubMed]
- Sokolenko, A.; Altschmied, L.; Herrmann, R.G. Sodium Dodecyl Sulfate-Stable Proteases in Chloroplasts. Plant Physiol. 1997, 115, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Christoff, A.P.; Margis, R. The Diversity of Rice Phytocystatins. Mol. Genet. Genom. 2014, 289, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-K.; Hwang, J.-E.; Park, T.-H.; Zang, Y.-X.; Lee, S.-C.; Kwon, S.-J.; Mun, J.-H.; Kim, H.-U.; Kim, J.-A.; Jin, M.-N.; et al. Identification and Characterization of the Phytocystatin Family from Brassica rapa. J. Plant Biotechnol. 2008, 35, 317–327. [Google Scholar] [CrossRef]
- Martinez, M.; Cambra, I.; Carrillo, L.; Diaz-Mendoza, M.; Diaz, I. Characterization of the Entire Cystatin Gene Family in Barley and Their Target Cathepsin L-Like Cysteine-Proteases, Partners in the Hordein Mobilization during Seed Germination. Plant Physiol. 2009, 151, 1531–1545. [Google Scholar] [CrossRef]
- Balbinott, N.; Margis, R. Review: Unraveling the Origin of the Structural and Functional Diversity of Plant Cystatins. Plant Sci. 2022, 321, 111342. [Google Scholar] [CrossRef]
- Valadares, N.F.; de Oliveira-Silva, R.; Cavini, I.A.; de Almeida Marques, I.; D’Muniz Pereira, H.; Soares-Costa, A.; Henrique-Silva, F.; Kalbitzer, H.R.; Munte, C.E.; Garratt, R.C. X-ray Crystallography and NMR Studies of Domain-swapped Canecystatin-1. FEBS J. 2013, 280, 1028–1038. [Google Scholar] [CrossRef]
- Valdes-Rodriguez, S.; Cedro-Tanda, A.; Aguilar-Hernandez, V.; Cortes-Onofre, E.; Blanco-Labra, A.; Guerrero-Rangel, A. Recombinant Amaranth Cystatin (AhCPI) Inhibits the Growth of Phytopathogenic Fungi. Plant Physiol. Biochem. 2010, 48, 469–475. [Google Scholar] [CrossRef]
- Abraham, Z.; Martinez, M.; Carbonero, P.; Diaz, I. Structural and Functional Diversity within the Cystatin Gene Family of Hordeum Vulgare. J. Exp. Bot. 2006, 57, 4245–4255. [Google Scholar] [CrossRef]
- Musil, D.; Zucic, D.; Turk, D.; Engh, R.A.; Mayr, I.; Huber, R.; Popovic, T.; Turk, V.; Towatari, T.; Katunuma, N. The Refined 2.15 A X-Ray Crystal Structure of Human Liver Cathepsin B: The Structural Basis for Its Specificity. EMBO J. 1991, 10, 2321–2330. [Google Scholar] [CrossRef]
- Nycander, M.; Estrada, S.; Mort, J.S.; Abrahamson, M.; Björk, I. Two-Step Mechanism of Inhibition of Cathepsin B by Cystatin C Due to Displacement of the Proteinase Occluding Loop. FEBS Lett. 1998, 422, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.; Krupa, J.C.; Mort, J.S.; Abrahamson, M.; Björk, I. Cystatin Inhibition of Cathepsin B Requires Dislocation of the Proteinase Occluding Loop. Demonstration by Release of Loop Anchoring through Mutation of His110. FEBS Lett. 2000, 487, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Björk, I.; Pol, E.; Raub-Segall, E.; Abrahamson, M.; Rowan, A.D.; Mort, J.S. Differential Changes in the Association and Dissociation Rate Constants for Binding of Cystatins to Target Proteinases Occurring on N-Terminal Truncation of the Inhibitors Indicate That the Interaction Mechanism Varies with Different Enzymes. Biochem. J. 1994, 299, 219–225. [Google Scholar] [CrossRef]
- Hall, A.; Ekiel, I.; Mason, R.W.; Kasprzykowski, F.; Grubb, A.; Abrahamson, M. Structural Basis for Different Inhibitory Specificities of Human Cystatins C and D. Biochemistry 1998, 37, 4071–4079. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Fei, X.; Xia, L.; Qin, Z.; Liang, Z. Parkinson’s Disease Involves Autophagy and Abnormal Distribution of Cathepsin L. Neurosci. Lett. 2011, 489, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Li, Y.; Lu, Y.; Zhong, H.; Jiang, W.; Chen, A.F.; Billiar, T.R.; Yuan, H.; Cai, J. Cathepsin L Activity Correlates with Proteinuria in Chronic Kidney Disease in Humans. Int. Urol. Nephrol. 2017, 49, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L Targeting in Cancer Treatment. Pharmacol. Ther. 2015, 155, 105–116. [Google Scholar] [CrossRef]
- Gomes, C.P.; Fernandes, D.E.; Casimiro, F.; da Mata, G.F.; Passos, M.T.; Varela, P.; Mastroianni-Kirsztajn, G.; Pesquero, J.B. Cathepsin L in COVID-19: From Pharmacological Evidences to Genetics. Front. Cell. Infect. Microbiol. 2020, 10, 589505. [Google Scholar] [CrossRef]
- Laurance-Young, P.; Bozec, L.; Gracia, L.; Rees, G.; Lippert, F.; Lynch, R.J.M.; Knowles, J.C. A Review of the Structure of Human and Bovine Dental Hard Tissues and Their Physicochemical Behaviour in Relation to Erosive Challenge and Remineralisation. J. Dent. 2011, 39, 266–272. [Google Scholar] [CrossRef]
- Monteiro Júnior, J.E.; Valadares, N.F.; Pereira, H.D.; Dyszy, F.H.; da Costa Filho, A.J.; Uchôa, A.F.; de Oliveira, A.S.; da Silveira Carvalho, C.P.; Grangeiro, T.B. Expression in Escherichia coli of Cysteine Protease Inhibitors from Cowpea (Vigna unguiculata): The Crystal Structure of a Single-Domain Cystatin Gives Insights on Its Thermal and PH Stability. Int. J. Biol. Macromol. 2017, 102, 29–41. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Araújo, T.T.; Ventura, T.M.O.; Dionizio, A.; Câmara, J.V.F.; Moraes, S.M.; Pelá, V.T.; Martini, T.; Leme, J.C.; Derbotolli, A.L.B.; et al. Acquired Pellicle Protein-Based Engineering Protects against Erosive Demineralization. J. Dent. 2020, 102, 103478. [Google Scholar] [CrossRef] [PubMed]
- Huysmans, M.-C.; Young, A.; Ganss, C. The Role of Fluoride in Erosion Therapy. Monogr. Oral Sci. 2014, 25, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Pelá, V.T.; Buzalaf, M.A.R.; Niemeyer, S.H.; Baumann, T.; Henrique-Silva, F.; Toyama, D.; Crusca, E.; Marchetto, R.; Lussi, A.; Carvalho, T.S. Acquired Pellicle Engineering with Proteins/Peptides: Mechanism of Action on Native Human Enamel Surface. J. Dent. 2021, 107, 103612. [Google Scholar] [CrossRef] [PubMed]
- Pelá, V.T.; Niemeyer, S.H.; Baumann, T.; Levy, F.M.; Henrique-Silva, F.; Lussi, A.; Carvalho, T.S.; Buzalaf, M.A.R. Acquired Pellicle Engineering Using a Combination of Organic (Sugarcane Cystatin) and Inorganic (Sodium Fluoride) Components against Dental Erosion. Caries Res. 2022, 56, 138–145. [Google Scholar] [CrossRef]
- Santos, L.A.; Martini, T.; Câmara, J.V.F.; Reis, F.N.; Ortiz, A.C.; Camiloti, G.D.; Levy, F.M.; Shibao, P.Y.T.; Honorio, H.M.; Henrique-Silva, F.; et al. Solutions and Gels Containing a Sugarcane-Derived Cystatin (CaneCPI-5) Reduce Enamel and Dentin Erosion in Vitro. Caries Res. 2021, 55, 594–602. [Google Scholar] [CrossRef]
- Pelá, V.T.; Ventura, T.M.O.; Taira, E.A.; Thomassian, L.T.G.; Brito, L.; Matuhara, Y.E.; Henrique-Silva, F.; Groisman, S.; Carvalho, T.S.; Lussi, A.; et al. Use of Reflectometer Optipen to Assess the Preventive Effect of a Sugarcane Cystatin on Initial Dental Erosion in Vivo. J. Mech. Behav. Biomed. Mater. 2023, 141, 105782. [Google Scholar] [CrossRef]
- Delecrode, T.R.; Siqueira, W.L.; Zaidan, F.C.; Bellini, M.R.; Moffa, E.B.; Mussi, M.C.M.; Xiao, Y.; Buzalaf, M.A.R. Identification of Acid-Resistant Proteins in Acquired Enamel Pellicle. J. Dent. 2015, 43, 1470–1475. [Google Scholar] [CrossRef]
- Schlueter, N.; Klimek, J.; Ganss, C. Effect of Stannous and Fluoride Concentration in a Mouth Rinse on Erosive Tissue Loss in Enamel in Vitro. Arch. Oral Biol. 2009, 54, 432–436. [Google Scholar] [CrossRef]
- Schlueter, N.; Klimek, J.; Ganss, C. In Vitro Efficacy of Experimental Tin- and Fluoride-Containing Mouth Rinses as Anti-Erosive Agents in Enamel. J. Dent. 2009, 37, 944–948. [Google Scholar] [CrossRef]
- Zarella, B.L.; Cardoso, C.A.B.; Pelá, V.T.; Kato, M.T.; Tjäderhane, L.; Buzalaf, M.A.R. The Role of Matrix Metalloproteinases and Cysteine-Cathepsins on the Progression of Dentine Erosion. Arch. Oral Biol. 2015, 60, 1340–1345. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Charone, S.; Tjäderhane, L. Role of Host-Derived Proteinases in Dentine Caries and Erosion. Caries Res. 2015, 49, 30–37. [Google Scholar] [CrossRef] [PubMed]
- De Souza, B.M.; Santi, L.R.P.; de Souza Silva, M.; Buzalaf, M.A.R.; Magalhães, A.C. Effect of an Experimental Mouth Rinse Containing NaF and TiF 4 on Tooth Erosion and Abrasion in Situ. J. Dent. 2018, 73, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wegehaupt, F.J.; Zaruba, M.; Becker, K.; Roos, M.; Attin, T.; Wiegand, A. Erosion-Inhibiting Potential of a Stannous Chloride-Containing Fluoride Solution under Acid Flow Conditions in Vitro. Arch. Oral Biol. 2010, 55, 702–705. [Google Scholar] [CrossRef]
- Szymańska, A.; Radulska, A.; Czaplewska, P.; Grubb, A.; Grzonka, Z.; Rodziewicz-Motowidło, S. Governing the Monomer-Dimer Ratio of Human Cystatin c by Single Amino Acid Substitution in the Hinge Region. Acta Biochim. Pol. 2009, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bangrak, P.; Chotigeat, W. Molecular Cloning and Biochemical Characterization of a Novel Cystatin from Hevea rubber Latex. Plant Physiol. Biochem. 2011, 49, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Shyu, D.J.H.; Yong, Y.-M.; LU, H.-C.; Cheng, Y.-M.; Tzen, J.T.C.; Chou, W.-M. Cloning, Functional Expression and Characterization of a Phytocystatin Gene from Jelly Fig (Ficus awkeotsang Makino) Achenes. Bot. Stud. 2011, 52, 407–416. [Google Scholar]
- Shyu, D.J.H.; Chyan, C.-L.; Tzen, J.T.C.; Chou, W.-M. Molecular Cloning, Expression, and Functional Characterization of a Cystatin from Pineapple Stem. Biosci. Biotechnol. Biochem. 2004, 68, 1681–1689. [Google Scholar] [CrossRef]
- Belenghi, B.; Acconcia, F.; Trovato, M.; Perazzolli, M.; Bocedi, A.; Polticelli, F.; Ascenzi, P.; Delledonne, M. AtCYS1, a Cystatin from Arabidopsis thaliana, Suppresses Hypersensitive Cell Death. Eur. J. Biochem. 2003, 270, 2593–2604. [Google Scholar] [CrossRef]
- Jerala, R.; Trstenjak, M.; Lenarčič, B.; Turk, V. Cloning a Synthetic Gene for Human Stefin B and Its Expression in E. coli. FEBS Lett. 1988, 239, 41–44. [Google Scholar] [CrossRef]
| Inhibition Constant—Ki (nM) | |||
|---|---|---|---|
| Inhibitor | Papain | Cathepsin L | Cathepsin B |
| MaquiCPI-1 | 7.13 ± 1.30 | 0.34 ± 0.07 | 35.74 ± 0.84 |
| MaquiCPI-2 | 1.42 ± 0.39 | 0.33 ± 0.07 | 20.97 ± 3.78 |
| MaquiCPI-3 | 3.29 ± 0.58 | 0.38 ± 0.08 | 21.94 ± 3.01 |
| MaquiCPI-4 | 2.99 ± 0.34 | 0.57 ± 0.11 | 876.70 ± 53.99 |
| MaquiCPI-5 | 5.05 ± 0.82 | 1.25 ± 0.25 | 5470 ± 630 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, E.P.; Ferro, M.; Pelá, V.T.; Fernanda-Carlos, T.; Borges, C.G.G.; Taira, E.A.; Ventura, T.M.O.; Arencibia, A.D.; Buzalaf, M.A.R.; Henrique-Silva, F. Maquiberry Cystatins: Recombinant Expression, Characterization, and Use to Protect Tooth Dentin and Enamel. Biomedicines 2023, 11, 1360. https://doi.org/10.3390/biomedicines11051360
de Souza EP, Ferro M, Pelá VT, Fernanda-Carlos T, Borges CGG, Taira EA, Ventura TMO, Arencibia AD, Buzalaf MAR, Henrique-Silva F. Maquiberry Cystatins: Recombinant Expression, Characterization, and Use to Protect Tooth Dentin and Enamel. Biomedicines. 2023; 11(5):1360. https://doi.org/10.3390/biomedicines11051360
Chicago/Turabian Stylede Souza, Eduardo Pereira, Milene Ferro, Vinicius Taioqui Pelá, Thais Fernanda-Carlos, Cecília Guimarães Giannico Borges, Even Akemi Taira, Talita Mendes Oliveira Ventura, Ariel Domingo Arencibia, Marília Afonso Rabelo Buzalaf, and Flávio Henrique-Silva. 2023. "Maquiberry Cystatins: Recombinant Expression, Characterization, and Use to Protect Tooth Dentin and Enamel" Biomedicines 11, no. 5: 1360. https://doi.org/10.3390/biomedicines11051360
APA Stylede Souza, E. P., Ferro, M., Pelá, V. T., Fernanda-Carlos, T., Borges, C. G. G., Taira, E. A., Ventura, T. M. O., Arencibia, A. D., Buzalaf, M. A. R., & Henrique-Silva, F. (2023). Maquiberry Cystatins: Recombinant Expression, Characterization, and Use to Protect Tooth Dentin and Enamel. Biomedicines, 11(5), 1360. https://doi.org/10.3390/biomedicines11051360







