Morphologic Findings in the Cerebral Cortex in COVID-19: Association of Microglial Changes with Clinical and Demographic Variables
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Ischemic Stroke in COVID-19
4.2. Evidence of Brain Damage in COVID-19
4.3. Neurotropic Effect of SARS-CoV-2 Virus
4.4. Functions and Diagnostic Significance of the NeuN Protein
4.5. The Importance of NeuN Localization in Neurons
4.6. Functions of Microglia
4.7. Role of Microglia in Viral Infections
4.8. Role of Iba-1 in Microglial Activation
4.9. Changes in Iba-1 Expression in Different Diseases
4.10. Influence of Sex on the Pathogenesis of COVID-19
4.11. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 24 March 2023).
- Sisniega, D.C.; Reynolds, A.S. Severe Neurologic Complications of SARS-CoV-2. Curr. Treat. Options. Neurol. 2021, 23, 14. [Google Scholar] [CrossRef]
- Tsygan, N.V.; Trashkov, A.P.; Ryabtsev, A.V.; Yakovleva, V.A.; Konevega, A.L.; Vasiliev, A.G.; Tsygan, V.N.; Odinak, M.M.; Litvinenko, I.V. Signs and Symptoms of Central Nervous System Involvement and Their Pathogenesis in COVID-19 According to the Clinical Data (Review). Gen. Reanimatol. 2021, 17, 65–77. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res. 2020, 12, 69. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Laksono, B.M.; de Vrij, F.M.S.; Kushner, S.A.; Harschnitz, O.; van Riel, D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022, 45, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Arbour, N.; Day, R.; Newcombe, J.; Talbot, P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000, 74, 8913–8921. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Lewis, A.; Frontera, J.; Placantonakis, D.G.; Lighter, J.; Galetta, S.; Balcer, L.; Melmed, K.R. Cerebrospinal fluid in COVID-19: A systematic review of the literature. J. Neurol. Sci. 2021, 421, 117316. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Yang, Y.; Yu, W.; Zhao, Y.; Long, H.; Gao, J.; Ding, K.; Ma, C.; Li, J.; Zhao, S.; et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduct. Target Ther. 2021, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021, 13, eabf8396. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Bertozzi, G.; Cipolloni, L.; Baldari, B.; Cantatore, S.; D’Errico, S.; Di Mizio, G.; Asmundo, A.; Castorina, S.; Salerno, M.; et al. Clinical-Forensic Autopsy Findings to Defeat COVID-19 Disease: A Literature Review. J. Clin. Med. 2020, 9, 2026. [Google Scholar] [CrossRef]
- Polak, S.B.; Van Gool, I.C.; Cohen, D.; von der Thüsen, J.H.; van Paassen, J. A systematic review of pathological findings in COVID-19: A pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020, 33, 2128–2138. [Google Scholar] [CrossRef]
- Deshmukh, V.; Motwani, R.; Kumar, A.; Kumari, C.; Raza, K. Histopathological observations in COVID-19: A systematic review. J. Clin. Pathol. 2021, 74, 76–83. [Google Scholar] [CrossRef]
- Hanley, B.; Lucas, S.B.; Youd, E.; Swift, B.; Osborn, M. Autopsy in suspected COVID-19 cases. J. Clin. Pathol. 2020, 73, 239–242. [Google Scholar] [CrossRef]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Mukerji, S.S.; Solomon, I.H. What can we learn from brain autopsies in COVID-19? Neurosci. Lett. 2021, 742, 135528. [Google Scholar] [CrossRef]
- Zhang, P.P.; He, Z.C.; Yao, X.H.; Tang, R.; Ma, J.; Luo, T.; Zhu, C.; Li, T.R.; Liu, X.; Zhang, D.; et al. COVID-19-associated monocytic encephalitis (CAME): Histological and proteomic evidence from autopsy. Signal Transduct. Target Ther. 2023, 8, 24. [Google Scholar] [CrossRef]
- Agrawal, S.; Farfel, J.M.; Arfanakis, K.; Al-Harthi, L.; Shull, T.; Teppen, T.L.; Evia, A.M.; Patel, M.B.; Ely, E.W.; Leurgans, S.E.; et al. Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profile of acute vascular injury, inflammation and age-linked chronic brain diseases. Acta Neuropathol. Commun. 2022, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.T.; Miller, E.H.; Glendinning, M.D.; Al-Dalahmah, O.; Banu, M.A.; Boehme, A.K.; Boubour, A.L.; Bruce, S.S.; Chong, A.M.; Claassen, J.; et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021, 144, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Cama, V.F.; Marín-Prida, J.; Acosta-Rivero, N.; Acosta, E.F.; Díaz, L.O.; Casadesús, A.V.; Fernández-Marrero, B.; Gilva-Rodríguez, N.; Cremata-García, D.; Cervantes-Llanos, M.; et al. The microglial NLRP3 inflammasome is involved in human SARS-CoV-2 cerebral pathogenicity: A report of three post-mortem cases. J. Neuroimmunol. 2021, 361, 577728. [Google Scholar] [CrossRef]
- Jeong, G.U.; Lyu, J.; Kim, K.D.; Chung, Y.C.; Yoon, G.Y.; Lee, S.; Hwang, I.; Shin, W.H.; Ko, J.; Lee, J.Y.; et al. SARS-CoV-2 Infection of Microglia Elicits Proinflammatory Activation and Apoptotic Cell Death. Microbiol. Spect. 2022, 10, e0109122. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, G.; Bocci, T.; Falleni, M.; Campiglio, L.; Coppola, S.; Tosi, D.; Chiumello, D.; Priori, A. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J. Neurol. 2021, 268, 4486–4491. [Google Scholar] [CrossRef]
- Poloni, T.E.; Moretti, M.; Medici, V.; Turturici, E.; Belli, G.; Cavriani, E.; Visonà, S.D.; Rossi, M.; Fantini, V.; Ferrari, R.R.; et al. COVID-19 Pathology in the Lung, Kidney, Heart and Brain: The Different Roles of T-Cells, Macrophages, and Microthrombosis. Cells 2022, 11, 3124. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Perl, D.P.; Steiner, J.; Pasternack, N.; Li, W.; Maric, D.; Safavi, F.; Horkayne-Szakaly, I.; Jones, R.; Stram, M. Net al Neurovascular injury with complement activation and inflammation in COVID-19. Brain 2022, 145, 2555–2568. [Google Scholar] [CrossRef]
- Stefano, G.B.; Büttiker, P.; Weissenberger, S.; Martin, A.; Ptacek, R.; Kream, R.M. Editorial: The Pathogenesis of Long-Term Neuropsychiatric COVID-19 and the Role of Microglia, Mitochondria, and Persistent Neuroinflammation: A Hypothesis. Med. Sci. Monit. 2021, 27, e933015. [Google Scholar] [CrossRef]
- Current Guidelines of the Ministry of Health of the Russian Federation (Version 11). Available online: https://xn--80aesfpebagmfblc0a.xn--p1ai/ai/doc/872/attach/Bmr_COVID-19_compressed.pdf (accessed on 24 March 2023). (In Russian).
- Current Guidelines of the Ministry of Health of the Russian Federation (Version 14). Available online: https://xn--80aesfpebagmfblc0a.xn--p1ai/ai/doc/1213/attach/vmr_COVID-19_V14_27-12-2021.pdf (accessed on 24 March 2023). (In Russian).
- Luo, W.; Liu, X.; Bao, K.; Huang, C. Ischemic stroke associated with COVID-19: A systematic review and meta-analysis. J. Neurol. 2022, 269, 1731–1740. [Google Scholar] [CrossRef]
- Beach, T.G.; Sue, L.I.; Intorcia, A.J.; Glass, M.J.; Walker, J.E.; Arce, R.; Nelson, C.M.; Serrano, G.E. Acute Brain Ischemia, Infarction and Hemorrhage in Subjects Dying with or without Autopsy-Proven Acute Pneumonia. Preprint. medRxiv 2021. [Google Scholar] [CrossRef]
- McIlwain, D.L.; Hoke, V.B. The role of the cytoskeleton in cell body enlargement, increased nuclear eccentricity and chromatolysis in axotomized spinal motor neurons. BMC Neurosci. 2005, 6, 19. [Google Scholar] [CrossRef]
- Dahmen, H.G.; Müller, W. Luxol-fast-blue-staining of nerve cell nucleoli as indicator for acidotic lesion in cases of intracranial hypertension? Acta Neuropathol. 1978, 42, 243–246. [Google Scholar] [CrossRef]
- Villadiego, J.; García-Arriaza, J.; Ramírez-Lorca, R.; García-Swinburn, R.; Cabello-Rivera, D.; Rosales-Nieves, A.E.; Álvarez-Vergara, M.I.; Cala-Fernández, F.; García-Roldán, E.; López-Ogáyar, J.L.; et al. Full protection from SARS-CoV-2 brain infection and damage in susceptible transgenic mice conferred by MVA-CoV2-S vaccine candidate. Nat. Neurosci. 2023, 26, 226–238. [Google Scholar] [CrossRef]
- Beckman, D.; Bonillas, A.; Diniz, G.B.; Ott, S.; Roh, J.W.; Elizaldi, S.R.; Schmidt, B.A.; Sammak, R.L.; Van Rompay, K.K.A.; Iyer, S.S.; et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 2022, 41, 111573. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Eschbacher, K.L.; Larsen, R.A.; Moyer, A.M.; Majumdar, R.; Reichard, R.R. Neuropathological findings in COVID-19: An autopsy cohort. J. Neuropathol. Exp. Neurol. 2022, 82, 21–28. [Google Scholar] [CrossRef]
- Alekseeva, O.S.; Guselnikova, V.V.; Beznin, G.V.; Korzhevsky, D.E. Prospects for the application of neun nuclear protein as a marker of the functional state of nerve cells in vertebrates. J. Evol. Biochem. Phys. 2015, 51, 357–369. [Google Scholar] [CrossRef]
- Shen, C.C.; Yang, Y.C.; Chiao, M.T.; Cheng, W.Y.; Tsuei, Y.S.; Ko, J.L. Characterization of endogenous neural progenitor cells after experimental ischemic stroke. Curr. Neurovasc. Res. 2010, 7, 6–14. [Google Scholar] [CrossRef]
- Davoli, M.A.; Fourtounis, J.; Tam, J.; Xanthoudakis, S.; Nicholson, D.; Robertson, G.S.; Ng, G.Y.; Xu, D. Immunohistochemical and biochemical assessment of caspase-3 activation and DNA fragmentation following transient focal ischemia in the rat. Neuroscience 2002, 115, 125–136. [Google Scholar] [CrossRef]
- Sugawara, T.; Lewén, A.; Noshita, N.; Gasche, Y.; Chan, P.H. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J. Neurotrauma 2002, 19, 85–98. [Google Scholar] [CrossRef]
- Luijerink, L.; Waters, K.A.; Machaalani, R. Immunostaining for NeuN Does Not Show all Mature and Healthy Neurons in the Human and Pig Brain: Focus on the Hippocampus. Appl. Immunohistochem. Mol. Morphol. 2021, 29, e46–e56. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Greenamyre, J.T. NeuN is not a reliable marker of dopamine neurons in rat substantia nigra. Neurosci. Lett. 2009, 464, 14–17. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, Y.P.; Hou, Z.; Huang, C.; Zhu, H.; Zhang, C.Q.; Yin, Q. Novel Insights into NeuN: From Neuronal Marker to Splicing Regulator. Mol. Neurobiol. 2016, 53, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Adelstein, R.S.; Kawamoto, S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J. Biol. Chem. 2009, 284, 31052–31061. [Google Scholar] [CrossRef] [PubMed]
- Unal-Cevik, I.; Kilinç, M.; Gürsoy-Ozdemir, Y.; Gurer, G.; Dalkara, T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: A cautionary note. Brain Res. 2004, 1015, 169–174. [Google Scholar] [CrossRef]
- Kornack, D.R.; Rakic, P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. USA 1999, 96, 5768–5773. [Google Scholar] [CrossRef]
- Van Nassauw, L.; Wu, M.; De Jonge, F.; Adriaensen, D.; Timmermans, J.P. Cytoplasmic, but not nuclear, expression of the neuronal nuclei (NeuN) antibody is an exclusive feature of Dogiel type II neurons in the guinea-pig gastrointestinal tract. Histochem. Cell. Biol. 2005, 124, 369–377. [Google Scholar] [CrossRef]
- Maxeiner, S.; Glassmann, A.; Kao, H.T.; Schilling, K. The molecular basis of the specificity and cross-reactivity of the NeuN epitope of the neuron-specific splicing regulator, Rbfox3. Histochem. Cell. Biol. 2014, 141, 43–55. [Google Scholar] [CrossRef]
- Eggen, B.J.; Raj, D.; Hanisch, U.K.; Boddeke, H.W. Microglial phenotype and adaptation. J. Neuroimmune Pharmacol. 2013, 8, 807–823. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: Unique stimuli, specialized responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Perkins, A.E.; Piazza, M.K.; Deak, T. Stereological Analysis of Microglia in Aged Male and Female Fischer 344 Rats in Socially Relevant Brain Regions. Neuroscience 2018, 377, 40–52. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Hovens, I.B.; Nyakas, C.; Schoemaker, R.G. A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining: Cell body to cell size ratio. Neuroimmunol. Neuroinflamm. 2014, 1, 82–88. [Google Scholar] [CrossRef]
- Filgueira, L.; Larionov, A.; Lannes, N. The Influence of Virus Infection on Microglia and Accelerated Brain Aging. Cells 2021, 10, 1836. [Google Scholar] [CrossRef]
- Enlow, W.; Bordeleau, M.; Piret, J.; Ibáñez, F.G.; Uyar, O.; Venable, M.-C.; Goyette, N.; Carbonneau, J.; Tremblay, M.-E.; Boivin, G. Microglia are involved in phagocytosis and extracellular digestion during Zika virus encephalitis in young adult immunodeficient mice. J. Neuroinflamm. 2021, 18, 178. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, D.; Li, G. The role of microglia in viral encephalitis: A review. J. Neuroinflamm. 2019, 16, 76. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 is an actin-cross-linking protein in macrophages/microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef]
- Shi, F.J.; Xie, H.; Zhang, C.Y.; Qin, H.F.; Zeng, X.W.; Lou, H.; Zhang, L.; Xu, G.T.; Zhang, J.F.; Xu, G.X. Is Iba-1 protein expression a sensitive marker for microglia activation in experimental diabetic retinopathy? Int. J. Ophthalmol. 2021, 14, 200–208. [Google Scholar] [CrossRef]
- Cserép, C.; Pósfai, B.; Dénes, Á. Shaping Neuronal Fate: Functional Heterogeneity of Direct Microglia-Neuron Interactions. Neuron 2021, 109, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Lituma, P.J.; Woo, E.; O’Hara, B.F.; Castillo, P.E.; Sibinga, N.E.S.; Nandi, S. Altered synaptic connectivity and brain function in mice lacking microglial adapter protein Iba1. Proc. Natl. Acad. Sci. USA 2021, 118, e2115539118. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.; Imai, Y.; Kohsaka, S.; Kimura, Y. Upregulated expression of Iba1 molecules in the central nervous system of mice in response to neurovirulent influenza A virus infection. Microbiol. Immunol. 2000, 44, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Tanaka, K.; Suzuki, S.; Dembo, T.; Fukuuchi, Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke 2021, 32, 1208–1215. [Google Scholar] [CrossRef]
- Serrano, G.E.; Walker, J.E.; Tremblay, C.; Piras, I.S.; Huentelman, M.J.; Belden, C.M.; Goldfarb, D.; Shprecher, D.; Atri, A.; Adler, C.H.; et al. SARS-CoV-2 Brain Regional Detection, Histopathology, Gene Expression, and Immunomodulatory Changes in Decedents with COVID-19. J. Neuropathol. Exp. Neurol. 2022, 81, 666–695. [Google Scholar] [CrossRef]
- Bechmann, N.; Barthel, A.; Schedl, A.; Herzig, S.; Varga, Z.; Gebhard, C.; Mayr, M.; Hantel, C.; Beuschlein, F.; Wolfrum, C.; et al. Sexual dimorphism in COVID-19: Potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022, 10, 221–230. [Google Scholar] [CrossRef]
- Escarcega, R.D.; Honarpisheh, P.; Colpo, G.D.; Ahnstedt, H.W.; Couture, L.; Juneja, S.; Torres, G.; Ortiz, G.J.; Sollome, J.; Tabor, N.; et al. Sex differences in global metabolomic profiles of COVID-19 patients. Cell Death Dis. 2022, 13, 461. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Long COVID syndrome-associated brain fog. J. Med. Virol. 2022, 94, 979–984. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, Ó.J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef]
- Michelutti, M.; Furlanis, G.; Buoite Stella, A.; Bellavita, G.; Frezza, N.; Torresin, G.; Ajčević, M.; Manganotti, P. Sex-dependent characteristics of Neuro-Long-COVID: Data from a dedicated neurology ambulatory service. J. Neurol. Sci. 2022, 441, 120355. [Google Scholar] [CrossRef]
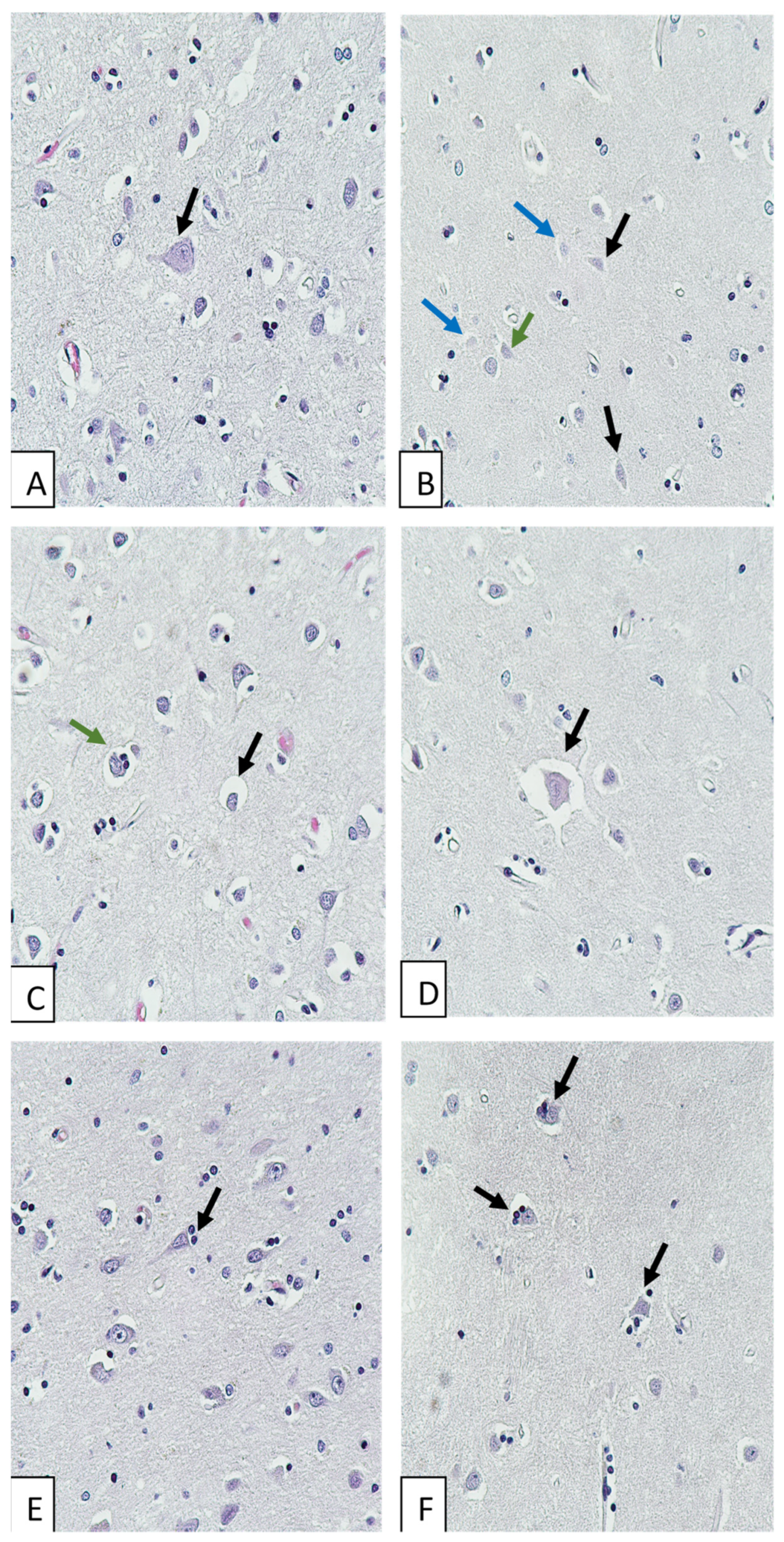
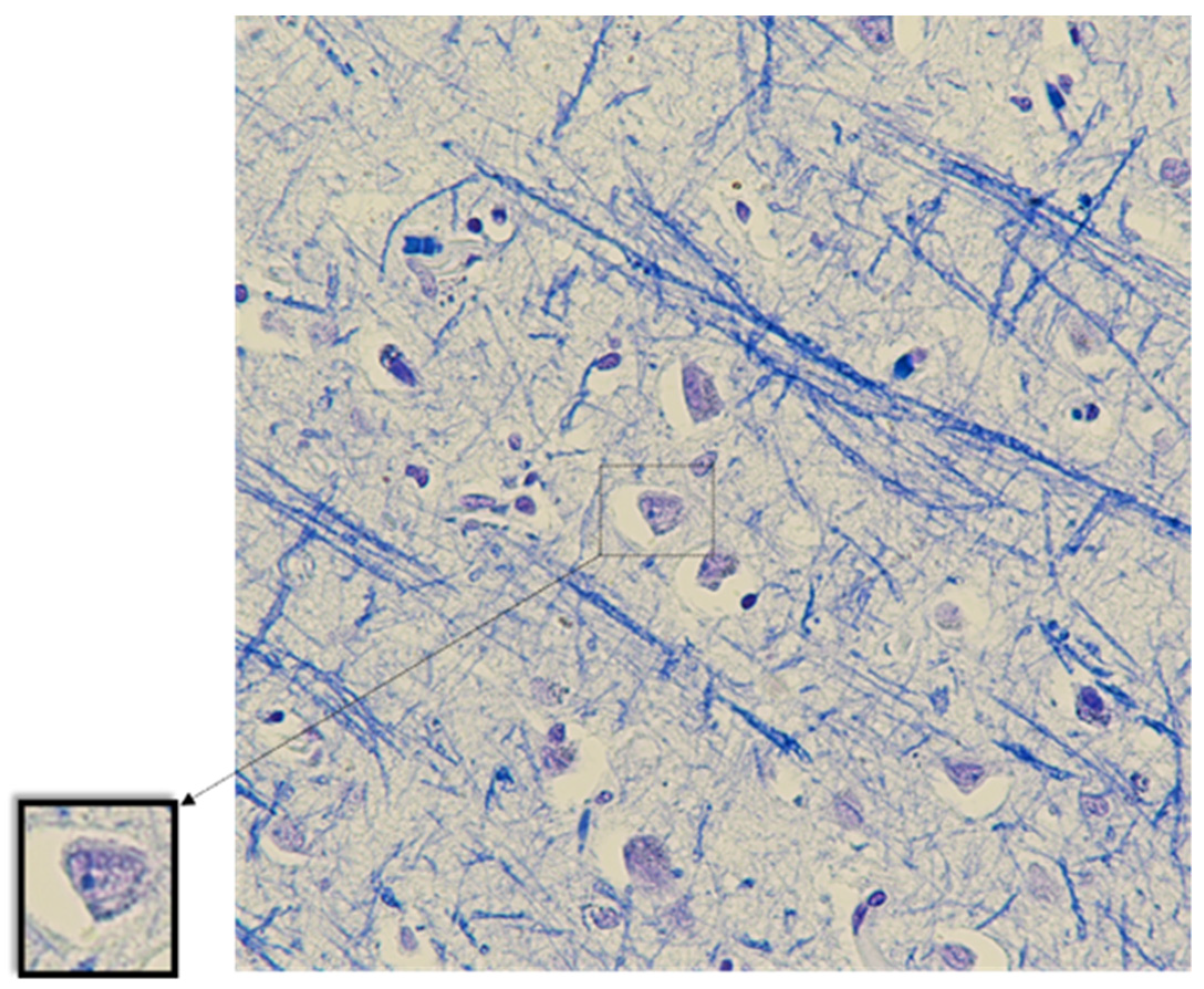



| No | Sex | Age, y | Direct Cause of Death | Comorbidity | Type of ACVA |
|---|---|---|---|---|---|
| 1 | M | 35 | Massive thromboembolism of the right and left pulmonary arteries. Right atrial auricular thrombosis. | Hypertension with predominant cardiac involvement: heart weight 560 g, concentric myocardial hypertrophy, left ventricular wall thickness 1.8 cm. Atherosclerosis of aorta and iliac arteries (stage II, grade 1), carotid arteries (stage I, grade 1), coronary arteries (stage II, grade 2, 40% stenosis). | Intracerebral hemorrhages of hematoma type in the region of subcortical nuclei of the left cerebral hemisphere. |
| 2 | F | 92 | Cerebral edema with compression of the cerebellum into the foramen magnum. Severe pulmonary edema. | Hypertension with predominant heart and kidney damage. Atherosclerosis of the cerebral basal arteries (stage II, grade 2, 40% stenosis). CHD. | Ischemic infarction in all lobes of the right cerebral hemisphere with hemorrhagic component. |
| 3 | F | 81 | Cerebral edema. Severe pulmonary edema. Ischemic infarction of kidney (according to histologic examination) | Hypertension with predominant heart and kidney damage. Atherosclerosis of the cerebral basal arteries (stage II, grade 2, 40% stenosis), mass lesion of the dura mater of the anterior cranial fossa (angiomatous meningioma microscopically). | Ischemic infarction of the frontal lobe of the right cerebral hemisphere. |
| 4 | M | 70 | Cerebral edema. Severe pulmonary edema | Previous myocardial infarction of the posterior wall of the left ventricle. 80% stenotic atherosclerosis of the coronary arteries (stage IV). Hypertension with predominant heart and kidney damage. Previous acute cerebrovascular accident in the right and left cerebral hemispheres: large brown cysts in the temporal lobe of the right hemisphere, lacunar cysts in the subcortical nuclei of the right and left hemispheres, in the white matter of the temporal lobe of the left hemisphere. Atherosclerosis of the cerebral basal arteries (stage II, grade 3, 70% stenosis). | Ischemic infarction of the occipital lobe of the right cerebral hemisphere with a hemorrhagic component. |
| 5 | F | 83 | Pulmonary edema. Pulmonary artery thrombosis | Hypertension: Atherosclerosis of coronary arteries (fibrous plaques, stenosis up to 50%); cerebral basal arteries (fibrous plaques, stenosis up to 50%). | Secondary focus of necrosis of the left occipital lobe. |
| Parameters | Value | |
|---|---|---|
| N | 18 | |
| Confirmed COVID-19 diagnosis | 18 (100%) | |
| Sex (M) | 9 (50%) | |
| Age (years) | 64.5 (44.8–73.3), range: 18–90 | |
| Disease duration (days), N = 13 | 14 (10–23), range: 9–28 | |
| Disease duration ≥ 14 days | 7/13 (54%) | |
| Concomitant/competing cause of death | 5 (28%) | |
| Year of death | 2021 2022 | 11 (61%) 7 (39%) |
| Intracranial artery stenosis | 0% 20% 40% 50% | 7 (39%) 2 (11%) 7 (39%) 2 (11%) |
| Computed tomography data | CT, 1–25% involvement | 3 (17%) |
| CT, 26–49% involvement | 3 (17%) | |
| CT, 50–75% involvement | 3 (17%) | |
| CT, 76–100% involvement | 8 (44%) | |
| No data | 1 (6%) | |
| Mechanical ventilation | 9 (50%) | |
| Hospital length of stay (days) | 8 (5–10), range: 1–18 | |
| Comorbidity | ||
| Diabetes mellitus | 4 (22%) | |
| Chronic obstructive pulmonary disease | 1 (6.0%) | |
| Coronary heart disease | 8 (44%) | |
| Hypertension | 14 (78%) | |
| Obesity | 7 (39%) | |
| Bacterial pneumonia | 6 (33%) | |
| Morphological Parameters | ||
| Number of neurons (NeuN), N/specimen | 40.7 (8.5–68.6) | |
| Number of microgliocytes, N/specimen | 27.8 (18.7–30.8) | |
| Microglia in close contact with neurons, N/specimen | 3.2 (2.2–4.4) | |
| Integral optical density of microglia, conv. units | 7.3 (6.7–8.1) | |
| Average microgliocyte area, µm2 | 56.5 (54.4–70.6) | |
| Parameters | Number of Neurons (NeuN), N/Specimen | Number of Microgliocytes, N/Specimen | Microglia in Close Contact with Neurons, N/Specimen | Int. OD, conv. Units | Average Microgliocyte Area, µm2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥40, N = 9 | <40, N = 9 | p | ≥28, N = 9 | <28, N = 9 | p | ≥3, N = 9 | < 3, N = 9 | p | ≥7.3, N = 9 | <7.3, N = 9 | p | ≥56.5, N = 9 | <56.5, N = 9 | p | |
| Sex (M) | 3, 33.3% | 6, 66.7% | 0.3 | 3, 33.3% | 6, 66.7% | 0.3 | 2, 22.2% | 7, 77.8% | 0.02 * | 4, 44.4 % | 5, 55.6% | 0.9 | 3, 33.3% | 6, 66.7% | 0.3 |
| Age (years) | 62 (45–72) | 71 (49–82) | 0.5 | 62 (32–71) | 71 (54–85) | 0.2 | 62 (45–71) | 71 (42–85) | 0.4 | 70 (61–77) | 47 (29–73) | 0.3 | 70 (41–73) | 62 (46–77) | 0.9 |
| Hospital length of stay (days) | 9 (5–14) | 6 (5–9) | 0.3 | 6 (4–11) | 8 (6–11) | 0.4 | 7 (4–11) | 8 (6–11) | 0.5 | 6 (4–8) | 10 (6–12) | 0.2 | 6 (3–10) | 8 (6–13) | 0.2 |
| Disease duration (days) | 18 (11–22) | 12 (10–25) | 0.8 | 11 (10–19) | 20 (10–24) | 0.4 | 11 (9–17) | 21 (12–25) | 0.09 | 10 (9–13) | 23 (15–25) | 0.01 * | 12 (10–25) | 18 (11–22) | 0.8 |
| Disease duration ≥ 14 days | 5/7, 71.4% | 2/6, 33.3% | 0.3 | 3/6, 50.0% | 4/7, 57.1% | 0.9 | 4/6, 66.7% | 3/7, 42.9% | 0.6 | 1/6, 16.7% | 6/7, 85.1% | 0.03 * | 3/7, 42.9% | 4/6, 66.7% | 0.6 |
| Concomitant/competing cause of death | 4, 44.4% | 1, 11.1% | 0.3 | 4, 44.4% | 1, 11.1% | 0.3 | 4, 44.4% | 1, 11.1% | 0.3 | 3, 33.3% | 2, 22.2% | 0.9 | 2, 22.2% | 3, 33.3% | 0.9 |
| Presence of intracranial artery stenosis | 5, 55.6% | 6, 66.7% | 0.9 | 5, 55.6% | 6, 66.7% | 0.9 | 6, 66.7% | 5, 55.6% | 0.9 | 8, 88.9% | 3, 33.3% | 0.05 | 5, 55.6% | 6, 66.7% | 0.9 |
| Year of death (2022) | 3, 33.3% | 4, 44.4% | 0.9 | 3, 33.3% | 4, 44.4% | 0.9 | 4, 44.4% | 3, 33.3% | 0.9 | 3, 33.3% | 4, 44.4% | 0.9 | 3, 33.3% | 4, 44.4% | 0.9 |
| CT, 76–100% involvement | 4/8, 50.0% | 4/9, 44.4% | 0.9 | 5/8, 62.5% | 3/9, 33.3% | 0.3 | 5/8, 62.5% | 3/9, 33.3% | 0.3 | 5/8, 62.5% | 3/9, 33.3% | 0.3 | 5/8, 62.5% | 3/9, 33.3% | 0.3 |
| Mechanical ventilation | 4, 44.4% | 5, 55.6% | 0.9 | 5, 55.6% | 4, 44.4% | 0.9 | 5, 55.6% | 4, 44.4% | 0.9 | 4, 44.4% | 5, 55.6% | 0.9 | 5, 55.6% | 4, 44.4% | 0.9 |
| Diabetes mellitus | 3, 33.3% | 1, 11.1% | 0.6 | 3, 33.3% | 1, 11.1% | 0.6 | 3, 33.3% | 1, 11.1% | 0.6 | 2, 22.2% | 2, 22.2% | 0.9 | 2, 22.2% | 2, 22.2% | 0.9 |
| Chronic obstructive pulmonary disease | 1, 11.1% | 0, 0% | 0.9 | 0, 0% | 1, 11.1% | 0.9 | 0, 0% | 1, 11.1% | 0.9 | 0, 0% | 1, 11.1% | 0.9 | 0, 0% | 1, 11.1% | 0.9 |
| Coronary heart disease | 3, 33.3% | 5, 55.6% | 0.7 | 3, 33.3% | 5, 55.6% | 0.7 | 3, 33.3% | 5, 55.6% | 0.7 | 6, 66.7% | 2, 22.2% | 0.2 | 4, 44.4% | 4, 44.4% | 0.9 |
| Hyperten-sion | 7, 77.8% | 7, 77.8% | 0.9 | 6, 66.7% | 8, 88.9% | 0.6 | 7, 77.8% | 7, 77.8% | 0.9 | 8, 88.9% | 6, 66.7% | 0.6 | 6, 66.7% | 8, 88.9% | 0.6 |
| Obesity | 3, 33.3% | 4, 44.4% | 0.9 | 2, 22.2% | 5, 55.6% | 0.3 | 3, 33.3% | 4, 44.4% | 0.9 | 3, 33.3% | 4, 44.4% | 0.9 | 5, 55.6% | 2, 22.2% | 0.3 |
| Bacterial pneumonia | 2, 22.2% | 4, 44.4% | 0.6 | 2, 22.2% | 4, 44.4% | 0.6 | 3, 33.3% | 3, 33.3% | 0.9 | 3, 33.3% | 3, 33.3% | 0.9 | 1, 11.1% | 5, 55.6% | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babkina, A.S.; Yadgarov, M.Y.; Lyubomudrov, M.A.; Ostrova, I.V.; Volkov, A.V.; Kuzovlev, A.N.; Grechko, A.V.; Golubev, A.M. Morphologic Findings in the Cerebral Cortex in COVID-19: Association of Microglial Changes with Clinical and Demographic Variables. Biomedicines 2023, 11, 1407. https://doi.org/10.3390/biomedicines11051407
Babkina AS, Yadgarov MY, Lyubomudrov MA, Ostrova IV, Volkov AV, Kuzovlev AN, Grechko AV, Golubev AM. Morphologic Findings in the Cerebral Cortex in COVID-19: Association of Microglial Changes with Clinical and Demographic Variables. Biomedicines. 2023; 11(5):1407. https://doi.org/10.3390/biomedicines11051407
Chicago/Turabian StyleBabkina, Anastasiya S., Mikhail Ya. Yadgarov, Maxim A. Lyubomudrov, Irina V. Ostrova, Alexey V. Volkov, Artem N. Kuzovlev, Andrey V. Grechko, and Arkady M. Golubev. 2023. "Morphologic Findings in the Cerebral Cortex in COVID-19: Association of Microglial Changes with Clinical and Demographic Variables" Biomedicines 11, no. 5: 1407. https://doi.org/10.3390/biomedicines11051407









