Evaluation of Folate-Functionalized Nanoparticle Drug Delivery Systems—Effectiveness and Concerns
Abstract
:1. Introduction
2. Current Updates on the Clinical Trials
3. Methodology
| References | Nanoparticles’ Composition | Payload | Disease | Summary |
|---|---|---|---|---|
| [11] | Folate-coated PEG-conjugated graphene oxide | Protocatechuic acid | Hepatocellular carcinoma | Folate-coated nanoparticles demonstrated better anticancer activity than non-targeting nanoparticles and free protocatechuic acid in vitro. |
| [12] | Folate-coated PEG-conjugated graphene oxide | Protocatechuic acid and chlorogenic acid | Hepatocellular carcinoma | Folate-coated nanoparticles demonstrated better anticancer activity than non-targeting nanoparticles and free drugs in vitro. |
| [13] | Folate-conjugated single-wall carbon nanotube | ITPA siRNA | Cancers | The folate- conjugated single-wall carbon nanotube with an average length of >450 nm is better for FR-mediated endocytosis in vitro. |
| [14] | 64Cu-radiolabeled folate-targeting liposomes | Copper chelator, DOTA | Cancers | Both tumor accumulation and circulation properties of liposomes may be lost due to functionalization. |
| [15] | Folate-functionalized mesoporous nanostructured silica systems | Triphenyl tin (IV) derivative | FR-overexpressed cancer cells | The nanoparticles are more active in targeting FR-overexpressed cancer cells when the quantity of functionalized folic acid is higher. |
| [16] | Folate-conjugated casein micelles | Monacan, Anka Flavin, and resveratrol | Breast cancer | The folate-conjugated micelle has similar cytotoxicity to the PEGylated phytozoa casein nanoparticle. Both nanoparticles have significantly higher cytotoxicity than free drugs. Significant reduction of body weight loss, tumor weight and volume, suppression of aromatase, NF-dB, VEGF, CD-1, and elevation of Caspase-3 demonstrated by both nanoparticles in vivo. |
| [17] | Folate-targeted liposomes composed of DOPE, cholesterol, DSPE-MPEG, and folate-peptide | Methotrexate | Arthritis | The folate-targeting liposomes have lower renal and hepatic elimination than the free methotrexate (MTX). The liposomes dosage equivalent to 2 mg/kg MTX, twice weekly, is similar to or better than 35 mg/kg MTX at reducing the swelling in the mice model. |
| [18] | Folate-targeting hyperbranched polyethylenimine-graft-polycaprolactone-block-PEG | siRNA | Ovarian cancer | The nanoparticles demonstrated excellent siRNA delivery profiles in vitro. The in vivo tumor uptake is affected by the route of administration. The intraperitoneal injection showed better tumor deposition over intravenous administration. |
| [19] | β-cyclodextrin and folic acid covalently conjugated to branched polyethylenimine | - | - | Developed new method for the nanoparticles’ synthesis. The FR-binding study using Lewis lung carcinoma suggests the conformation of the folate ligand on the surface is essential to maximize the binding. |
| [20] | Folate-chitosan-lipid conjugate | Cisplatin | Cancers | Significant increase in cytotoxicity, cell cycle arrest, and cellular uptake for folate-functionalized nanoparticles than the non-targeting nanoparticles. |
| [21] | Folic acid and fluorescein isothiocyanate conjugated to a PEG core | - | Hepatocellular carcinoma | The PEG enhanced the solubility of folic acid. The study suggests that intra-arterial administration is more efficient for targeted HCC detection than intravenous delivery. |
| [22] | Nanosuspension composed of DSPE-PEG-FA and soybean lecithin | Annonaceous acetogenins (ACGs) | Cancers | The folate-functionalized nanosuspension demonstrated significantly higher cytotoxicity against HeLa cells and better tumor growth inhibition in vivo than the non-targeting nanosuspension. |
| [23] | Folate-chitosan- selenium nanoparticles | FLuc mRNA | Cancers | Significant transgene expression for the FR-positive KB cells than other cells with little or negative FR. |
| [24] | Folate-functionalized PEG-modified PAMAM G4 dendrimers | 5-fluorouracil (5-FU) and technetium-99 m (99mTc) | Breast cancer | The nanoparticles are highly internalized by the 4 T1 cells than C2C12. The data suggest that PEG helps to reduce cytotoxicity. The complex significantly reduced the tumor volume in the mice model compared to the control group. The complex significantly accumulates in the liver and tumor tissue. |
| [25] | Folate-modified PEG liposomes, primarily using SPC, DC- Cholesterol, and DSPE-PEG | Oleuropein | Prostate cancer | The nanoparticles significantly inhibit cell viability more than plain oleuropein solution. The intravenous pharmacokinetic profile shows six times increase in AUC for nanoparticles than free oleuropein. The folate-modified nanoparticles increase weight loss resistance, tumor suppression, and survival probability in the mice. |
| [26] | Folate-functionalized pluronic micelles | Fisetin | Breast cancer | The folate-functionalized micelles demonstrated higher anticancer activity than non-targeting micelles and free fisetin in vitro against FR-overexpressed human breast cancer MCF-7 cell line. |
| [27] | Folate-functionalized PEG-coated liposomes | Fluorescent DiD/ 3H-cholesteryl hexadecyl ether/ Betamethasone | FR-positive immune cells in inflammatory diseases (e.g., colitis and atherosclerosis | The folate-targeted liposomes selectively bind to the FR-positive RAW 264.7 murine macrophage cell line in vitro and accumulate at inflammation sites in atherosclerosis and colitis murine models. |
| [28] | Folate-decorated poly(ε-caprolactone)-poly(ethylene glycol), PEG-PCL | - | - | PEG length particularly affects folate exposition and protein interaction. The nanoparticles with PEG length (2.0 kDa) are smaller than a shorter PEG length (1.0 kDa). The zeta potential is slightly negative, as typically exhibited by PEGylated nanoparticles. The characterization suggests the PEG moiety is in a mushroom conformation. The study suggests reduced nanoparticle uptake in human macrophages due to PEGylation. Significant uptake in FR+ KB cells than FR– A549. |
| [29] | Folic acid-dimethyl indole red (Dir)- bovine serum albumin (BSA) | siRNA | Cancers | Dir demonstrated selective noncovalent interaction with BSA than human serum albumin (HSA). The nanoparticles demonstrated fluorescent properties suitable for the targeted tumor cell imaging in vitro. |
| [30] | Folate-displaying exosome | siRNA | Cancers | Demonstrated that the delivery of the siRNA payload is unaffected by endosomal trapping. |
| [31] | Folate-functionalized PEGylated cyclodextrin | Docetaxel and siRNA | Colorectal cancer | The folate-functionalized nanoparticles demonstrated an enhanced apoptotic effect of docetaxel and downregulation of Re1A expression against CT26 cell lines and mouse model. |

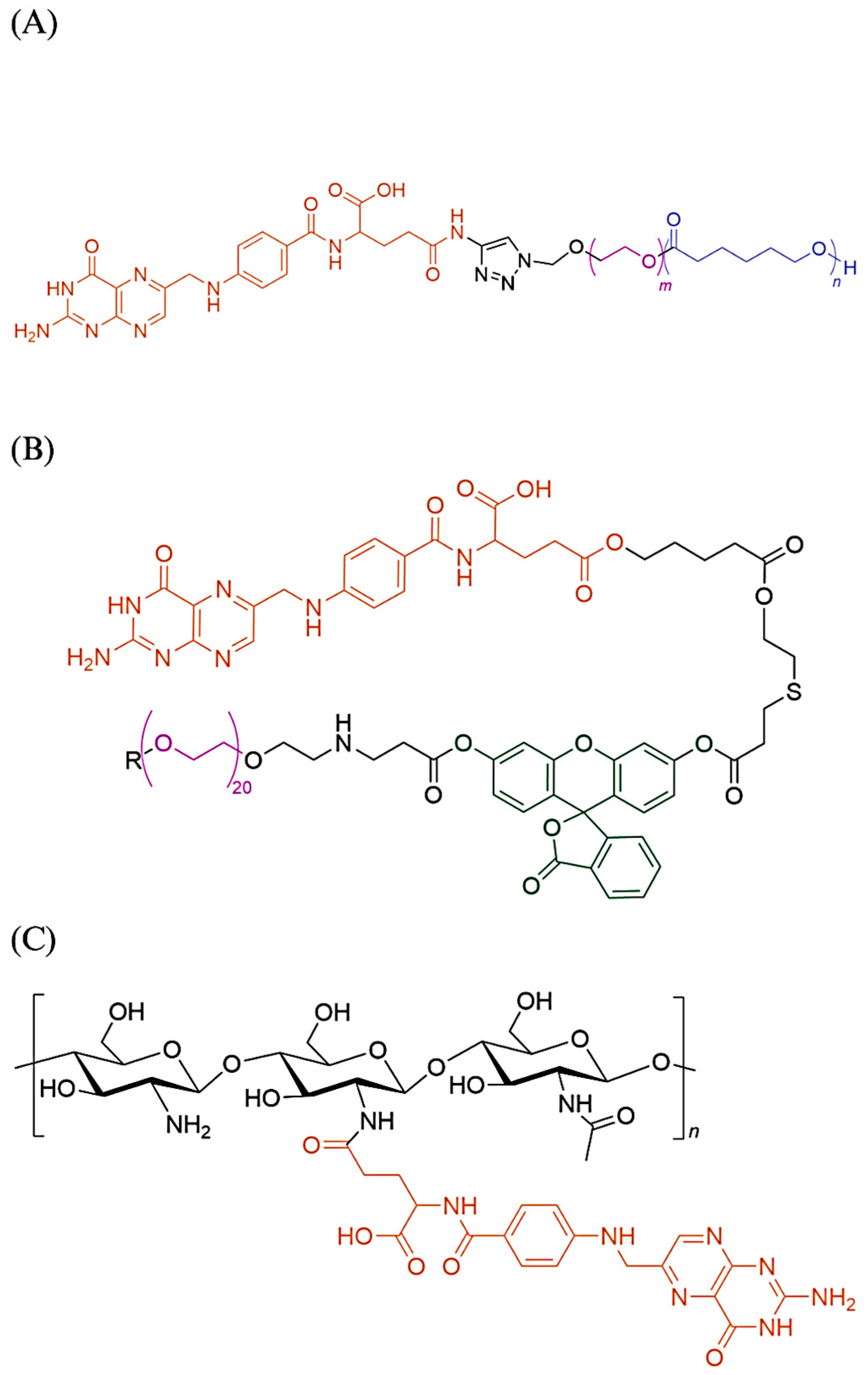
4. Discussion
4.1. Factors Affecting Folate-Functionalized Nanocarrier Effectiveness
4.2. Concerns Regarding Folate-Functionalized Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Rana, A.; Bhatnagar, S. Advancements in folate receptor targeting for anti-cancer therapy: A small molecule-drug conjugate approach. Bioorg. Chem. 2021, 112, 104946. [Google Scholar] [CrossRef]
- Leamon, C.P.; Reddy, J.A. Folate-targeted chemotherapy. Adv. Drug Deliv. Rev. 2004, 56, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Kamen, B.A.; Capdevila, A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc. Natl. Acad. Sci. USA 1986, 83, 5983–5987. [Google Scholar] [CrossRef]
- Cheng, X.; Li, J.; Tanaka, K.; Majumder, U.; Milinichik, A.Z.; Verdi, A.C.; Maddage, C.J.; Rybinski, K.A.; Fernando, S.; Fernando, D.; et al. MORAb-202, an antibody–drug conjugate utilizing humanized anti-human FRa farletuzumab and the microtubule-targeting agent eribulin, has potent antitumor activity. Mol. Cancer Ther. 2018, 17, 2665–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, T.; Fujiwara, Y.; Yonemori, K.; Koyama, T.; Sato, J.; Tamura, K.; Shimomura, A.; Ikezawa, H.; Nomoto, M.; Furuuchi, K.; et al. First-in-human phase 1 study of MORAb-202, an antibody–Drug conjugate comprising farletuzumab linked to Eribulin Mesylate, in patients with folate receptor-a–Positive advanced solid tumors. Clin. Cancer Res. 2021, 27, 3905–3915. [Google Scholar] [CrossRef]
- Moore, K.N.; Borghaei, H.; O’Malley, D.M.; Jeong, W.; Seward, S.M.; Bauer, T.M.; Perez, R.P.; Matulonis, U.A.; Running, K.L.; Zhang, X.; et al. Phase 1 Dose-Escalation Study of Mirvetuximab Soravtansine (IMGN853), a Folate Receptor α-Targeting Antibody-Drug Conjugate, in Patients With Solid Tumors. Cancer 2017, 123, 3080–3087. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.N.; Martin, L.P.; O’Malley, D.M.; Matulonis, U.A.; Konner, J.A.; Perez, R.P.; Bauer, T.M.; Ruiz-Soto, R.; Birrer, M.J. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: A phase i expansion study. J. Clin. Oncol. 2017, 35, 1112–1118. [Google Scholar] [CrossRef] [Green Version]
- O’Malley, D.M.; Matulonis, U.A.; Birrer, M.J.; Castro, C.M.; Gilbert, L.; Vergote, I.; Martin, L.P.; Mantia-Smaldone, G.M.; Martin, A.G.; Bratos, R.; et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2020, 157, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; O’Malley, D.M.; Vergote, I.; Martin, L.P.; Gonzalez-Martin, A.; Malek, K.; Birrer, M.J. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 2018, 151, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buskaran, K.; Bullo, S.; Hussein, M.Z.; Masarudin, M.J.; Mohd Moklas, M.A.; Fakurazi, S. Anticancer molecular mechanism of protocatechuic acid loaded on folate coated functionalized graphene oxide nanocomposite delivery system in human hepatocellular carcinoma. Materials 2021, 14, 817. [Google Scholar] [CrossRef]
- Buskaran, K.; Hussein, M.Z.; Moklas, M.A.M.; Masarudin, M.J.; Fakurazi, S. Graphene oxide loaded with protocatechuic acid and chlorogenic acid dual drug nanodelivery system for human hepatocellular carcinoma therapeutic application. Int. J. Mol. Sci. 2021, 22, 5786. [Google Scholar] [CrossRef]
- Charbgoo, F.; Nikkhah, M.; Behmanesh, M. Size of single-wall carbon nanotube affects the folate receptor-mediated cancer cell targeting. Biotechnol. Appl. Biochem. 2018, 65, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Henriksen, J.R.; Jørgensen, J.T.; Amitay, Y.; Shmeeda, H.; Gabizon, A.A.; Kjær, A.; Andresen, T.L.; Hansen, A.E. Folate receptor targeting of radiolabeled liposomes reduces intratumoral liposome accumulation in human KB carcinoma xenografts. Int. J. Nanomed. 2018, 13, 7647–7656. [Google Scholar] [CrossRef] [Green Version]
- Díaz-García, D.; Montalbán-Hernández, K.; Mena-Palomo, I.; Achimas-Cadariu, P.; Rodríguez-Diéguez, A.; López-Collazo, E.; Prashar, S.; Paredes, K.O.; Filice, M.; Fischer-Fodor, E.; et al. Role of folic acid in the therapeutic action of nanostructured porous silica functionalized with organotin(IV) compounds against different cancer cell lines. Pharmaceutics 2020, 12, 512. [Google Scholar] [CrossRef]
- El-Far, S.W.; Helmy, M.W.; Khattab, S.N.; Bekhit, A.A.; Hussein, A.A.; Elzoghby, A.O. Folate conjugated vs PEGylated phytosomal casein nanocarriers for codelivery of fungal- and herbal-derived anticancer drugs. Nanomedicine 2018, 13, 1463–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, D.; Lager, F.; Renault, G.; Guezguez, J.; Burnet, M.; Cunha, J.; Cavaco-Paulo, A.; Nogueira, E. Folate-Targeted Liposomal Formulations Improve Effects of Methotrexate in Murine Collagen-Induced Arthritis. Biomedicines 2022, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.K.; Douglas, K.; Shields, A.F.; Merkel, O.M. Correlating quantitative tumor accumulation and gene knockdown using SPECT/CT and bioluminescence imaging within an orthotopic ovarian cancer model. Biomaterials 2018, 178, 183–192. [Google Scholar] [CrossRef]
- Kasprzak, A.; Grudzinski, I.P.; Bamburowicz-Klimkowska, M.; Parzonko, A.; Gawlak, M.; Poplawska, M. New Insight into the Synthesis and Biological Activity of the Polymeric Materials Consisting of Folic Acid and β-Cyclodextrin. Macromol. Biosci. 2018, 18, 1700289. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Filipczak, N.; Pan, J.; Rehman, M.; Rai, N.; Attia, S.A.; Torchilin, V.P. Folate targeted lipid chitosan hybrid nanoparticles for enhanced anti-tumor efficacy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102228. [Google Scholar] [CrossRef]
- Koirala, N.; Das, D.; Fayazzadeh, E.; Sen, S.; McClain, A.; Puskas, J.E.; Drazba, J.A.; McLennan, G. Folic acid conjugated polymeric drug delivery vehicle for targeted cancer detection in hepatocellular carcinoma. J. Biomed. Mater. Res. Part A 2019, 107, 2522–2535. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Ao, H.; Bi, D.; Han, M.; Guo, Y.; Wang, X. Folate-targeting annonaceous acetogenins nanosuspensions: Significantly enhanced antitumor efficacy in hela tumor-bearing mice. Drug Deliv. 2018, 25, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Maiyo, F.; Singh, M. Folate-targeted mRNA delivery using chitosan- functionalized selenium nanoparticles: Potential in cancer immunotherapy. Pharmaceuticals 2019, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Narmani, A.; Arani, M.A.A.; Mohammadnejad, J.; Vaziri, A.Z.; Solymani, S.; Yavari, K.; Talebi, F.; Darzi, S.J. Breast Tumor Targeting with PAMAM-PEG-5FU-99mTc as a New Therapeutic Nanocomplex: In In-vitro and In-vivo studies. Biomed. Microdevices 2020, 22, 31. [Google Scholar] [CrossRef]
- Nassir, A.M.; Ibrahim, I.A.A.; Md, S.; Waris, M.; Tanuja Ain, M.R.; Ahmad, I.; Shahzad, N. Surface functionalized folate targeted oleuropein nano-liposomes for prostate tumor targeting: In vitro and in vivo activity. Life Sci. 2019, 220, 136–146. [Google Scholar] [CrossRef]
- Pawar, A.; Singh, S.; Rajalakshmi, S.; Shaikh, K.; Bothiraja, C. Development of fisetin-loaded folate functionalized pluronic micelles for breast cancer targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 347–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poh, S.; Chelvam, V.; Ayala-López, W.; Putt, K.S.; Low, P.S. Selective liposome targeting of folate receptor positive immune cells in inflammatory diseases. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Venuta, A.; Moret, F.; Poggetto, G.D.; Esposito, D.; Fraix, A.; Avitabile, C.; Ungaro, F.; Malinconico, M.; Sortino, S.; Romanelli, A.; et al. Shedding light on surface exposition of poly(ethylene glycol) and folate targeting units on nanoparticles of poly(ε-caprolactone) diblock copolymers: Beyond a paradigm. Eur. J. Pharm. Sci. 2018, 111, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, G.; Chen, H.; Zan, Y.; Hong, S.; Zhang, T.; Zhang, Y.; Pei, R. Folic acid-modified fluorescent dye-protein nanoparticles for the targeted tumor cell imaging. Talanta 2019, 194, 643–648. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Z.; Xu, C.; Guo, B.; Guo, P. Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping. J. Control. Release 2019, 311–312, 43–49. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Fotticchia, I.; Tirino, P.; Moret, F.; Pagano, B.; Gref, R.; Ungaro, F.; Reddi, E.; Giancola, C.; Quaglia, F. Cyclodextrin-assisted assembly of PEGylated polyester nanoparticles decorated with folate. Colloids Surf. B Biointerfaces 2016, 141, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Buyanova, M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjug. Chem. 2019, 30, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Tzemach, D.; Gorin, J.; Mak, L.; Amitay, Y.; Shmeeda, H.; Zalipsky, S. Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models. Cancer Chemother. Pharmacol. 2010, 66, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Leamon, C.P.; Reddy, J.A.; Dorton, R.; Bloomfield, A.; Emsweller, K.; Parker, N.; Westrick, E. Impact of high and low folate diets on tissue folate receptor levels and antitumor responses toward folate-drug conjugates. J. Pharmacol. Exp. Ther. 2008, 327, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, A.S.; Singh, M.; Reeder, K.M.; Carter, J.J.; Kovach, A.R.; Meng, W.; Ratnam, M.; Zhang, F.; Dann, C.E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. USA 2013, 110, 15180–15188. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Doshi, N.; Mitragotri, S. Adaptive micro and nanoparticles: Temporal control over carrier properties to facilitate drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- McNeeley, K.M.; Karathanasis, E.; Annapragada, A.V.; Bellamkonda, R.V. Masking and triggered unmasking of targeting ligands on nanocarriers to improve drug delivery to brain tumors. Biomaterials 2009, 30, 3986–3995. [Google Scholar] [CrossRef]

| Problems | Potential Solutions |
|---|---|
| Significant liver accumulation | Alternative administration route [18]. PEGylated nanoparticles are less efficiently taken by liver phagocytes. PEG-shielded surface capable of dePEGylation at the target site [38]. Multiple doses of pre-injected folic acid or sustained-release folic acid [14]. An inhibitor selectively targeting activated FR-β macrophages instead of FR-α tumor. |
| Formation of protein corona | Introducing (2-hydroxypropyl)-β-cyclodextrin (HPβCD) into the formulation to promote the PEG chain extension and folate exposition. The usage of shorter PEG length [28]. |
| Endosomal trapping | More investigation is required to understand the endosomal escape mechanism [33]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.A.I.; Othman, R.; Chee, C.F.; Ahmad Fisol, F. Evaluation of Folate-Functionalized Nanoparticle Drug Delivery Systems—Effectiveness and Concerns. Biomedicines 2023, 11, 2080. https://doi.org/10.3390/biomedicines11072080
Ibrahim MAI, Othman R, Chee CF, Ahmad Fisol F. Evaluation of Folate-Functionalized Nanoparticle Drug Delivery Systems—Effectiveness and Concerns. Biomedicines. 2023; 11(7):2080. https://doi.org/10.3390/biomedicines11072080
Chicago/Turabian StyleIbrahim, Muhammad Aiman Irfan, Rozana Othman, Chin Fei Chee, and Faisalina Ahmad Fisol. 2023. "Evaluation of Folate-Functionalized Nanoparticle Drug Delivery Systems—Effectiveness and Concerns" Biomedicines 11, no. 7: 2080. https://doi.org/10.3390/biomedicines11072080





