Effects of Mesenchymal Stem Cell Injection into Healed Myocardial Infarction Scar Border Zone on the Risk of Ventricular Tachycardia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Creation of a Rabbit MI Model
2.2. Rabbit MSC Isolation and Culture
2.3. Identification and Differentiation of MSCs
| GAPDH | F | aacatcatccctgcctctactg |
| R | ctccgacgcctgcttcac | |
| CD44 | F | acaccacggatttctgaccac |
| R | actgctgccacttctctctacat | |
| CD29 | F | cgagtacaccatgagccactatta |
| R | gatgacattgctggagtttgc |
2.4. Electrophysiologic Study (EPS) and Echocardiography
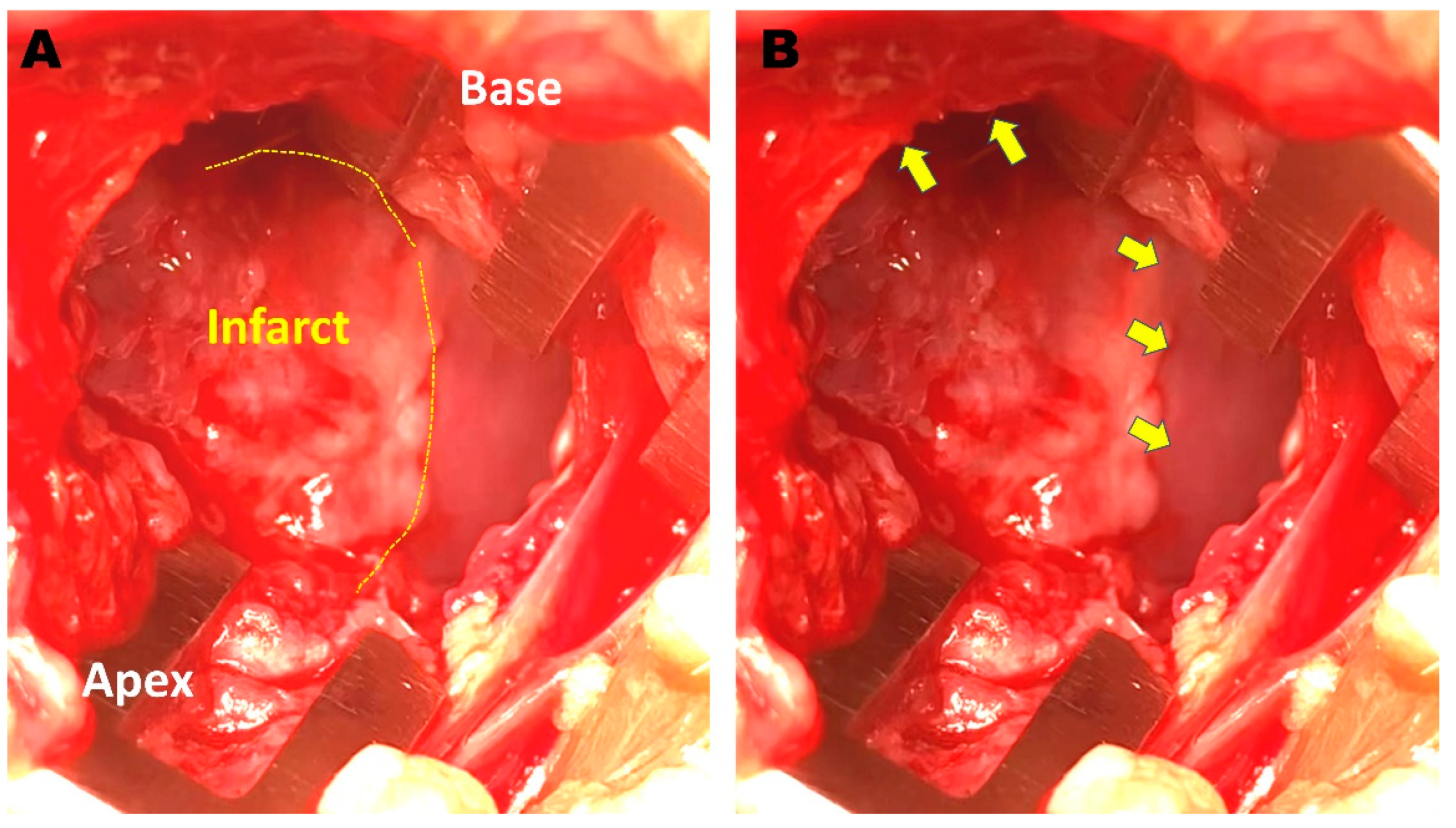
2.5. Cell Injection and ILR Implantation
2.6. Repeated EPS and Histologic Examination
2.7. Statistical Analysis
3. Results
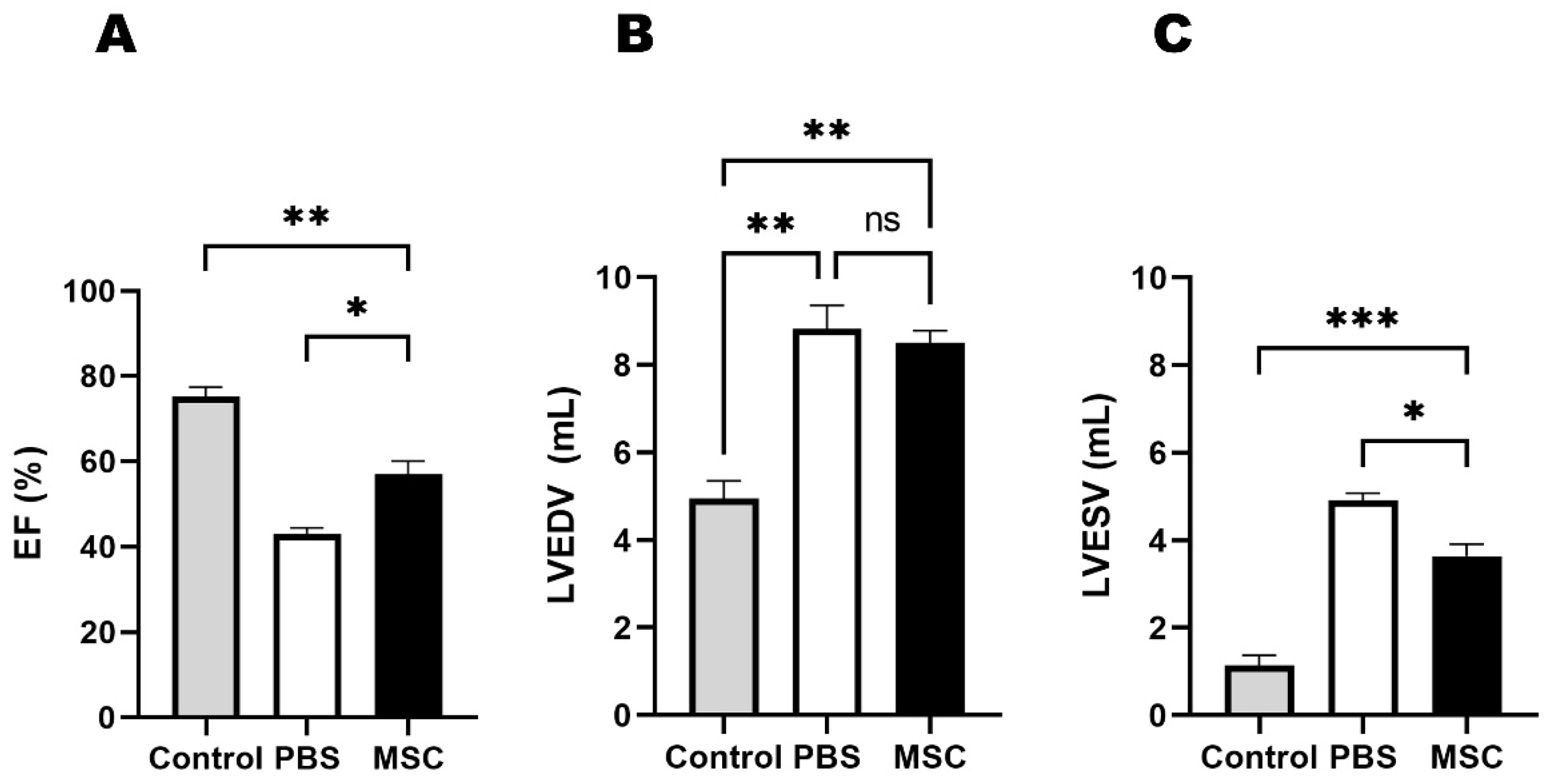
3.1. Echocardiographic Results
3.2. VT Inducibility
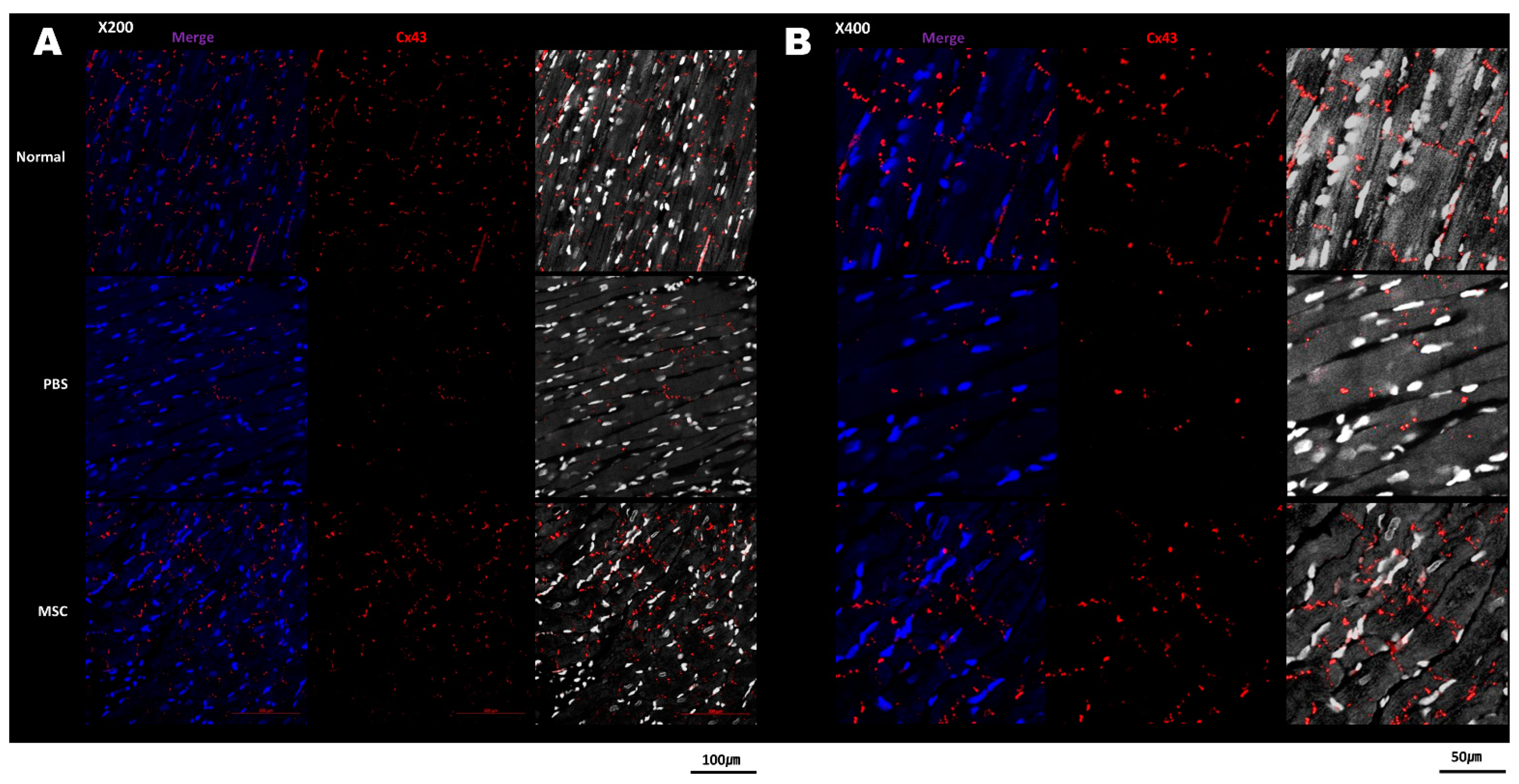
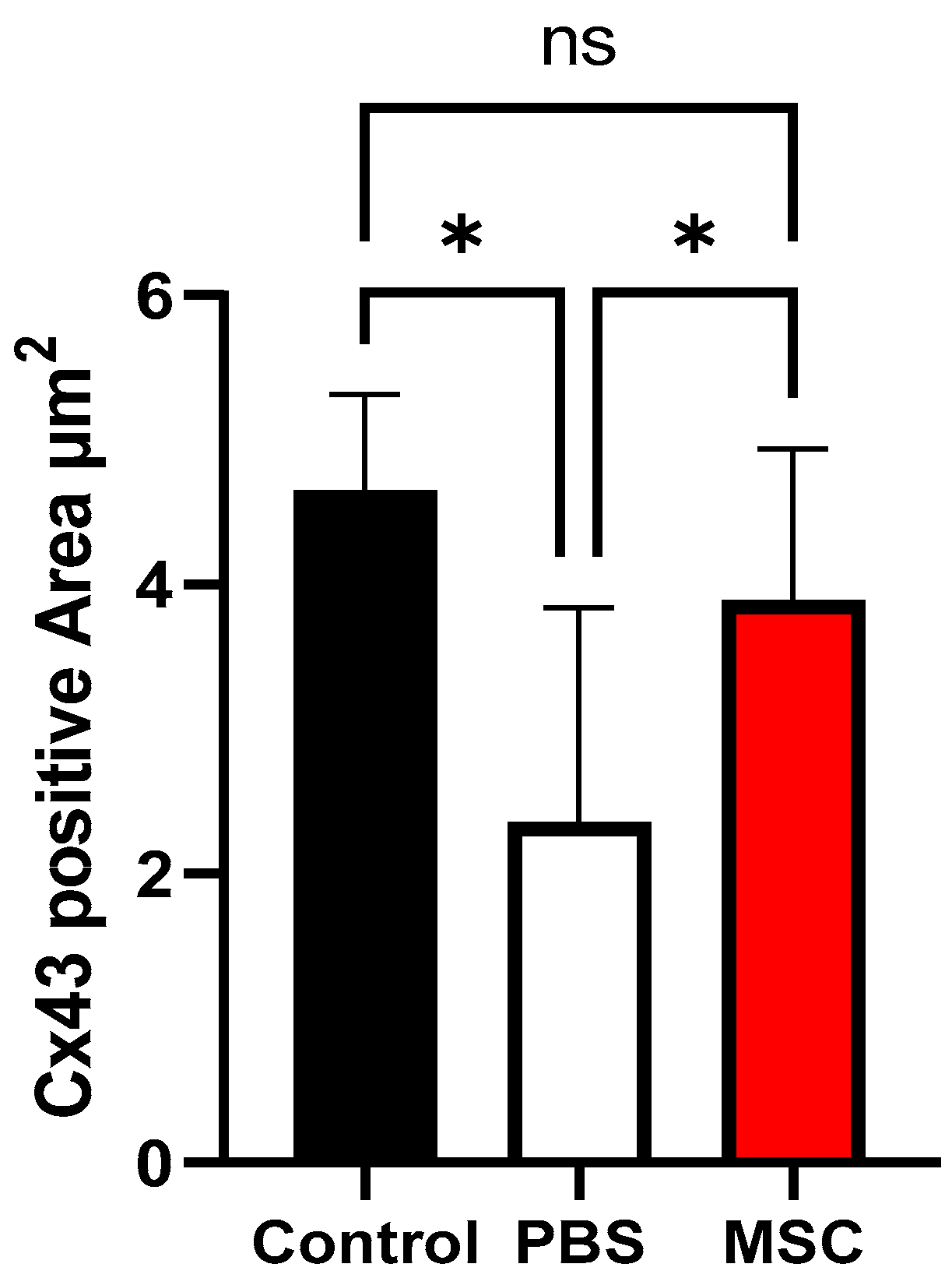
3.3. Histology and Cx43 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shivkumar, K. Catheter Ablation of Ventricular Arrhythmias. N. Engl. J. Med. 2019, 380, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabes, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Josephson, M.E.; Anter, E. Substrate Mapping for Ventricular Tachycardia: Assumptions and Misconceptions. JACC Clin. Electrophysiol. 2015, 1, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.G.; Friedman, P.L.; Sager, P.T.; Saxon, L.A.; Kocovic, D.; Harada, T.; Wiener, I.; Khan, H. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J. Am. Coll. Cardiol. 1997, 29, 1180–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palaniswamy, C.; Kolte, D.; Harikrishnan, P.; Khera, S.; Aronow, W.S.; Mujib, M.; Mellana, W.M.; Eugenio, P.; Lessner, S.; Ferrick, A.; et al. Catheter ablation of postinfarction ventricular tachycardia: Ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm 2014, 11, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Vaseghi, M.; Frankel, D.S.; Vergara, P.; Di Biase, L.; Nagashima, K.; Yu, R.; Vangala, S.; Tseng, C.H.; Choi, E.K.; et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm 2015, 12, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Dinov, B.; Fiedler, L.; Schonbauer, R.; Bollmann, A.; Rolf, S.; Piorkowski, C.; Hindricks, G.; Arya, A. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: Results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014, 129, 728–736. [Google Scholar] [CrossRef]
- Karantalis, V.; Schulman, I.H.; Balkan, W.; Hare, J.M. Allogeneic cell therapy: A new paradigm in therapeutics. Circ. Res. 2015, 116, 12–15. [Google Scholar] [CrossRef]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA 2014, 311, 62–73. [Google Scholar] [CrossRef]
- Amoni, M.; Vermoortele, D.; Ekhteraei-Tousi, S.; Donate Puertas, R.; Gilbert, G.; Youness, M.; Thienpont, B.; Willems, R.; Roderick, H.L.; Claus, P.; et al. Heterogeneity of Repolarization and Cell-Cell Variability of Cardiomyocyte Remodeling Within the Myocardial Infarction Border Zone Contribute to Arrhythmia Susceptibility. Circ. Arrhythm. Electrophysiol. 2023, 16, e011677. [Google Scholar] [CrossRef]
- Chang, M.G.; Tung, L.; Sekar, R.B.; Chang, C.Y.; Cysyk, J.; Dong, P.; Marban, E.; Abraham, M.R. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation 2006, 113, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Dib, N.; Michler, R.E.; Pagani, F.D.; Wright, S.; Kereiakes, D.J.; Lengerich, R.; Binkley, P.; Buchele, D.; Anand, I.; Swingen, C.; et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: Four-year follow-up. Circulation 2005, 112, 1748–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, G.P.; Wollert, K.C.; Lotz, J.; Steffens, J.; Lippolt, P.; Fichtner, S.; Hecker, H.; Schaefer, A.; Arseniev, L.; Hertenstein, B.; et al. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 2006, 113, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepstein, L. Electrophysiologic implications of myocardial stem cell therapies. Heart Rhythm 2008, 5, S48–S52. [Google Scholar] [CrossRef]
- Roell, W.; Lewalter, T.; Sasse, P.; Tallini, Y.N.; Choi, B.R.; Breitbach, M.; Doran, R.; Becher, U.M.; Hwang, S.M.; Bostani, T.; et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 2007, 450, 819–824. [Google Scholar] [CrossRef]
- Panda, N.C.; Zuckerman, S.T.; Mesubi, O.O.; Rosenbaum, D.S.; Penn, M.S.; Donahue, J.K.; Alsberg, E.; Laurita, K.R. Improved conduction and increased cell retention in healed MI using mesenchymal stem cells suspended in alginate hydrogel. J. Interv. Card. Electrophysiol. 2014, 41, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhang, F.; Shen, W.; Chen, M.; Yang, B.; Zhang, Y.; Cao, K. Mesenchymal stem cell injection ameliorates the inducibility of ventricular arrhythmias after myocardial infarction in rats. Int. J. Cardiol. 2011, 152, 314–320. [Google Scholar] [CrossRef]
- Maizels, L.; Gepstein, L. Gap junctions, stem cells, and cell therapy: Rhythmic/arrhythmic implications. Heart Rhythm 2012, 9, 1512–1516. [Google Scholar] [CrossRef]
- Macia, E.; Boyden, P.A. Stem cell therapy is proarrhythmic. Circulation 2009, 119, 1814–1823. [Google Scholar] [CrossRef] [Green Version]
- De Ferrari, G.M.; Rordorf, R.; Frattini, F.; Petracci, B.; De Filippo, P.; Landolina, M. Predictive value of programmed ventricular stimulation in patients with ischaemic cardiomyopathy: Implications for the selection of candidates for an implantable defibrillator. Europace 2007, 9, 1151–1157. [Google Scholar] [CrossRef]
- Rutherford, S.L.; Trew, M.L.; Sands, G.B.; LeGrice, I.J.; Smaill, B.H. High-resolution 3-dimensional reconstruction of the infarct border zone: Impact of structural remodeling on electrical activation. Circ. Res. 2012, 111, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Temirkhanova, K.; Saparov, A. Novel Therapies for the Treatment of Cardiac Fibrosis Following Myocardial Infarction. Biomedicines 2022, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Ursell, P.C.; Gardner, P.I.; Albala, A.; Fenoglio, J.J., Jr.; Wit, A.L. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ. Res. 1985, 56, 436–451. [Google Scholar] [CrossRef]
- Gutstein, D.E.; Morley, G.E.; Tamaddon, H.; Vaidya, D.; Schneider, M.D.; Chen, J.; Chien, K.R.; Stuhlmann, H.; Fishman, G.I. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res. 2001, 88, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mills, W.R.; Mal, N.; Kiedrowski, M.J.; Unger, R.; Forudi, F.; Popovic, Z.B.; Penn, M.S.; Laurita, K.R. Stem cell therapy enhances electrical viability in myocardial infarction. J. Mol. Cell Cardiol. 2007, 42, 304–314. [Google Scholar] [CrossRef]
- Leobon, B.; Garcin, I.; Menasche, P.; Vilquin, J.T.; Audinat, E.; Charpak, S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. P. Natl. Acad. Sci. USA 2003, 100, 7808–7811. [Google Scholar] [CrossRef]
- Martinez-Falguera, D.; Iborra-Egea, O.; Galvez-Monton, C. iPSC Therapy for Myocardial Infarction in Large Animal Models: Land of Hope and Dreams. Biomedicines 2021, 9, 1836. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.J.; Park, J.H.; Lee, S.; Park, B.W.; Lee, S.M.; Hwang, J.W.; Kim, J.J.; Kang, B.; Sim, W.S.; et al. Enhancement strategy for effective vascular regeneration following myocardial infarction through a dual stem cell approach. Exp. Mol. Med. 2022, 54, 1165–1178. [Google Scholar] [CrossRef]
- Choo, E.H.; Lee, J.H.; Park, E.H.; Park, H.E.; Jung, N.C.; Kim, T.H.; Koh, Y.S.; Kim, E.; Seung, K.B.; Park, C.; et al. Infarcted Myocardium-Primed Dendritic Cells Improve Remodeling and Cardiac Function After Myocardial Infarction by Modulating the Regulatory T Cell and Macrophage Polarization. Circulation 2017, 135, 1444–1457. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, R.; Nakayama, K.; Itoh, M.; Kamohara, K.; Furukawa, K.; Oyama, J.I.; Node, K.; Morita, S. Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease. J. Heart Lung Transpl. 2016, 35, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Spoel, T.I.G.; Vrijsen, K.R.; Koudstaal, S.; Sluijter, J.P.G.; Nijsen, J.F.W.; de Jong, H.W.; Hoefer, I.E.; Cramer, M.J.M.; Doevendans, P.A.; van Belle, E.; et al. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: A study on delivery efficiency. J. Cell Mol. Med. 2012, 16, 2768–2776. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.-H.; Kim, J.-M.; Seong, E.; Lee, E.; Chang, K.; Choi, Y. Effects of Mesenchymal Stem Cell Injection into Healed Myocardial Infarction Scar Border Zone on the Risk of Ventricular Tachycardia. Biomedicines 2023, 11, 2141. https://doi.org/10.3390/biomedicines11082141
Park E-H, Kim J-M, Seong E, Lee E, Chang K, Choi Y. Effects of Mesenchymal Stem Cell Injection into Healed Myocardial Infarction Scar Border Zone on the Risk of Ventricular Tachycardia. Biomedicines. 2023; 11(8):2141. https://doi.org/10.3390/biomedicines11082141
Chicago/Turabian StylePark, Eun-Hye, Jin-Moo Kim, EunHwa Seong, Eunmi Lee, Kiyuk Chang, and Young Choi. 2023. "Effects of Mesenchymal Stem Cell Injection into Healed Myocardial Infarction Scar Border Zone on the Risk of Ventricular Tachycardia" Biomedicines 11, no. 8: 2141. https://doi.org/10.3390/biomedicines11082141





