The Pig as a Translational Animal Model for Biobehavioral and Neurotrauma Research
Abstract
:1. Introduction
2. Review
2.1. Preclinical Research
2.2. Translational Considerations
2.3. Similarities of the Pig Brain
2.4. Pig Cognition and Behavior
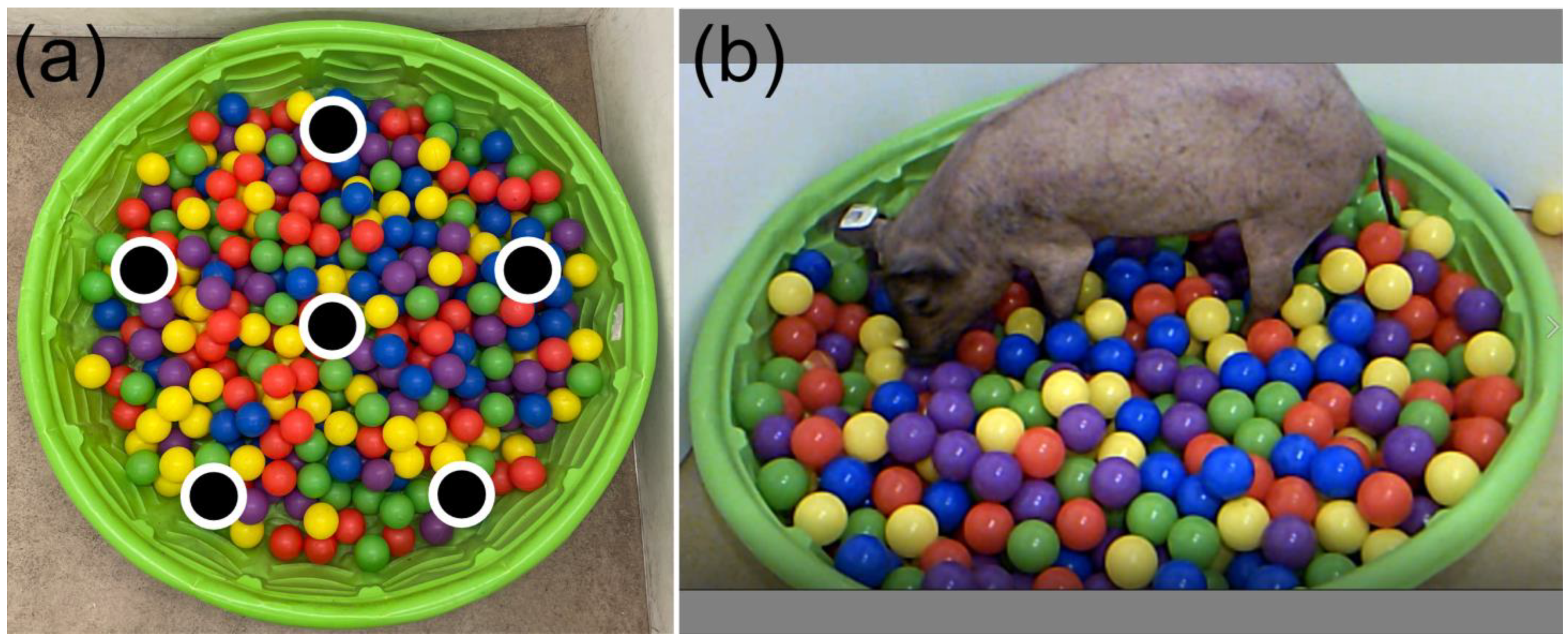
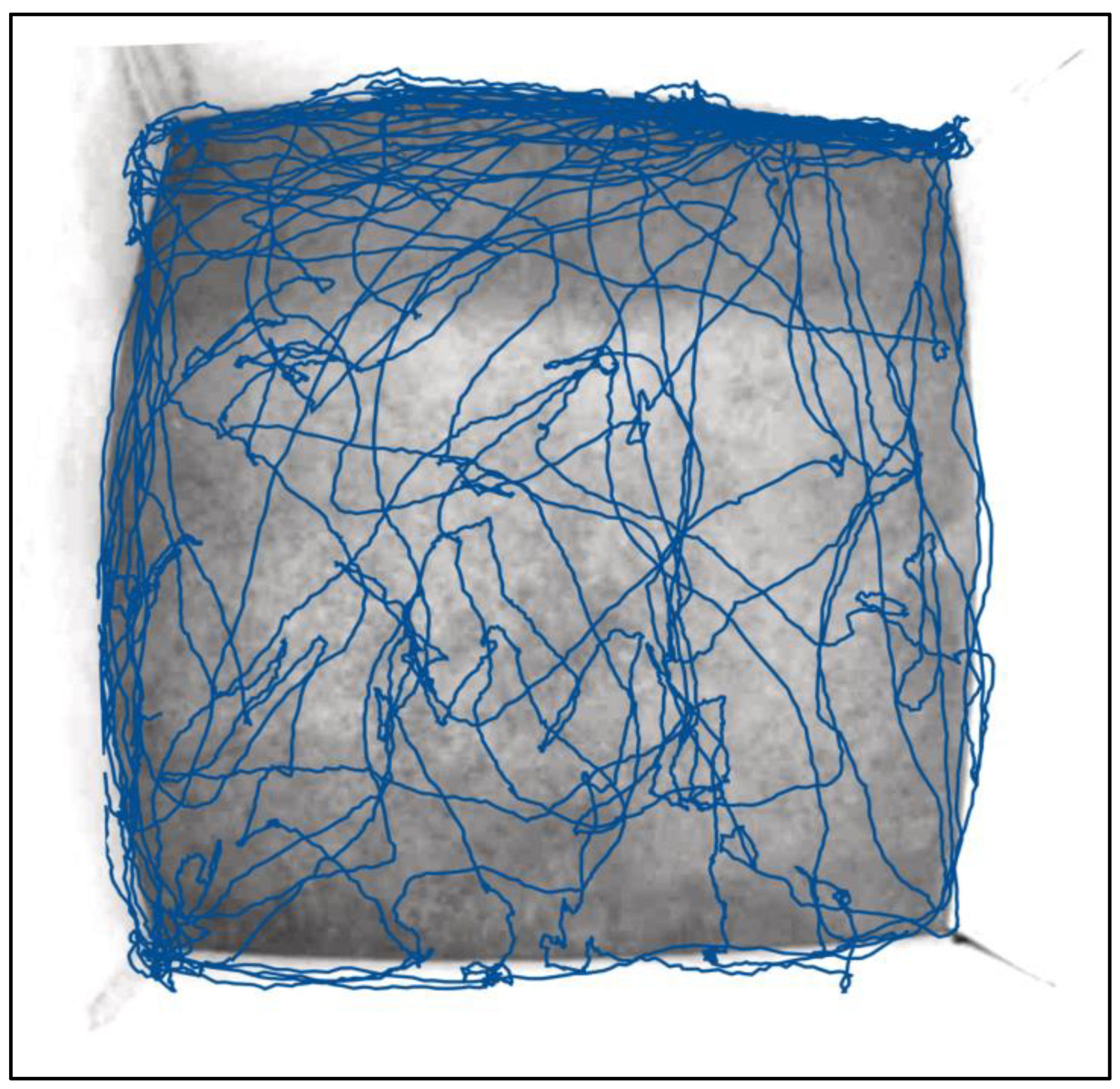
2.4.1. Spatial Memory and Maze Tests
2.4.2. Object Recognition and Discrimination Tests
2.4.3. Social Interaction and Aggression Tasks
2.4.4. Vocalization Analysis
2.4.5. Fear and Anxiety Tasks
2.5. Physiological Assessments
2.5.1. Real-Time Physiological Monitoring
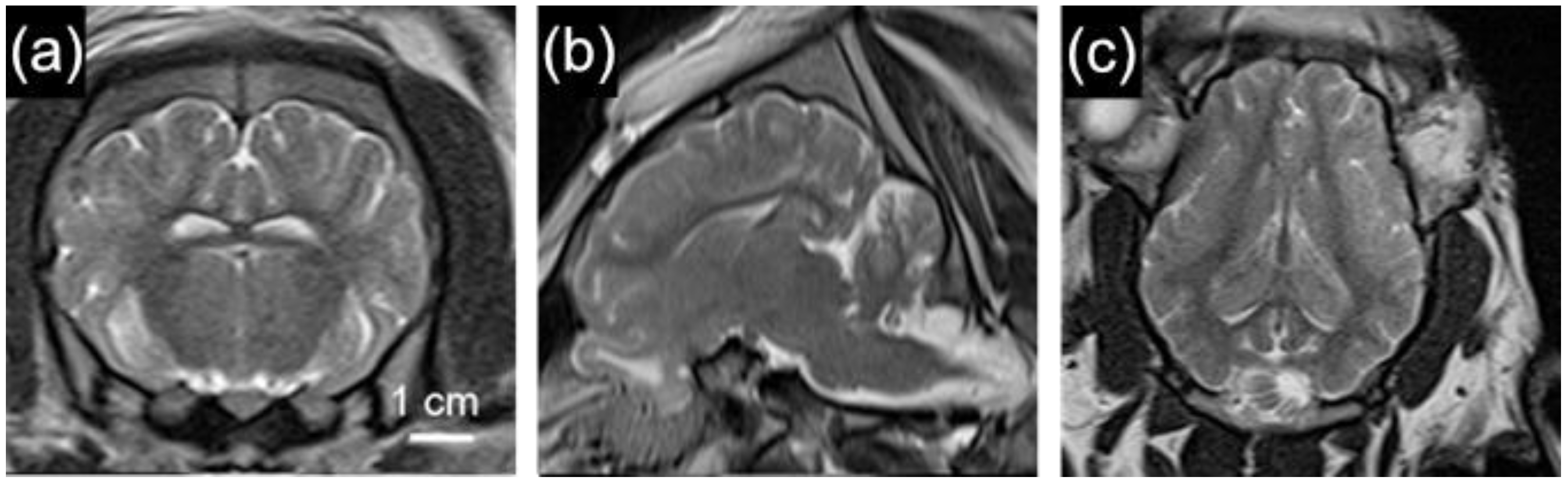
2.5.2. Neuroimaging
2.6. Pigs as a Model Animal for Traumatic Brain Injury Research
Studies That Use Pigs for TBI Research
2.7. Considerations for Conducting Pig Research
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davies, C.; Hamilton, O.K.L.; Hooley, M.; Ritakari, T.E.; Stevenson, A.J.; Wheater, E.N.W. Translational neuroscience: The state of the nation (a Ph.D. student perspective). Brain Commun. 2020, 2, fcaa038. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.R.; Fisher, P.A.; Pfeifer, J.H.; Allen, N.B.; Berkman, E.T. Levers and barriers to success in the use of translational neuroscience for the prevention and treatment of mental health and promotion of well-being across the lifespan. J. Abnorm. Psychol. 2020, 129, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M., Jr. Animal research as a basis for clinical trials. Eur. J. Oral Sci. 2015, 123, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Hishikawa, S.; Teratani, T.; Lefor, A.T. The pig as a model for translational research: Overview of porcine animal models at Jichi Medical University. Transplant. Res. 2012, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.X.; Ma, Y.B.; Le, N.Y.; Cao, J.; Wang, Y. Large animal models of traumatic brain injury. Int. J. Neurosci. 2018, 128, 243–254. [Google Scholar] [CrossRef]
- Sorby-Adams, A.J.; Vink, R.; Turner, R.J. Large animal models of stroke and traumatic brain injury as translational tools. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R165–R190. [Google Scholar] [CrossRef] [PubMed]
- Vink, R. Large animal models of traumatic brain injury. J. Neurosci. Res. 2018, 96, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Gieling, E.T.; Nordquist, R.E.; van der Staay, F.J. Assessing learning and memory in pigs. Anim. Cogn. 2011, 14, 151–173. [Google Scholar] [CrossRef] [Green Version]
- Gieling, E.T.; Schuurman, T.; Nordquist, R.E.; van der Staay, F.J. The pig as a model animal for studying cognition and neurobehavioral disorders. Curr. Top. Behav. Neurosci. 2011, 7, 359–383. [Google Scholar] [CrossRef]
- Puccinelli, E.; Gervasi, P.G.; Longo, V. Xenobiotic metabolizing cytochrome P450 in pig, a promising animal model. Curr. Drug Metab. 2011, 12, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.B.; Wood, V.A.; Schreiber, W.; Williams, B.B.; Gallez, B.; Swartz, H.M. Guidance to Transfer ‘Bench-Ready’ Medical Technology into Usual Clinical Practice: Case Study—Sensors and Spectrometer Used in EPR Oximetry. Adv. Exp. Med. Biol. 2018, 1072, 233–239. [Google Scholar] [CrossRef]
- Huang, W.; Percie du Sert, N.; Vollert, J.; Rice, A.S.C. General Principles of Preclinical Study Design. Handb. Exp. Pharmacol. 2020, 257, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Bovenkerk, B.; Kaldewaij, F. The use of animal models in behavioural neuroscience research. Curr. Top. Behav. Neurosci. 2015, 19, 17–46. [Google Scholar] [CrossRef]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Models Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.S.; Krishnan, V.; Stokes, W.; Robertson, C.; Celnik, P.; Chen, Y.; Song, X.; Lu, H.; Liu, P.; Pelled, G. Transcranial magnetic stimulation and environmental enrichment enhances cortical excitability and functional outcomes after traumatic brain injury. Brain Stimul. 2018, 11, 1306–1313. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Yan, L.; Zhang, Y.; Yang, Z.; Wang, J.; Song, C. EPA is More Effective than DHA to Improve Depression-Like Behavior, Glia Cell Dysfunction and Hippcampal Apoptosis Signaling in a Chronic Stress-Induced Rat Model of Depression. Int. J. Mol. Sci. 2020, 21, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughson, A.R.; Horvath, A.P.; Holl, K.; Palmer, A.A.; Solberg Woods, L.C.; Robinson, T.E.; Flagel, S.B. Incentive salience attribution, “sensation-seeking” and “novelty-seeking” are independent traits in a large sample of male and female heterogeneous stock rats. Sci. Rep. 2019, 9, 2351. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; O’Neill, N.; Maffei, B.; Zourray, C.; Almacellas-Barbanoj, A.; Carpenter, J.C.; Jones, S.P.; Leite, M.; Turner, T.J.; Moreira, F.C.; et al. On-demand cell-autonomous gene therapy for brain circuit disorders. Science 2022, 378, 523–532. [Google Scholar] [CrossRef]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G.; Geddes, R.I.; Sribnick, E.A. Recent developments in clinical trials for the treatment of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 433–451. [Google Scholar] [CrossRef]
- Todea, R.A.; Lu, P.J.; Fartaria, M.J.; Bonnier, G.; Du Pasquier, R.; Krueger, G.; Bach Cuadra, M.; Psychogios, M.N.; Kappos, L.; Kuhle, J.; et al. Evolution of Cortical and White Matter Lesion Load in Early-Stage Multiple Sclerosis: Correlation With Neuroaxonal Damage and Clinical Changes. Front. Neurol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Cloots, R.J.; Gervaise, H.M.; van Dommelen, J.A.; Geers, M.G. Biomechanics of traumatic brain injury: Influences of the morphologic heterogeneities of the cerebral cortex. Ann. Biomed. Eng 2008, 36, 1203–1215. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Antunes, L.; Mota, B.; Herculano-Houzel, S. Different scaling of white matter volume, cortical connectivity, and gyrification across rodent and primate brains. Front. Neuroanat. 2013, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Ryan, M.C.; Sherman, P.; Rowland, L.M.; Wijtenburg, S.A.; Acheson, A.; Fieremans, E.; Veraart, J.; Novikov, D.S.; Hong, L.E.; Sladky, J.; et al. Miniature pig model of human adolescent brain white matter development. J. Neurosci. Methods 2018, 296, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Henry, Y.; Sève, B.; Mounier, A.; Ganier, P. Growth performance and brain neurotransmitters in pigs as affected by tryptophan, protein, and sex. J. Anim. Sci. 1996, 74, 2700–2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, J.W.; Dobbing, J. Prenatal and postnatal growth and development of the central nervous system of the pig. Proc. R. Soc. Lond. B Biol. Sci. 1967, 166, 384–395. [Google Scholar] [PubMed]
- Holm, I.E.; West, M.J. Hippocampus of the domestic pig: A stereological study of subdivisional volumes and neuron numbers. Hippocampus 1994, 4, 115–125. [Google Scholar] [CrossRef]
- Jelsing, J.; Nielsen, R.; Olsen, A.K.; Grand, N.; Hemmingsen, R.; Pakkenberg, B. The postnatal development of neocortical neurons and glial cells in the Göttingen minipig and the domestic pig brain. J. Exp. Biol. 2006, 209, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef]
- Pond, W.G.; Boleman, S.L.; Fiorotto, M.L.; Ho, H.; Knabe, D.A.; Mersmann, H.J.; Savell, J.W.; Su, D.R. Perinatal ontogeny of brain growth in the domestic pig. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 2000, 223, 102–108. [Google Scholar] [CrossRef]
- Croney, C.; Adams, K.; Washington, C.; Stricklin, W. A note on visual, olfactory and spatial cue use in foraging behavior of pigs: Indirectly assessing cognitive abilities. Appl. Anim. Behav. Sci. 2003, 83, 303–308. [Google Scholar] [CrossRef]
- de Jong, I.C.; Prelle, I.T.; van de Burgwal, J.A.; Lambooij, E.; Korte, S.M.; Blokhuis, H.J.; Koolhaas, J.M. Effects of environmental enrichment on behavioral responses to novelty, learning, and memory, and the circadian rhythm in cortisol in growing pigs. Physiol. Behav. 2000, 68, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Kornum, B.R.; Knudsen, G.M. Cognitive testing of pigs (Sus scrofa) in translational biobehavioral research. Neurosci. Biobehav. Rev. 2011, 35, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.T.; Ashwell, M.S.; Baynes, R.E.; Brooks, J.D.; Yeatts, J.L.; Maltecca, C. Genetic Parameter Estimates for Metabolizing Two Common Pharmaceuticals in Swine. Front. Genet. 2018, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Wolf, E.; Braun-Reichhart, C.; Streckel, E.; Renner, S. Genetically engineered pig models for diabetes research. Transgenic Res. 2014, 23, 27–38. [Google Scholar] [CrossRef]
- Kinder, H.A.; Baker, E.W.; Howerth, E.W.; Duberstein, K.J.; West, F.D. Controlled Cortical Impact Leads to Cognitive and Motor Function Deficits that Correspond to Cellular Pathology in a Piglet Traumatic Brain Injury Model. J. Neurotrauma 2019, 36, 2810–2826. [Google Scholar] [CrossRef]
- Singh, R.; Kulikowicz, E.; Santos, P.T.; Koehler, R.C.; Martin, L.J.; Lee, J.K. Spatial T-maze identifies cognitive deficits in piglets 1 month after hypoxia-ischemia in a model of hippocampal pyramidal neuron loss and interneuron attrition. Behav. Brain Res. 2019, 369, 111921. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Yang, C.; Shi, Y.; Dong, Z.; Troy, F.A., 2nd. Functional Correlates and Impact of Dietary Lactoferrin Intervention and its Concentration-dependence on Neurodevelopment and Cognition in Neonatal Piglets. Mol. Nutr. Food Res. 2021, 65, e2001099. [Google Scholar] [CrossRef]
- Haagensen, A.M.; Klein, A.B.; Ettrup, A.; Matthews, L.R.; Sørensen, D.B. Cognitive performance of Göttingen minipigs is affected by diet in a spatial hole-board discrimination test. PLoS ONE 2013, 8, e79429. [Google Scholar] [CrossRef] [Green Version]
- Grimberg-Henrici, C.G.; Vermaak, P.; Bolhuis, J.E.; Nordquist, R.E.; van der Staay, F.J. Effects of environmental enrichment on cognitive performance of pigs in a spatial holeboard discrimination task. Anim. Cogn. 2016, 19, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Clouard, C.; Reimert, I.; Fleming, S.A.; Koopmans, S.J.; Schuurman, T.; Hauser, J. Dietary sialylated oligosaccharides in early-life may promote cognitive flexibility during development in context of obesogenic dietary intake. Nutr. Neurosci. 2022, 25, 2461–2478. [Google Scholar] [CrossRef]
- Netzley, A.H.; Hunt, R.D.; Franco-Arellano, J.; Arnold, N.; Vazquez, A.I.; Munoz, K.A.; Colbath, A.C.; Bush, T.R.; Pelled, G. Multimodal characterization of Yucatan minipig behavior and physiology through maturation. Sci. Rep. 2021, 11, 22688. [Google Scholar] [CrossRef] [PubMed]
- Schramke, S.; Schuldenzucker, V.; Schubert, R.; Frank, F.; Wirsig, M.; Ott, S.; Motlik, J.; Fels, M.; Kemper, N.; Hölzner, E.; et al. Behavioral phenotyping of minipigs transgenic for the Huntington gene. J. Neurosci. Methods 2016, 265, 34–45. [Google Scholar] [CrossRef]
- Ao, W.; Grace, M.; Floyd, C.L.; Vonder Haar, C. A Touchscreen Device for Behavioral Testing in Pigs. Biomedicines 2022, 10, 2612. [Google Scholar] [CrossRef]
- Wegner, B.; Spiekermeier, I.; Nienhoff, H.; Große-Kleimann, J.; Rohn, K.; Meyer, H.; Plate, H.; Gerhardy, H.; Kreienbrock, L.; Beilage, E.G.; et al. Application of the voluntary human approach test on commercial pig fattening farms: A meaningful tool? Porc. Health Manag. 2020, 6, 19. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, S.; Chen, M.; Zhao, Y.; Wang, Y.; Zhao, X.; Li, D.; Pu, H. Combined spectral and speech features for pig speech recognition. PLoS ONE 2022, 17, e0276778. [Google Scholar] [CrossRef]
- Briefer, E.F.; Sypherd, C.C.; Linhart, P.; Leliveld, L.M.C.; Padilla de la Torre, M.; Read, E.R.; Guérin, C.; Deiss, V.; Monestier, C.; Rasmussen, J.H.; et al. Classification of pig calls produced from birth to slaughter according to their emotional valence and context of production. Sci. Rep. 2022, 12, 3409. [Google Scholar] [CrossRef]
- Haigh, A.; Chou, J.-Y.; O’Driscoll, K. Variations in the Behavior of Pigs During an Open Field and Novel Object Test. Front. Vet. Sci. 2020, 7, 607. [Google Scholar] [CrossRef]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef]
- Nachman, D.; Constantini, K.; Poris, G.; Wagnert-Avraham, L.; Gertz, S.D.; Littman, R.; Kabakov, E.; Eisenkraft, A.; Gepner, Y. Wireless, non-invasive, wearable device for continuous remote monitoring of hemodynamic parameters in a swine model of controlled hemorrhagic shock. Sci. Rep. 2020, 10, 17684. [Google Scholar] [CrossRef]
- Martinez-Ramirez, L.; Slate, A.; Price, G.; Duhaime, A.C.; Staley, K.; Costine-Bartell, B.A. Robust, long-term video EEG monitoring in a porcine model of post-traumatic epilepsy. eNeuro 2022, 9, ENEURO.0025-22.2022. [Google Scholar] [CrossRef]
- Simchick, G.; Shen, A.; Campbell, B.; Park, H.J.; West, F.D.; Zhao, Q. Pig Brains Have Homologous Resting-State Networks with Human Brains. Brain Connect. 2019, 9, 566–579. [Google Scholar] [CrossRef]
- Lee, J.K.; Liu, D.; Jiang, D.; Kulikowicz, E.; Tekes, A.; Liu, P.; Qin, Q.; Koehler, R.C.; Aggarwal, M.; Zhang, J.; et al. Fractional anisotropy from diffusion tensor imaging correlates with acute astrocyte and myelin swelling in neonatal swine models of excitotoxic and hypoxic-ischemic brain injury. J. Comp. Neurol. 2021, 529, 2750–2770. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Tenovuo, O.; Diaz-Arrastia, R.; Goldstein, L.E.; Sharp, D.J.; van der Naalt, J.; Zasler, N.D. Assessing the Severity of Traumatic Brain Injury-Time for a Change? J. Clin. Med. 2021, 10, 148. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S. Recent Advances in Pathophysiology of Traumatic Brain Injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Laskowitz, D.T. Cellular and Molecular Mechanisms of Secondary Neuronal Injury Following Traumatic Brain Injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Marklund, N.; Bellander, B.M.; Godbolt, A.K.; Levin, H.; McCrory, P.; Thelin, E.P. Treatments and rehabilitation in the acute and chronic state of traumatic brain injury. J. Intern. Med. 2019, 285, 608–623. [Google Scholar] [CrossRef] [Green Version]
- Osier, N.D.; Korpon, J.R.; Dixon, C.E. Controlled Cortical Impact Model. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Pareja, J.C.; Keeley, K.; Duhaime, A.C.; Dodge, C.P. Modeling Pediatric Brain Trauma: Piglet Model of Controlled Cortical Impact. Methods Mol. Biol. 2016, 1462, 345–356. [Google Scholar] [CrossRef]
- Simchick, G.; Scheulin, K.M.; Sun, W.; Sneed, S.E.; Fagan, M.M.; Cheek, S.R.; West, F.D.; Zhao, Q. Detecting functional connectivity disruptions in a translational pediatric traumatic brain injury porcine model using resting-state and task-based fMRI. Sci. Rep. 2021, 11, 12406. [Google Scholar] [CrossRef]
- Baker, E.W.; Kinder, H.A.; Hutcheson, J.M.; Duberstein, K.J.J.; Platt, S.R.; Howerth, E.W.; West, F.D. Controlled Cortical Impact Severity Results in Graded Cellular, Tissue, and Functional Responses in a Piglet Traumatic Brain Injury Model. J. Neurotrauma 2019, 36, 61–73. [Google Scholar] [CrossRef]
- Manley, G.T.; Rosenthal, G.; Lam, M.; Morabito, D.; Yan, D.; Derugin, N.; Bollen, A.; Knudson, M.M.; Panter, S.S. Controlled cortical impact in swine: Pathophysiology and biomechanics. J. Neurotrauma 2006, 23, 128–139. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Cao, S.; Liu, X.; Martin, L.J.; Simoni, J.; Soltys, B.J.; Hsia, C.J.C.; Koehler, R.C. Polynitroxylated PEGylated hemoglobin protects pig brain neocortical gray and white matter after traumatic brain injury and hemorrhagic shock. Front. Med. Technol. 2023, 5, 1074643. [Google Scholar] [CrossRef]
- Ginsburg, J.; Huff, J.S. Closed Head Trauma; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shin, S.S.; Gottschalk, A.C.; Mazandi, V.M.; Kilbaugh, T.J.; Hefti, M.M. Transcriptional Profiling in a Novel Swine Model of Traumatic Brain Injury. Neurotrauma Rep. 2022, 3, 178–184. [Google Scholar] [CrossRef]
- Shin, S.S.; Chawla, S.; Jang, D.H.; Mazandi, V.M.; Weeks, M.K.; Kilbaugh, T.J. Imaging of White Matter Injury Correlates with Plasma and Tissue Biomarkers in Pediatric Porcine Model of Traumatic Brain Injury. J. Neurotrauma 2023, 40, 74–85. [Google Scholar] [CrossRef]
- Cullen, D.K.; Harris, J.P.; Browne, K.D.; Wolf, J.A.; Duda, J.E.; Meaney, D.F.; Margulies, S.S.; Smith, D.H. A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration. Methods Mol. Biol. 2016, 1462, 289–324. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.R.; Ling, J.M.; Patton, D.A.; Stephenson, D.D.; Dodd, A.B.; Dodd, R.J.; Rannou-Latella, J.G.; Smith, D.H.; Johnson, V.E.; Cullen, D.K.; et al. Non-Linear Device Head Coupling and Temporal Delays in Large Animal Acceleration Models of Traumatic Brain Injury. Ann. Biomed. Eng. 2022, 50, 728–739. [Google Scholar] [CrossRef]
- Mayer, A.R.; Ling, J.M.; Dodd, A.B.; Rannou-Latella, J.G.; Stephenson, D.D.; Dodd, R.J.; Mehos, C.J.; Patton, D.A.; Cullen, D.K.; Johnson, V.E.; et al. Reproducibility and Characterization of Head Kinematics During a Large Animal Acceleration Model of Traumatic Brain Injury. Front. Neurol. 2021, 12, 658461. [Google Scholar] [CrossRef]
- O’Donnell, J.C.; Browne, K.D.; Kvint, S.; Makaron, L.; Grovola, M.R.; Karandikar, S.; Kilbaugh, T.J.; Cullen, D.K.; Petrov, D. Multimodal Neuromonitoring and Neurocritical Care in Swine to Enhance Translational Relevance in Brain Trauma Research—Neurocritical Care in Swine. Biomedicines 2023, 11, 5. [Google Scholar]
- Chen, C.; Zhou, C.; Cavanaugh, J.M.; Kallakuri, S.; Desai, A.; Zhang, L.; King, A.I. Quantitative electroencephalography in a swine model of blast-induced brain injury. Brain Inj. 2017, 31, 120–126. [Google Scholar] [CrossRef]
- Kallakuri, S.; Desai, A.; Feng, K.; Tummala, S.; Saif, T.; Chen, C.; Zhang, L.; Cavanaugh, J.M.; King, A.I. Neuronal Injury and Glial Changes Are Hallmarks of Open Field Blast Exposure in Swine Frontal Lobe. PLoS ONE 2017, 12, e0169239. [Google Scholar] [CrossRef] [Green Version]
- Cralley, A.L.; Moore, E.E.; Kissau, D.; Coleman, J.R.; Vigneshwar, N.; DeBot, M.; Schaid, T.R., Jr.; Moore, H.B.; Cohen, M.J.; Hansen, K.; et al. A combat casualty relevant dismounted complex blast injury model in swine. J. Trauma Acute Care Surg. 2022, 93, S110–S118. [Google Scholar] [CrossRef]
- Wendt, M.; Bickhardt, K.; Herzog, A.; Fischer, A.; Martens, H.; Richter, T. Porcine stress syndrome and PSE meat: Clinical symptoms, pathogenesis, etiology and animal rights aspects. Berl. Und Munch. Tierarztl. Wochenschr. 2000, 113, 173–190. [Google Scholar]

| Type of Test | Authors | Experimental Condition |
|---|---|---|
| Maze Tasks | Kinder et al., 2019 [37] | Traumatic Brain Injury |
| Singh et al., 2019 [38] | Hypoxia-Ischemia | |
| Chen et al., 2021 [39] | Diet | |
| Spatial and Hole board | Clouard et al., 2022 [42] | Diet |
| Netzley et al., 2021 [43] | Healthy | |
| Object Discrimination | Schramke et al., 2016 [44] | Huntington’s Disease |
| Ao et al., 2022 [45] | Healthy | |
| Socialization | Wegner et al., 2020 [46] | Healthy |
| Schramke et al., 2016 [44] | Huntington’s Disease | |
| Vocalization | Wu et al., 2022 [47] | Healthy |
| Briefer et al., 2022 [48] | Healthy | |
| Open Field | Haigh et al., 2020 [49] | Tail biting |
| Input Methods | Authors |
|---|---|
| Controlled Cortical Impact | Kinder et al., 2019 [37] |
| Simchick et al., 2021 [62] | |
| Baker et al., 2019 [63] | |
| Manley et al., 2006 [64] | |
| Wang et al., 2023 [65] | |
| Rotational Acceleration | Cullen et al., 2016 [69] |
| Mayer et al., 2021 [71] | |
| Mayer et al., 2022 [70] | |
| O’Donnell et al., 2023 [72] | |
| Blast Injury | Chen et al., 2017 [73] |
| Kallakuri et al., 2017 [74] | |
| Cralley et al., 2022 [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netzley, A.H.; Pelled, G. The Pig as a Translational Animal Model for Biobehavioral and Neurotrauma Research. Biomedicines 2023, 11, 2165. https://doi.org/10.3390/biomedicines11082165
Netzley AH, Pelled G. The Pig as a Translational Animal Model for Biobehavioral and Neurotrauma Research. Biomedicines. 2023; 11(8):2165. https://doi.org/10.3390/biomedicines11082165
Chicago/Turabian StyleNetzley, Alesa H., and Galit Pelled. 2023. "The Pig as a Translational Animal Model for Biobehavioral and Neurotrauma Research" Biomedicines 11, no. 8: 2165. https://doi.org/10.3390/biomedicines11082165





