Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Laser Photocoagulation
2.3. Multimodal Imaging and Focal Electroretinography Recording
2.4. Retinal Pigment Epithelium, Choroid, and Sclera Flat-Mounts
2.5. Real-Time PCR
2.6. Histology and Immunohistochemistry
2.7. Exclusion Criteria
2.8. Statistics
3. Results
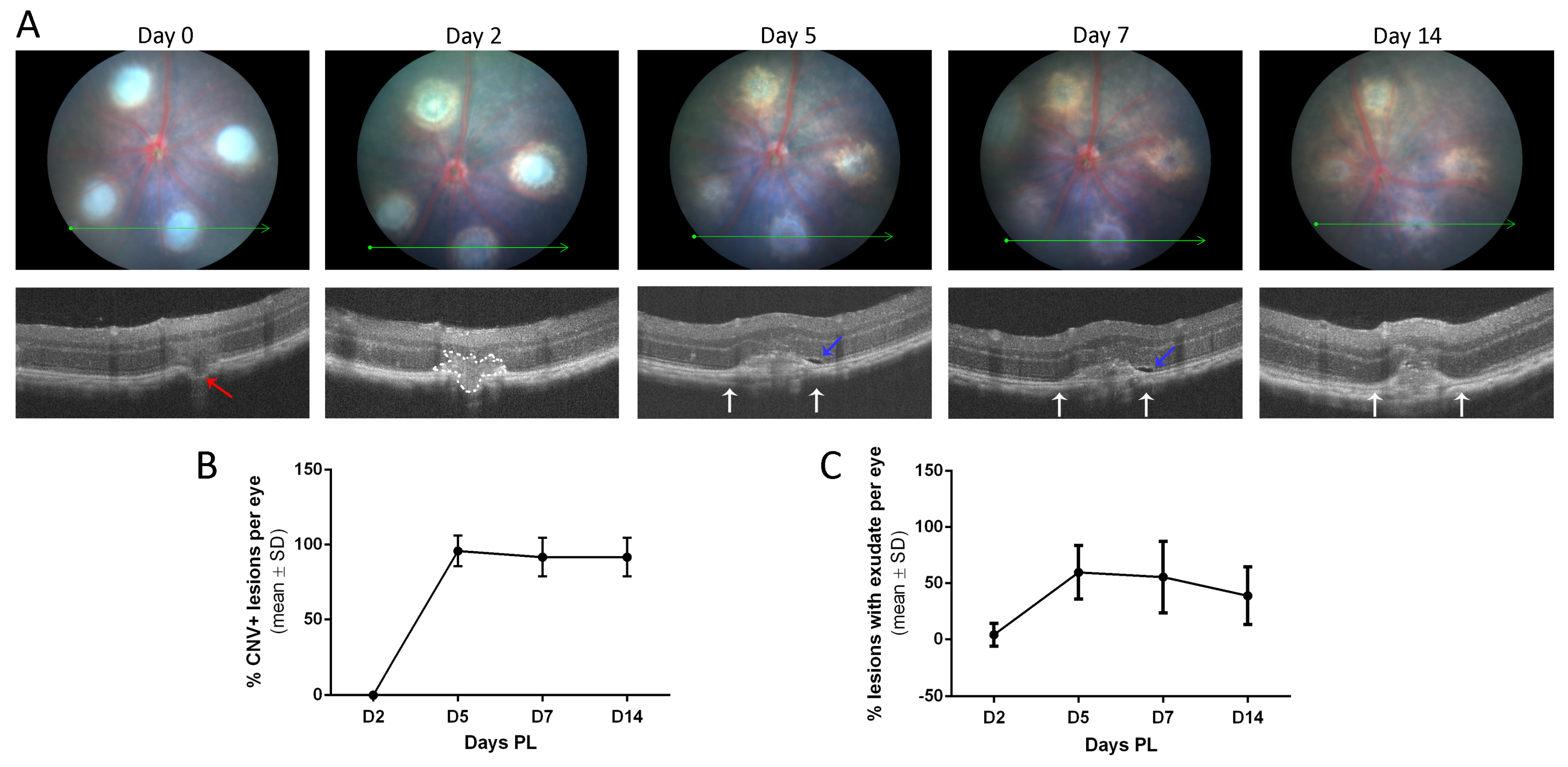
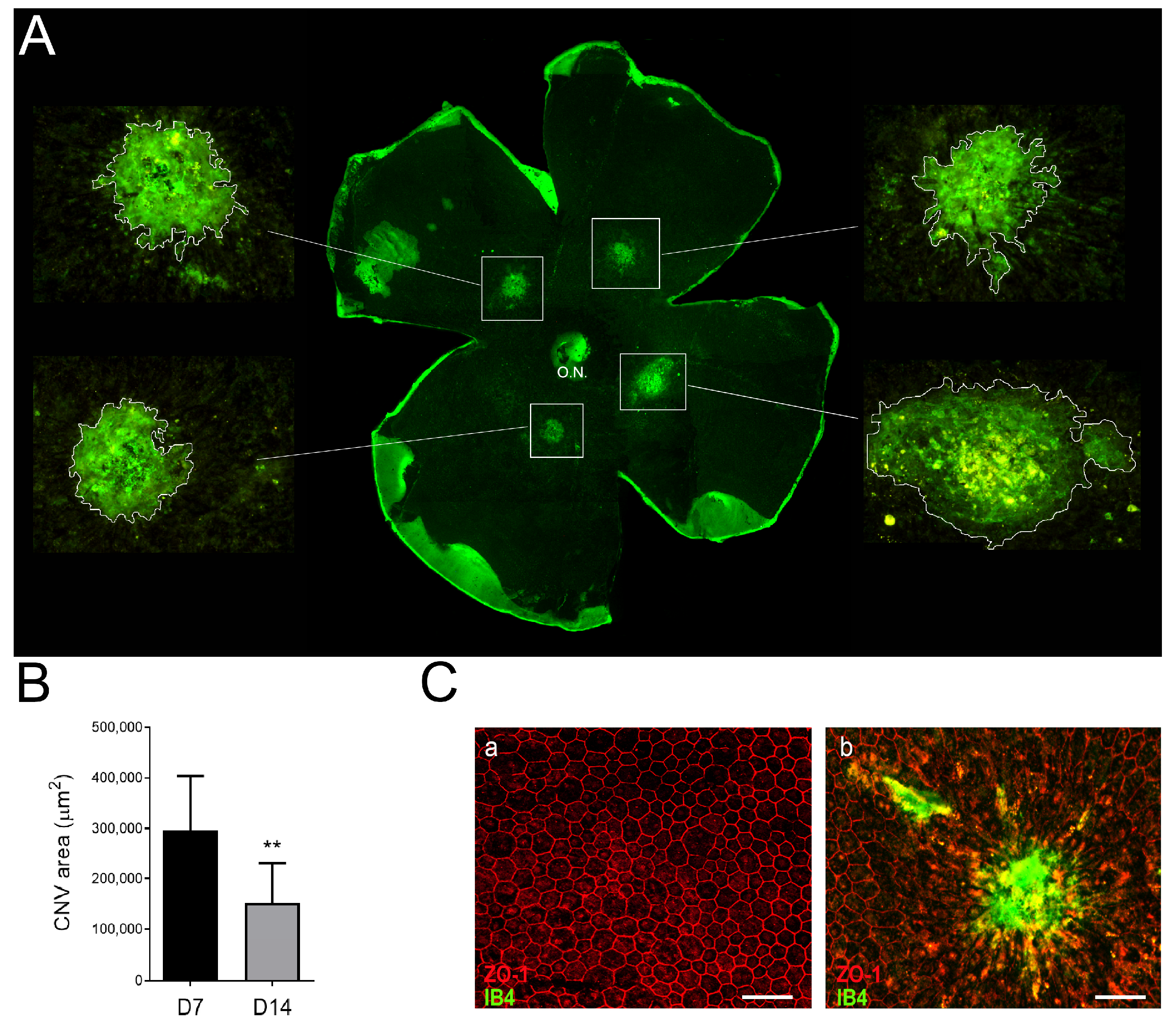
3.1. LI-CNV Lesion Size Stabilized from Day 7 Post-Induction
3.2. The LI-CNV Mouse Model Presents a Retinal Dysfunction in Lesioned Areas
3.3. Neovascular Areas Are Maximal at 7 Days Post-Laser
3.4. Laser Photocoagulation Induces a Pro-Inflammatory but Not a Pro-Angiogenic Response
3.5. Inflammation and Fibrosis Are Present in the LI-CNV Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia-Pacific J. Ophthalmol. 2017, 6, 493–497. [Google Scholar]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021, 7, 31. [Google Scholar] [CrossRef]
- Coleman, H.R.; Chan, C.C.; Ferris, F.L.; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Sacconi, R.; Fragiotta, S.; Sarraf, D.; Sadda, S.V.R.; Freund, K.B.; Parravano, M.; Corradetti, G.; Cabral, D.; Capuano, V.; Miere, A.; et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog. Retin. Eye Res. 2023, 92, 101113. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular Pathogenesis of Retinal and Choroidal Vascular Diseases. Prog. Retin. Eye Res. 2015, 49, 67. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Forooghian, F.; Razavi, R.; Timms, L. Hypoxia-inducible factor expression in human RPE cells. Br. J. Ophthalmol. 2007, 91, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Khachigian, L.M.; Liew, G.; Teo, K.Y.C.; Wong, T.Y.; Mitchell, P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J. Transl. Med. 2023, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.; Kaur, I. Molecular Mediators and Regulators of Retinal Angiogenesis. Semin. Ophthalmol. 2023, 38, 124–133. [Google Scholar] [CrossRef]
- Daniel, E.; Pan, W.; Ying, G.S.; Kim, B.J.; Grunwald, J.E.; Ferris, F.L.; Jaffe, G.J.; Toth, C.A.; Martin, D.F.; Fine, S.L.; et al. Development and Course of Scars in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2018, 125, 1037–1046. [Google Scholar] [CrossRef]
- Daniel, E.; Toth, C.A.; Grunwald, J.E.; Jaffe, G.J.; Martin, D.F.; Fine, S.L.; Huang, J.; Ying, G.S.; Hagstrom, S.A.; Winter, K.; et al. Risk of scar in the comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2014, 121, 656–666. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Martinez, J.A.; Brown, V.B.; Lambert, H.M.; Sternberg, P.; Capone, A.; Aaberg, T.M.; Lopez, P.F. Immunohistochemical and histochemical properties of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am. J. Ophthalmol. 1992, 114, 464–472. [Google Scholar] [CrossRef]
- Schlecht, A.; Boneva, S.; Gruber, M.; Zhang, P.; Horres, R.; Bucher, F.; Auw-Haedrich, C.; Hansen, L.; Stahl, A.; Hilgendorf, I.; et al. Transcriptomic Characterization of Human Choroidal Neovascular Membranes Identifies Calprotectin as a Novel Biomarker for Patients with Age-Related Macular Degeneration. Am. J. Pathol. 2020, 190, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Little, K.; Llorián-Salvador, M.; Tang, M.; Du, X.; Marry, S.; Chen, M.; Xu, H. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J. Neuroinflammation 2020, 17, 355. [Google Scholar] [CrossRef]
- Brandli, A.; Khong, F.L.; Kong, R.C.K.; Kelly, D.J.; Fletcher, E.L. Transcriptomic analysis of choroidal neovascularization reveals dysregulation of immune and fibrosis pathways that are attenuated by a novel anti-fibrotic treatment. Sci. Rep. 2022, 12, 859. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Kang, S.J.; Berglin, L. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 2010, 29, 500–519. [Google Scholar] [CrossRef]
- Ryan, S.J. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans. Am. Ophthalmol. Soc. 1979, 77, 707–745. [Google Scholar]
- Miller, H.; Miller, B.; Ryan, S.J. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1644–1652. [Google Scholar]
- Criswell, M.H.; Ciulla, T.A.; Hill, T.E.; Small, W.; Danis, R.P.; Snyder, W.J.; Lowseth, L.A.; Carson, D.L. The Squirrel Monkey: Characterization of a New-World Primate Model of Experimental Choroidal Neovascularization and Comparison with the Macaque. Investig. Ophthalmol. Vis. Sci. 2004, 45, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Tobe, T.; Ortega, S.; Luna, J.D.; Ozaki, H.; Okamoto, N.; Derevjanik, N.L.; Vinores, S.A.; Basilico, C.; Campochiaro, P.A. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am. J. Pathol. 1998, 153, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kwak, N.; Ozaki, H.; Yamada, H.; Okamoto, N.; Yamada, E.; Fabbro, D.; Hofmann, F.; Wood, J.M.; Campochiaro, P.A. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am. J. Pathol. 1999, 154, 1743–1753. [Google Scholar] [CrossRef]
- Dobi, E.T.; Puliafito, C.A.; Destro, M. A New Model of Experimental Choroidal Neovascularization in the Rat. Arch. Ophthalmol. 1989, 107, 264–269. [Google Scholar] [CrossRef]
- Frank, R.N.; Das, A.; Weber, M.L. A model of subretinal neovascularization in the pigmented rat. Curr. Eye Res. 1989, 8, 239–247. [Google Scholar] [CrossRef]
- Kimura, H.; Sakamoto, T.; Hinton, D.R.; Spee, C.; Ogura, Y.; Tabata, Y.; Ikada, Y.; Ryan, S.J. A new model of subretinal neovascularization in the rabbit. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2110–2119. [Google Scholar]
- Saishin, Y.; Silva, R.L.; Saishin, Y.; Callahan, K.; Schoch, C.; Ahlheim, M.; Lai, H.; Kane, F.; Brazzell, R.K.; Bodmer, D.; et al. Periocular Injection of Microspheres Containing PKC412 Inhibits Choroidal Neovascularization in a Porcine Model. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4989–4993. [Google Scholar] [CrossRef]
- Kiilgaard, J.F.; Andersen, M.V.N.; Wiencke, A.K.; Scherfig, E.; la Cour, M.; Tezel, T.H.; Prause, J.U. A new animal model of choroidal neovascularization. Acta Ophthalmol. Scand. 2005, 83, 697–704. [Google Scholar] [CrossRef]
- Spilsbury, K.; Garrett, K.L.; Shen, W.Y.; Constable, I.J.; Rakoczy, P.E. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 2000, 157, 135–144. [Google Scholar] [CrossRef]
- Rastoin, O.; Pagès, G.; Dufies, M. Experimental models in neovascular age related macular degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lu, Q.; Shen, J.; Zhang, L.; Gao, Y.; Shen, X.; Xie, B. Improvement and optimization of standards for a preclinical animal test model of laser induced choroidal neovascularization. PLoS ONE 2014, 9, e94743. [Google Scholar] [CrossRef]
- Poor, S.H.; Qiu, Y.; Fassbender, E.S.; Shen, S.; Woolfenden, A.; Delpero, A.; Kim, Y.; Buchanan, N.; Gebuhr, T.C.; Hanks, S.M.; et al. Reliability of the mouse model of choroidal neovascularization induced by laser photocoagulation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6525–6534. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Heidmann, D.G.; Marin-Castano, M.E.; Pereira-Simon, S.; Hernandez, E.P.; Elliot, S.; Cousins, S.W. Gender and estrogen supplementation increases severity of experimental choroidal neovascularization. Exp. Eye Res. 2005, 80, 413–423. [Google Scholar] [CrossRef]
- Schnabolk, G.; Stauffer, K.; O’Quinn, E.; Coughlin, B.; Kunchithapautham, K.; Rohrer, B. A comparative analysis of C57BL/6J and 6N substrains; chemokine/cytokine expression and susceptibility to laser-induced choroidal neovascularization. Exp. Eye Res. 2014, 129, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Heidmann, D.G.; Suner, I.; Hernandez, E.P.; Frazier, W.D.; Csaky, K.G.; Cousins, S.W. Age as an Independent Risk Factor for Severity of Experimental Choroidal Neovascularization | IOVS | ARVO Journals. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1567–1573. [Google Scholar]
- Edelman, J.L.; Castro, M.R. Quantitative image analysis of laser-induced choroidal neovascularization in rat. Exp. Eye Res. 2000, 71, 523–533. [Google Scholar] [CrossRef]
- Giani, A.; Thanos, A.; Roh, M.I.; Connolly, E.; Trichonas, G.; Kim, I.; Gragoudas, E.; Vavvas, D.; Miller, J.W. In Vivo Evaluation of Laser-Induced Choroidal Neovascularization Using Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3880. [Google Scholar] [CrossRef]
- Espinosa-Heidmann, D.G.; Suner, I.J.; Hernandez, E.P.; Monroy, D.; Csaky, K.G.; Cousins, S.W. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3586–3592. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Ling, J.X.; Wallace, T.M.; Dithmar, S.; Lawson, D.H.; Cohen, C.; Elner, V.M.; Elner, S.G.; Sternberg, P., Jr. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization-PubMed. Mol. Vis. 2002, 8, 119–126. [Google Scholar] [PubMed]
- Fabian-Jessing, B.K.; Jakobsen, T.S.; Jensen, E.G.; Alsing, S.; Hansen, S.; Aagaard, L.; Askou, A.L.; Bek, T.; Corydon, T.J. Animal Models of Choroidal Neovascularization: A Systematic Review. Investig. Ophthalmol. Vis. Sci. 2022, 63, 11. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Aspects Med. 2012, 33, 487–509. [Google Scholar] [CrossRef]
- Badia, A.; Duarri, A.; Salas, A.; Rosell, J.; Ramis, J.; Gusta, M.F.; Casals, E.; Zapata, M.A.; Puntes, V.; García-Arumí, J. Repeated Topical Administration of 3 nm Cerium Oxide Nanoparticles Reverts Disease Atrophic Phenotype and Arrests Neovascular Degeneration in AMD Mouse Models. ACS Nano 2023, 17, 910–926. [Google Scholar] [CrossRef]
- Ragauskas, S.; Kielczewski, E.; Vance, J.; Kaja, S.; Kalesnykas, G. In vivo multimodal imaging and analysis of mouse laser-induced choroidal neovascularization model. J. Vis. Exp. 2018, 2018, 56173. [Google Scholar] [CrossRef]
- Zhao, M.; Xie, W.; Hein, T.W.; Kuo, L.; Rosa, R.H. Laser-Induced Choroidal Neovascularization in Rats. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2021; Volume 2319, pp. 77–85. [Google Scholar]
- Shah, R.S.; Soetikno, B.T.; Lajko, M.; Fawzi, A.A. A Mouse Model for Laser-induced Choroidal Neovascularization. J. Vis. Exp 2015, 106, 53502. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.L.A.; Struman, I.; Sounni, N.E.; Rozet, E.; De Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Badia, A.; Salas, A.; Duarri, A.; Ferreira-de-Souza, B.; Zapata, M.Á.; Fontrodona, L.; García-Arumí, J. Transcriptomics analysis of Ccl2/Cx3cr1/Crb1rd8 deficient mice provides new insights into the pathophysiology of progressive retinal degeneration. Exp. Eye Res. 2021, 203, 108424. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Sun, Y.; Fu, Z.; Liu, C.H.; Evans, L.; Tian, K.; Saba, N.; Fredrick, T.; Morss, P.; et al. Optimization of an image-guided laser-induced choroidal neovascularization model in mice. PLoS ONE 2015, 10, e0132643. [Google Scholar] [CrossRef]
- Congdon, N. Causes and Prevalence of Visual Impairment among Adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Mattapallil, M.J.; Wawrousek, E.F.; Chan, C.C.; Zhao, H.; Roychoudhury, J.; Ferguson, T.A.; Caspi, R.R. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2921–2927. [Google Scholar] [CrossRef]
- Aredo, B.; Zhang, K.; Chen, X.; Wang, C.X.Z.; Li, T.; Ufret-Vincenty, R.L. Differences in the distribution, phenotype and gene expression of subretinal microglia/macrophages in C57BL/6N (Crb1rd8/rd8) versus C57BL6/J (Crb1wt/wt) mice. J. Neuroinflamm. 2015, 12, 6. [Google Scholar] [CrossRef]
- Jawad, S.; Liu, B.; Li, Z.; Katamay, R.; Campos, M.; Wei, L.; Sen, H.N.; Ling, D.; Estrada, F.M.; Amaral, J.; et al. The role of macrophage class a scavenger receptors in a laser-induced murine choroidal neovascularization model. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5959–5970. [Google Scholar] [CrossRef]
- Caicedo, A.; Espinosa-Heidmann, D.G.; Hamasaki, D.; Piña, Y.; Cousins, S.W. Photoreceptor synapses degenerate early in experimental choroidal neovascularization. J. Comp. Neurol. 2005, 483, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Hoerster, R.; Muether, P.S.; Vierkotten, S.; Schröder, S.; Kirchhof, B.; Fauser, S. In-vivo and ex-vivo characterization of laser-induced choroidal neovascularization variability in mice. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1579–1586. [Google Scholar] [CrossRef]
- Shansky, R.M. Sex differences in mechanisms of disease. Genes Brain Behav. 2020, 19, e12646. [Google Scholar] [CrossRef]
- Ramsey, D.J.; Ripps, H.; Qian, H. An electrophysiological study of retinal function in the diabetic female rat. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5116–5124. [Google Scholar] [CrossRef]
- Toker, E.; Yenice, Ö.; Akpinar, I.; Aribal, E.; Kazokoglu, H. The influence of sex hormones on ocular blood flow in women. Acta Ophthalmol. Scand. 2003, 81, 617–624. [Google Scholar] [CrossRef]
- Sulaiman, R.S.; Quigley, J.; Qi, X.; O’Hare, M.N.; Grant, M.B.; Boulton, M.E.; Corson, T.W. A simple optical coherence tomography quantification method for choroidal neovascularization. J. Ocul. Pharmacol. Ther. 2015, 31, 447–454. [Google Scholar] [CrossRef] [PubMed]
- André, H.; Tunik, S.; Aronsson, M.; Kvanta, A. Hypoxia-inducible factor-1α Is associated with sprouting angiogenesis in the murine laser-induced Choroidal neovascularization model. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6591–6604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yoshida, S.; Kubo, Y.; Yoshimura, T.; Kobayashi, Y.; Nakama, T.; Yamaguchi, M.; Ishikawa, K.; Oshima, Y.; Ishibashi, T. Different distributions of M1 and M2 macrophages in a mouse model of laser-induced choroidal neovascularization. Mol. Med. Rep. 2017, 15, 3949–3956. [Google Scholar] [CrossRef]
- Yang, C.; Tang, D. Patient-Specific Carotid Plaque Progression Simulation. C. Model. Eng. Sci. 2000, 1, 119–131. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Wittchen, E.S.; Hartnett, M.E. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol. Vis. 2016, 22, 116–128. [Google Scholar]
- Xu, J.; Zhu, D.; Sonoda, S.; He, S.; Spee, C.; Ryan, S.J.; Hinton, D.R. Over-expression of BMP4 inhibits experimental choroidal neovascularization by modulating VEGF and MMP-9. Angiogenesis 2012, 15, 213–227. [Google Scholar] [CrossRef]
- Bora, P.S.; Sohn, J.-H.; Cruz, J.M.C.; Jha, P.; Nishihori, H.; Wang, Y.; Kaliappan, S.; Kaplan, H.J.; Bora, N.S. Role of Complement and Complement Membrane Attack Complex in Laser-Induced Choroidal Neovascularization. J. Immunol. 2005, 174, 491–497. [Google Scholar] [CrossRef]
- Yanai, R.; Chen, S.; Uchi, S.H.; Nanri, T.; Connor, K.M.; Kimura, K. Attenuation of choroidal neovascularization by dietary intake of ω-3 long-chain polyunsaturated fatty acids and lutein in mice. PLoS ONE 2018, 13, e0196037. [Google Scholar] [CrossRef]
- Hasegawa, E.; Inafuku, S.; Mulki, L.; Okunuki, Y.; Yanai, R.; Smith, K.E.; Kim, C.B.; Klokman, G.; Bielenberg, D.R.; Puli, N.; et al. Cytochrome P450 monooxygenase lipid metabolites are significant second messengers in the resolution of choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2017, 114, E7545–E7553. [Google Scholar] [CrossRef]
- Jeong, J.H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharm. Res. 2021, 44, 1. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, X.; Huang, L.; Huang, X.; Gu, J.; Yu, X.; Zhu, Y.; Zhou, Y.; Song, Y.; Zhu, M. Lycopene inhibits endothelial-to-mesenchymal transition of choroidal vascular endothelial cells in laser-induced mouse choroidal neovascularization. J. Cell. Mol. Med. 2023, 27, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Liang, I.C.; Ko, W.C.; Hsu, Y.J.; Lin, Y.R.; Chang, Y.H.; Zong, X.H.; Lai, P.C.; Chang, D.C.; Hung, C.F. The anti-inflammatory effect of hydrogen gas inhalation and its influence on laser-induced choroidal neovascularization in a mouse model of neovascular age-related macular degeneration. Int. J. Mol. Sci. 2021, 22, 2049. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhu, M.; Guo, Y.; Tu, Y.; Zhou, Y.; Zeng, J.; Zhu, L.; Du, S.; Wang, Z.; et al. Shikonin alleviates choroidal neovascularization by inhibiting proangiogenic factor production from infiltrating macrophages. Exp. Eye Res. 2021, 213, 108823. [Google Scholar] [CrossRef] [PubMed]
- Do, J.Y.; Kim, J.; Kim, M.J.; Lee, J.Y.; Park, S.Y.; Yanai, R.; Lee, I.K.; Park, S.; Park, D.H. Fursultiamine alleviates choroidal neovascularization by suppressing inflammation and metabolic reprogramming. Investig. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Liu, J.; Huang, L.; Qin, B.; Yang, X.; Li, L.; Liu, Y.; Gu, J.; Wu, W.; Yu, Y.; et al. Andrographolide attenuates choroidal neovascularization by inhibiting the HIF-1α/VEGF signaling pathway. Biochem. Biophys. Res. Commun. 2020, 530, 60–66. [Google Scholar] [CrossRef]
- Narendran, S.; Pereira, F.; Yerramothu, P.; Apicella, I.; Wang, S.B.; Varshney, A.; Baker, K.L.; Marion, K.M.; Ambati, M.; Ambati, V.L.; et al. A clinical metabolite of azidothymidine inhibits experimental choroidal neovascularization and retinal pigmented epithelium degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Bao, Y.; Huang, L.; Huang, X.; Gao, C.; Chen, Y.; Wu, L.; Zhu, S.; Song, Y. Pirfenidone ameliorates the formation of choroidal neovascularization in mice. Mol. Med. Rep. 2020, 21, 2162–2170. [Google Scholar] [CrossRef]
- Heloterä, H.; Kaarniranta, K. A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration. Cells 2022, 11, 3453. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, V.M.; Chan, C.C. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye 2011, 25, 127–139. [Google Scholar] [CrossRef]
- Kuppermann, B.D.; Goldstein, M.; Maturi, R.K.; Pollack, A.; Singer, M.; Tufail, A.; Weinberger, D.; Li, X.Y.; Liu, C.C.; Lou, J.; et al. Dexamethasone Intravitreal Implant as Adjunctive Therapy to Ranibizumab in Neovascular Age-Related Macular Degeneration: A Multicenter Randomized Controlled Trial. Ophthalmologica 2015, 234, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Becerra, E.M.; Morescalchi, F.; Gandolfo, F.; Danzi, P.; Nascimbeni, G.; Arcidiacono, B.; Semeraro, F. Clinical evidence of intravitreal triamcinolone acetonide in the management of age-related macular degeneration. Curr. Drug Targets 2011, 12, 149–172. [Google Scholar] [CrossRef]
- Ando, R.; Hirooka, K.; Saito, M.; Kase, S.; Noda, K.; Ishida, S. Two-year clinical outcomes of triple therapy with photodynamic therapy, anti-vascular endothelial growth factor agent, and triamcinolone acetonide for neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2023, 67, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Forte, R.; Bonavolontà, P.; Benayoun, Y.; Adenis, J.P.; Robert, P.Y. Intravitreal ranibizumab and bevacizumab in combination with full-fluence verteporfin therapy and dexamethasone for exudative age-related macular degeneration. Ophthalmic Res. 2011, 45, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Frimpong-Boateng, A.; Bunse, A.; Rüfer, F.; Roider, J. Photodynamic therapy with intravitreal application of triamcinolone acetonide in age-related macular degeneration: Functional results in 54 patients. Acta Ophthalmol. 2009, 87, 183–187. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas, A.; Badia, A.; Fontrodona, L.; Zapata, M.; García-Arumí, J.; Duarri, A. Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model. Biomedicines 2023, 11, 2445. https://doi.org/10.3390/biomedicines11092445
Salas A, Badia A, Fontrodona L, Zapata M, García-Arumí J, Duarri A. Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model. Biomedicines. 2023; 11(9):2445. https://doi.org/10.3390/biomedicines11092445
Chicago/Turabian StyleSalas, Anna, Anna Badia, Laura Fontrodona, Miguel Zapata, José García-Arumí, and Anna Duarri. 2023. "Neovascular Progression and Retinal Dysfunction in the Laser-Induced Choroidal Neovascularization Mouse Model" Biomedicines 11, no. 9: 2445. https://doi.org/10.3390/biomedicines11092445



