7-Hydroxy Frullanolide Ameliorates Isoproterenol-Induced Myocardial Injury through Modification of iNOS and Nrf2 Genes
Abstract
:1. Introduction
2. Methods
2.1. Chemicals and Reagents
2.2. Source of 7-Hydroxy Frullanolide (7-HF)
2.3. Ethical Approval
2.4. MI Induction in Rats
2.5. Animals and Experimental Design
2.6. Electocardioghrapy
2.7. Myocardial Infarct Quantification
2.8. Histopathological Evaluation
2.9. Estimation of Cardiac Markers
2.10. Determination of Oxidative Stress and Antioxidant Activity
2.11. PCR Analysis for iNOS and Nrf2
2.12. Principal Mechanism on Isolated Rat Atria
2.13. Vascular Activity on Isolated Rat Aorta
2.13.1. Effect of 7-Hydroxy Frullanolide on Phenylephrine, K+ (80 mM) and Ang II Pre-Contraction
2.13.2. Evaluating the Effect of 7-Hydroxy Frullanolide in L-NAME, Atropine and Indomethacin Presence
2.13.3. Effect of 7-Hydroxy Frullanolide on Voltage-Dependent Calcium Channel
2.13.4. Effect of 7-Hydroxy Frullanolide on Intracellular Ca2+ Stores
2.13.5. Effect of 7-Hydroxy Frullanolide on K+-Channels
2.13.6. Statistical Analysis
3. Results
3.1. In Vivo Studies
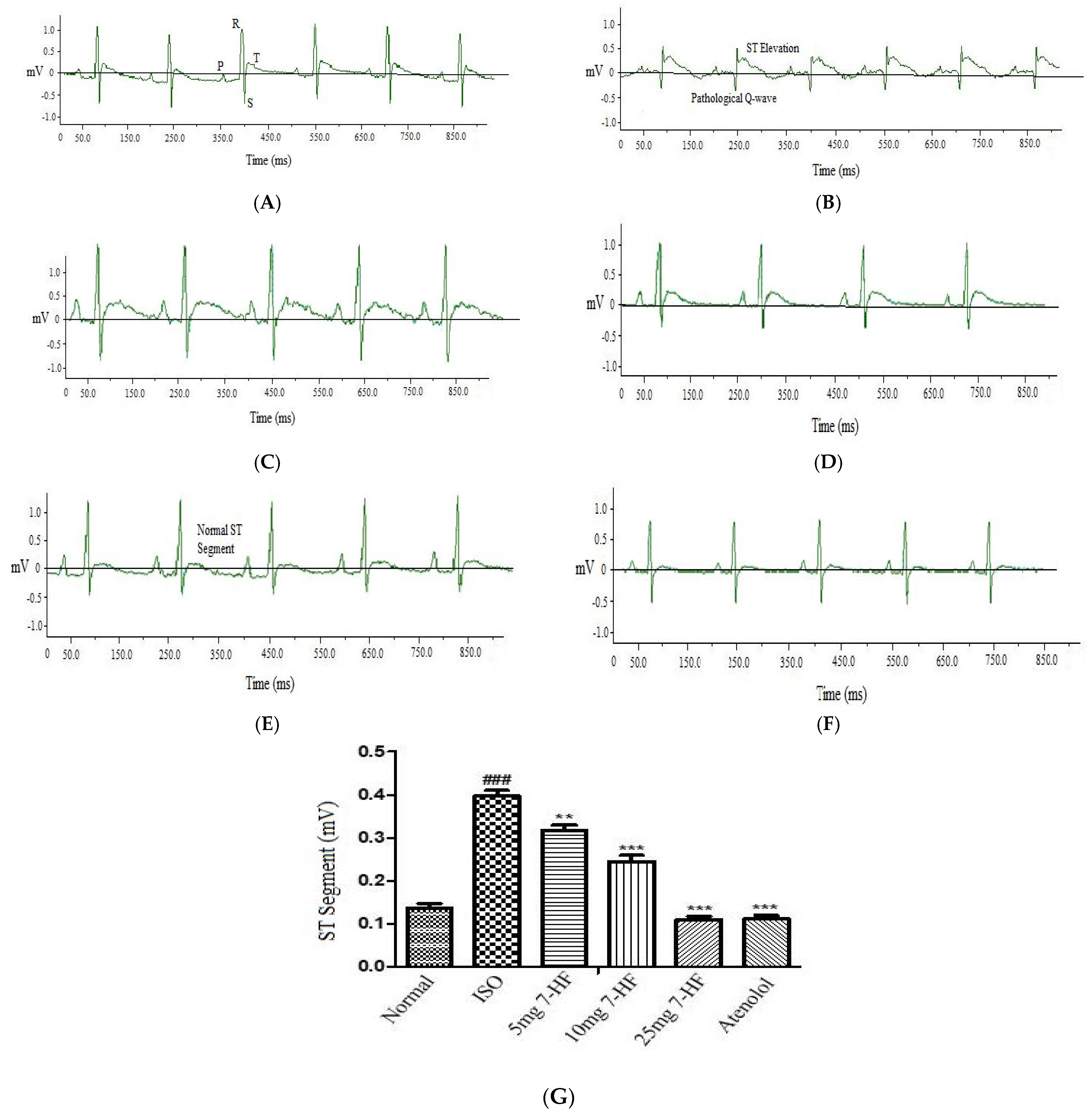
Effect of 7-Hydroxy Frullanolide on Electrocardiograph (ECG) Pattern
3.2. In Vitro Studies
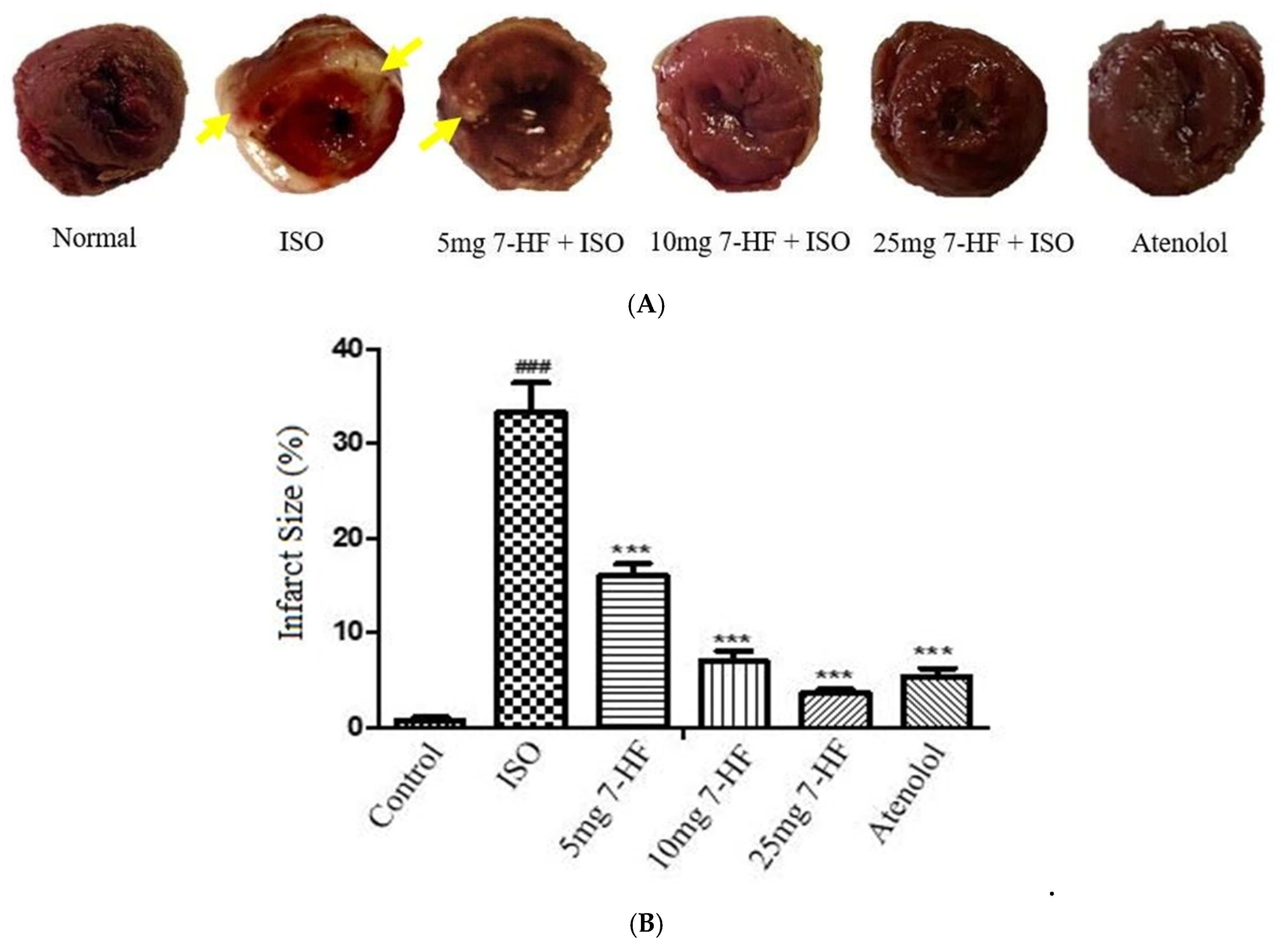
3.2.1. Effect of 7-Hydroxy Frullanolide on Infarct Size
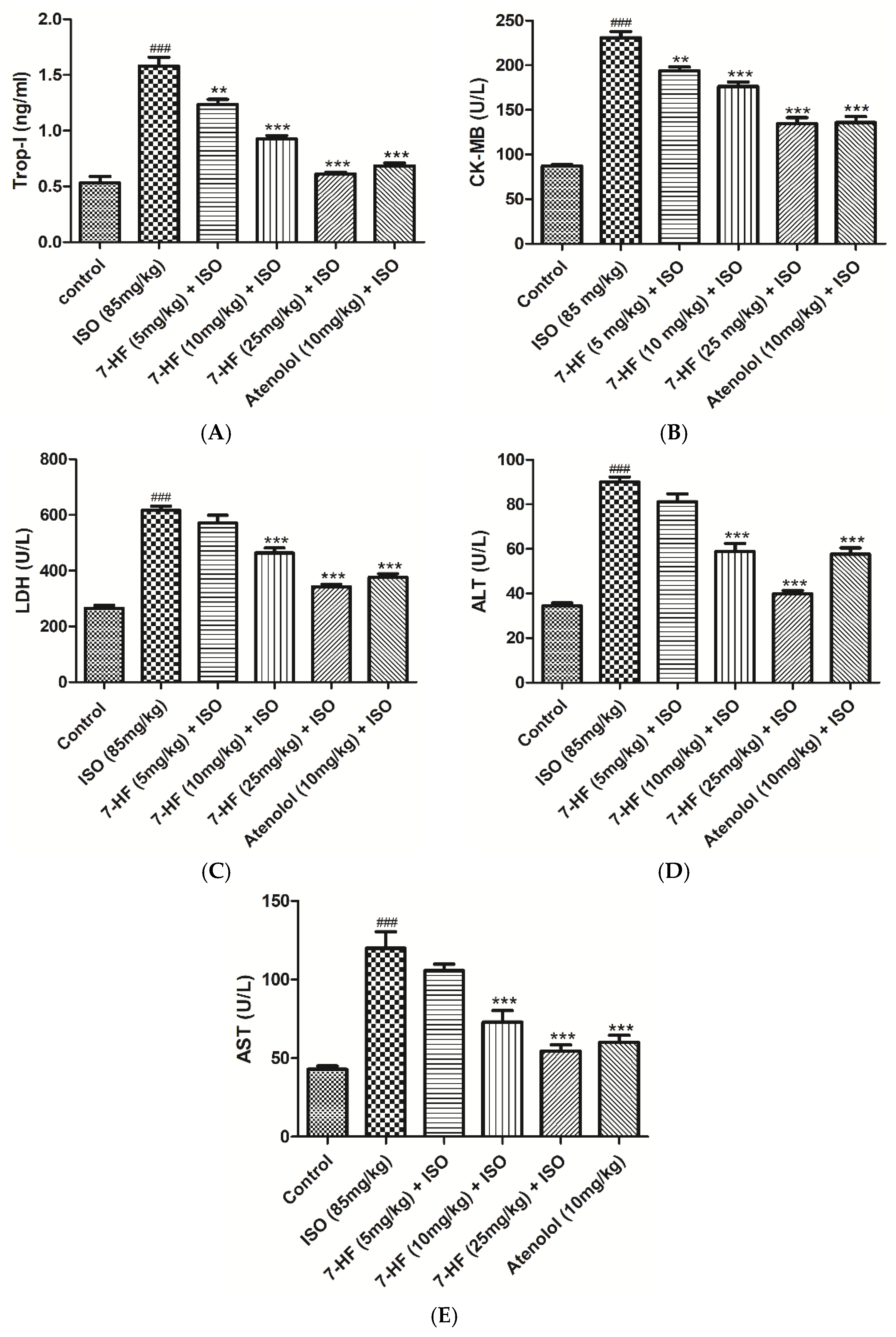
3.2.2. Effect of 7-Hydroxy Frullanolide on Cardiac Biomarkers
3.2.3. Histopathological Examination of Heart Tissue
3.2.4. Effect of 7-HF on Oxidative Stress and Antioxidant Enzymes
3.2.5. PCR Analysis of Nrf2 and iNOS
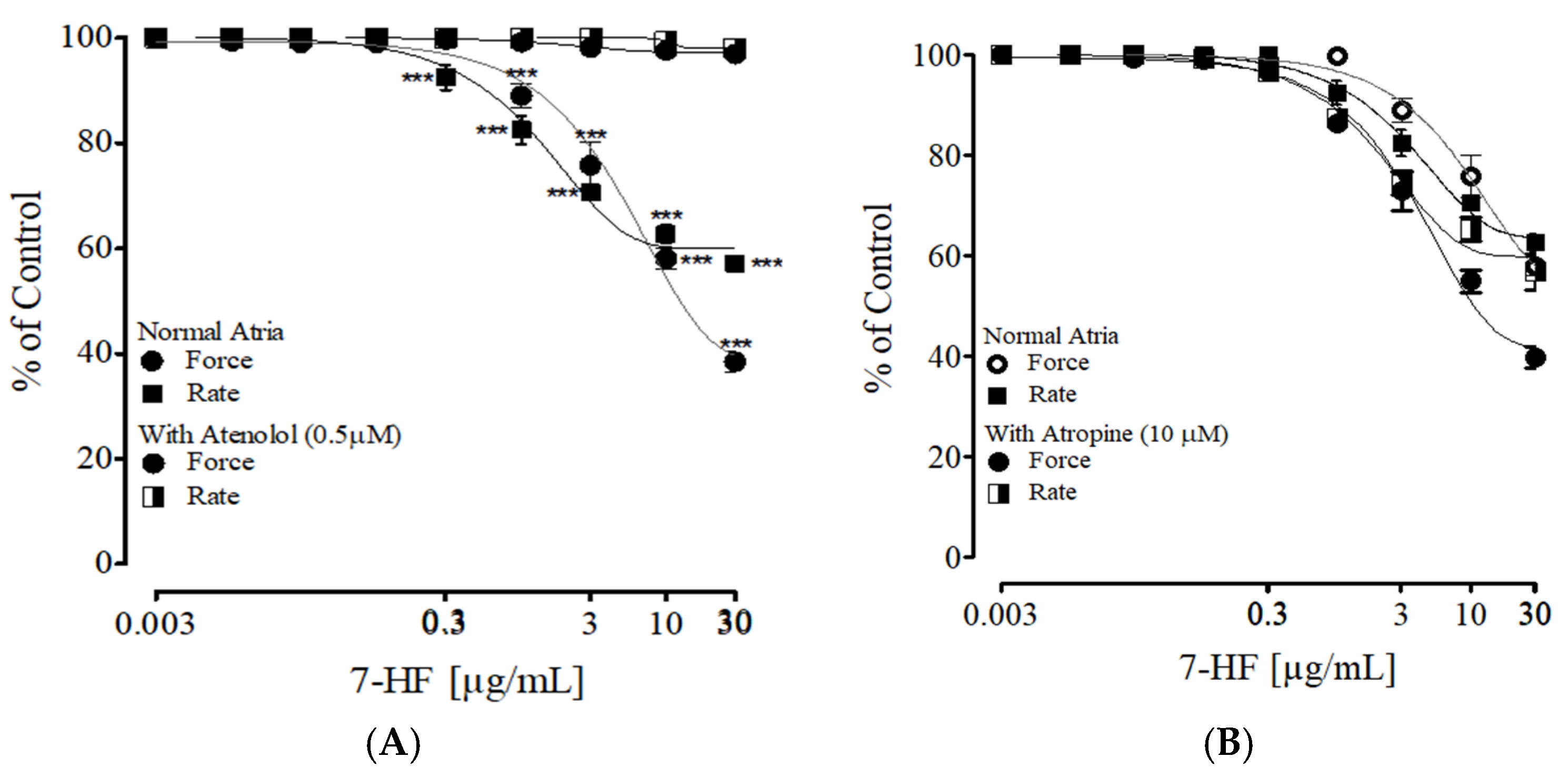
3.2.6. Effect of 7-HF on Isolated Rat Atria
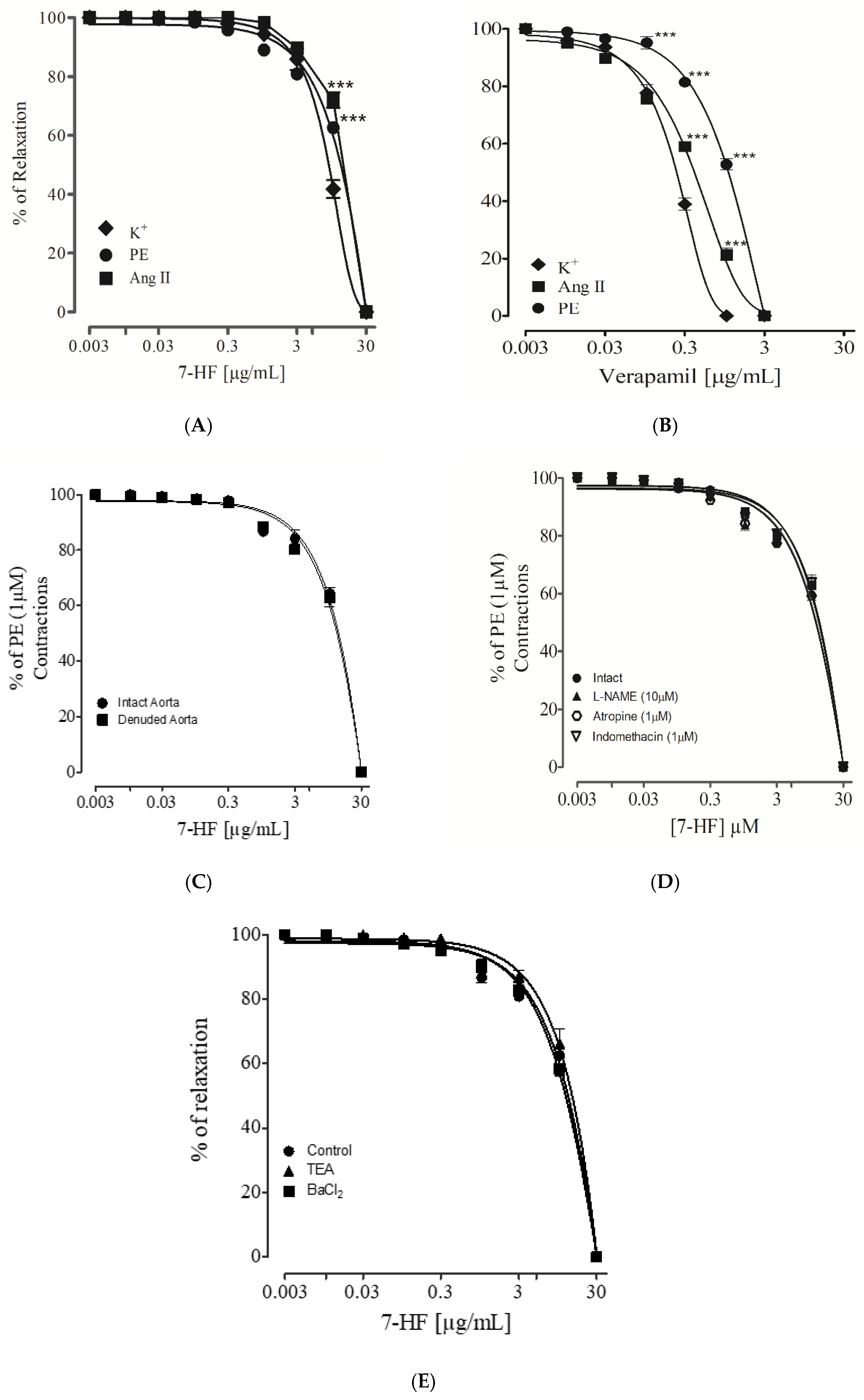
3.2.7. Effect of 7-Hydroxy Frullanolide on Pre-Contraction Produced by Phenylephrine, K+ (80 mM) and Ang II
3.2.8. Endothelium-Dependent and Independent Effect of 7-Hydroxy Frullanolide
3.2.9. Evaluating the Effect of 7-Hydroxy Frullanolide in Aorta Pre-Incubated with L-NAME, Atropine and Indomethacin
3.2.10. Effect of 7-Hydroxy Frullanolide on K+-Channels
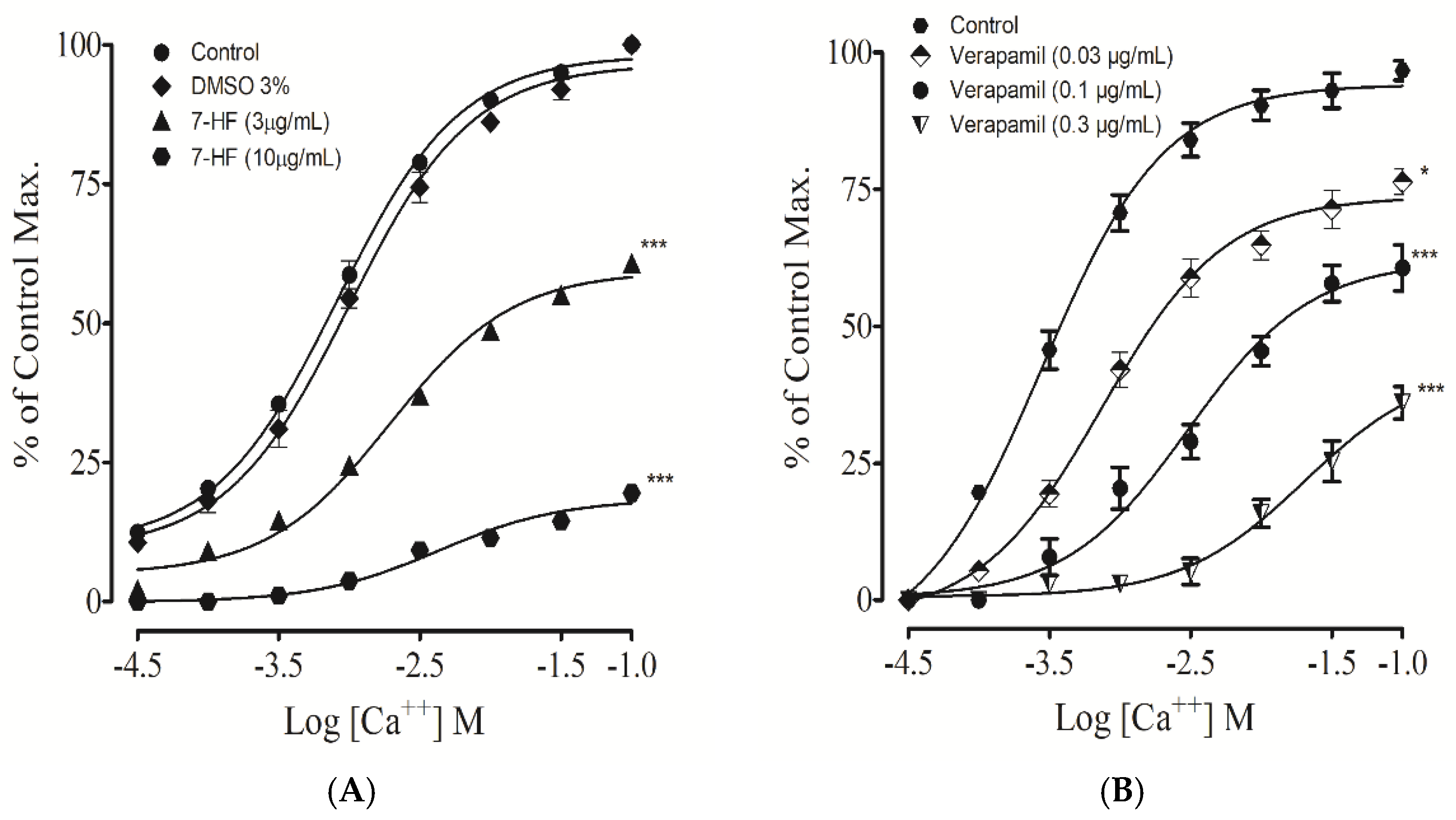
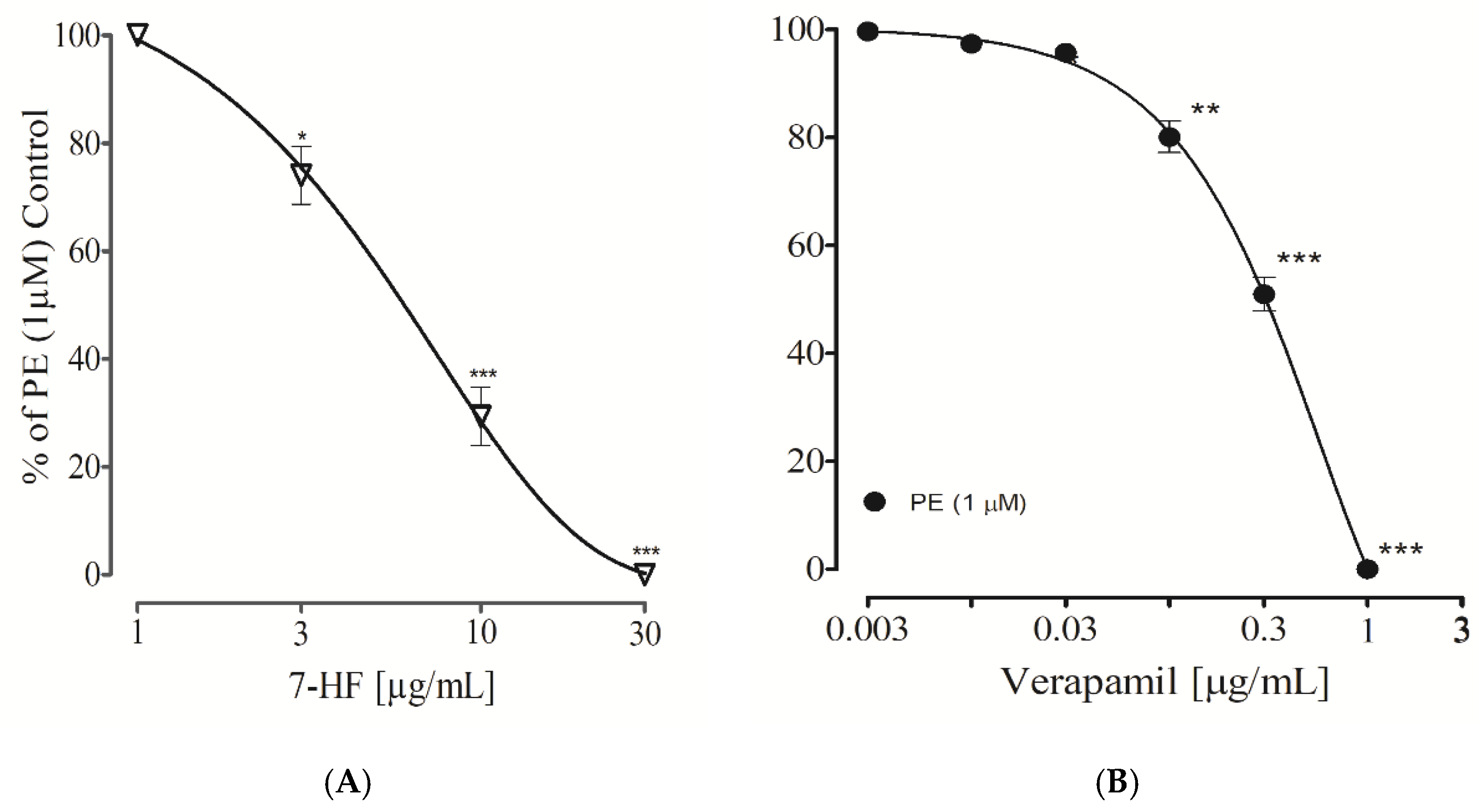
3.2.11. Effect of 7-Hydroxy Frulanolide on Calcium Channels
3.2.12. Effect of 7-Hydroxy Frulanolide on Intracellular Ca2+ Stores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jayaraj, J.C.; Davatyan, K.; Subramanian, S.V.; Priya, J.V.S. Epidemiology of Myocardial Infarction. In Myocardial Infarction; IntechOpen: London, UK, 2018. [Google Scholar]
- Institute of Medicine (US) Committee on Social Security Cardiovascular Disability Criteria. Cardiovascular Disability: Updating the Social Security Listings; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Mendis, S.; Abegunde, D.; Yusuf, S.; Ebrahim, S.; Shaper, G.; Ghannem, H.; Shengelia, B. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE). Bull. World Health Organ. 2005, 83, 820–829. [Google Scholar] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, X.; Shi, J.; Wu, X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int. J. Biol. Macromol. 2019, 125, 496–502. [Google Scholar] [CrossRef]
- Wu, J.; Hecker, J.G.; Chiamvimonvat, N. Antioxidant enzyme gene transfer for ischemic diseases. Adv. Drug Deliv. Rev. 2009, 61, 351–363. [Google Scholar] [CrossRef]
- Zhang, J.; Knapton, A.; Lipshultz, S.E.; Weaver, J.L.; Herman, E.H. Isoproterenol-induced cardiotoxicity in sprague-dawley rats: Correlation of reversible and irreversible myocardial injury with release of cardiac troponin T and roles of iNOS in myocardial injury. Toxicol. Pathol. 2008, 36, 277–278. [Google Scholar] [CrossRef]
- Talukder, M.A.; Zweier, J.L.; Periasamy, M. Targeting calcium transport in ischaemic heart disease. Cardiovasc. Res. 2009, 84, 345–352. [Google Scholar] [CrossRef]
- Yang, Z.; Min Zhou, D. Cardiac markers and their point-of-care testing for diagnosis of acute myocardial infarction. Clin. Biochem. 2006, 39, 771–780. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ahn, J.K.; Shin, K.A.; Kim, C.H.; Lee, Y.H.; Park, K.M. Correlation of Cardiac Markers and Biomarkers with Blood Pressure of Middle-Aged Marathon Runners. J. Clin. Hypertens. 2015, 17, 868–873. [Google Scholar] [CrossRef]
- Ertracht, O.; Liani, E.; Bachner-Hinenzon, N.; Bar-Am, O.; Frolov, L.; Ovcharenko, E.; Awad, H.; Blum, S.; Barac, Y.; Amit, T.; et al. The cardioprotective efficacy of TVP1022 in a rat model of ischaemia/reperfusion. Br. J. Pharmacol. 2011, 163, 755–769. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef]
- Zingarelli, B.; Hake, P.W.; Denenberg, A.; Wong, H.R. Sesquiterpene lactone parthenolide, an inhibitor of IkappaB kinase complex and nuclear factor-kappaB, exerts beneficial effects in myocardial reperfusion injury. Shock 2002, 17, 127–134. [Google Scholar] [CrossRef]
- Fonseca, L.C.; Dadarkar, S.S.; Lobo, A.S.; Suthar, A.C.; Chauhan, V.S.; Chandrababu, S.; Sharma, S.D.; Dagia, N.M.; Padigaru, M. 7-hydroxyfrullanolide, a sesquiterpene lactone, inhibits pro-inflammatory cytokine production from immune cells and is orally efficacious in animal models of inflammation. Eur. J. Pharmacol. 2010, 644, 220–229. [Google Scholar] [CrossRef]
- Srivastava, R.A.; Mistry, S.; Sharma, S. A novel anti-inflammatory natural product from Sphaeranthus indicus inhibits expression of VCAM1 and ICAM1, and slows atherosclerosis progression independent of lipid changes. Nutr. Metab. 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Gokhroo, A.; Kumar Dubey, A.; Majumdar, S.; Gupta, S.; Almeida, A.; Mahajan, G.B.; Kate, A.; Mishra, P.; Sharma, R.; et al. 7-Hydroxy Frullanolide, a sesquiterpene lactone, increases intracellular calcium amounts, lowers CD4+ T cell and macrophage responses, and ameliorates DSS-induced colitis. Int. Immunopharmacol. 2021, 97, 107655. [Google Scholar] [CrossRef] [PubMed]
- Marleau, S.; Harb, D.; Bujold, K.; Avallone, R.; Iken, K.; Wang, Y.; Demers, A.; Sirois, M.G.; Febbraio, M.; Silverstein, R.L.; et al. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J. 2005, 19, 1869–1871. [Google Scholar] [CrossRef]
- Bessi, V.L.; Labbé, S.M.; Huynh, D.N.; Ménard, L.; Jossart, C.; Febbraio, M.; Guérin, B.; Bentourkia, M.; Lecomte, R.; Carpentier, A.C.; et al. EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovasc. Res. 2012, 96, 99–108. [Google Scholar] [CrossRef]
- Nagendran, J.; Pulinilkunnil, T.; Kienesberger, P.C.; Sung, M.M.; Fung, D.; Febbraio, M.; Dyck, J.R. Cardiomyocyte-specific ablation of CD36 improves post-ischemic functional recovery. J. Mol. Cell Cardiol. 2013, 63, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.M.; Wahid, F.; Ikram, M.; Qaisar, M.; Shah, A.J.; Khan, T. Chemical profiling and anti-breast cancer potential of hexane fraction of Sphaeranthus indicus flowers. Trop. J. Pharm. Res. 2021, 20, 1931–1939. [Google Scholar] [CrossRef]
- Konopelski, P.; Ufnal, M. Electrocardiography in rats: A comparison to human. Physiol. Res. 2016, 65, 717–725. [Google Scholar] [CrossRef]
- Tiwari, R.; Mohan, M.; Kasture, S.; Maxia, A.; Ballero, M. Cardioprotective potential of myricetin in isoproterenol-induced myocardial infarction in Wistar rats. Phytother. Res. 2009, 23, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Dianita, R.; Jantan, I.; Amran, A.Z.; Jalil, J. Protective effects of Labisia pumila var. alata on biochemical and histopathological alterations of cardiac muscle cells in isoproterenol-induced myocardial infarction rats. Molecules 2015, 20, 4746–4763. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.F.; Sawyer, B.C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem. J. 1971, 123, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Salma, U.; Khan, T.; Shah, A.J. Antihypertensive effect of the methanolic extract from Eruca sativa Mill., (Brassicaceae) in rats: Muscarinic receptor-linked vasorelaxant and cardiotonic effects. J. Ethnopharmacol. 2018, 224, 409–420. [Google Scholar] [CrossRef]
- Bucci, M.; Roviezzo, F.; Cicala, C.; Pinto, A.; Cirino, G. 17-beta-oestradiol-induced vasorelaxation in vitro is mediated by eNOS through hsp90 and akt/pkb dependent mechanism. Br. J. Pharmacol. 2002, 135, 1695–1700. [Google Scholar] [CrossRef]
- Chan, S.S.; Choi, A.O.; Jones, R.L.; Lin, G. Mechanisms underlying the vasorelaxing effects of butylidenephthalide, an active constituent of Ligusticum chuanxiong, in rat isolated aorta. Eur. J. Pharmacol. 2006, 537, 111–117. [Google Scholar] [CrossRef]
- Ahmad, T.; Qayyum, R.; Khan, T.; Mahnashi, M.H.; Jalal, M.M.; Altayar, M.A.; Alshehri, O.M.; Shah, A.J. Vasorelaxant and Antihypertensive Effects of Bergenin on Isolated Rat Aorta and High Salt-Induced Hypertensive Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 4886193. [Google Scholar] [CrossRef]
- Faraci, F.M.; Heistad, D.D. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiol. Rev. 1998, 78, 53–97. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Senejoux, F.; Girard, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Demougeot, C. Mechanisms of vasorelaxation induced by Ziziphora clinopodioides Lam. (Lamiaceae) extract in rat thoracic aorta. J. Ethnopharmacol. 2010, 132, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.; Palade, P.T.; Marsh, J.D.; Telemaque, S.; Pesic, A.; Rusch, N.J. Vascular calcium channels and high blood pressure: Pathophysiology and therapeutic implications. Vasc. Pharmacol. 2006, 44, 131–142. [Google Scholar] [CrossRef]
- Qayyum, R.; Qamar, H.M.; Khan, S.; Salma, U.; Khan, T.; Shah, A.J. Mechanisms underlying the antihypertensive properties of Urtica dioica. J. Transl. Med. 2016, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Yan, L.; Iwatsubo, K.; Vatner, D.E.; Vatner, S.F. Modulation of beta-adrenergic receptor signaling in heart failure and longevity: Targeting adenylyl cyclase type 5. Heart Fail. Rev. 2010, 15, 495–512. [Google Scholar] [CrossRef] [PubMed]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Tian, S.; Yang, F.; Yang, H.G.; Yang, X.Y.; Du, G.H. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur. J. Pharmacol. 2009, 615, 125–132. [Google Scholar] [CrossRef]
- Yeager, J.C.; Whitehurst, M.E. Verapamil prevents isoproterenol-induced cardiac failure in the rat. Life Sci. 1982, 30, 299–306. [Google Scholar] [CrossRef]
- Kondo, T.; Ogawa, Y.; Sugiyama, S.; Ito, T.; Satake, T.; Ozawa, T. Mechanism of isoproterenol induced myocardial damage. Cardiovasc. Res. 1987, 21, 248–254. [Google Scholar] [CrossRef]
- Wexler, B.C. Myocardial infarction in young vs old male rats: Pathophysiologic changes. Am. Heart J. 1978, 96, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A.; Biswas, S. Preventive effect of Agnucastoside C against Isoproterenol-induced myocardial injury. Sci. Rep. 2017, 7, 16146. [Google Scholar] [CrossRef] [PubMed]
- Suchalatha, S.; Shyamala Devi, C.S. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian. J. Exp. Biol. 2004, 42, 174–178. [Google Scholar] [PubMed]
- Roos, A.; Hellgren, A.; Rafatnia, F.; Hammarsten, O.; Ljung, R.; Carlsson, A.C.; Holzmann, M.J. Investigations, findings, and follow-up in patients with chest pain and elevated high-sensitivity cardiac troponin T levels but no myocardial infarction. Int. J. Cardiol. 2017, 232, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Laurent, M.; Lepoivre, M.; Tenu, J.P. Kinetic modelling of the nitric oxide gradient generated in vitro by adherent cells expressing inducible nitric oxide synthase. Biochem. J. 1996, 314 Pt 1, 109–113. [Google Scholar] [CrossRef]
- Roghani-Dehkordi, F.; Roghani, M. The vasorelaxant effect of simvastatin in isolated aorta from diabetic rats. ARYA Atheroscler. 2016, 12, 104–108. [Google Scholar]
- Bolton, T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979, 59, 606–718. [Google Scholar] [CrossRef]
- Karaki, H.; Ozaki, H.; Hori, M.; Mitsui-Saito, M.; Amano, K.; Harada, K.; Miyamoto, S.; Nakazawa, H.; Won, K.J.; Sato, K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997, 49, 157–230. [Google Scholar]
- Ross, G.R.; Yallampalli, C. Endothelium-independent relaxation by adrenomedullin in pregnant rat mesenteric artery: Role of cAMP-dependent protein kinase A and calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2006, 317, 1269–1275. [Google Scholar] [CrossRef] [PubMed]




| Accession No | Genes | Primer Sequence (5′-3′) |
|---|---|---|
| NM_031789.2 | Nrf2 | F-TTGTAGATGACCATGAGTCGC R-ACTTCCAGGGGCACTGTCTA |
| XM_039085203.1 | iNOS | F-GCAGGGCCACCTCTATGTTT R-TGGTCACCCAAAGTGCTTCA |
| NM_017008.4 | Gapdh | F-GGGTGTGAACCACGAGAAAT R-ACTGTGGTCAATGAGCCCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Ahmad, T.; Ikram, M.; Rasheed, H.M.; Khan, M.I.; Khan, T.; Alsahli, T.G.; Alzarea, S.I.; Althobaiti, M.; Shah, A.J. 7-Hydroxy Frullanolide Ameliorates Isoproterenol-Induced Myocardial Injury through Modification of iNOS and Nrf2 Genes. Biomedicines 2023, 11, 2470. https://doi.org/10.3390/biomedicines11092470
Ullah S, Ahmad T, Ikram M, Rasheed HM, Khan MI, Khan T, Alsahli TG, Alzarea SI, Althobaiti M, Shah AJ. 7-Hydroxy Frullanolide Ameliorates Isoproterenol-Induced Myocardial Injury through Modification of iNOS and Nrf2 Genes. Biomedicines. 2023; 11(9):2470. https://doi.org/10.3390/biomedicines11092470
Chicago/Turabian StyleUllah, Saif, Taseer Ahmad, Muhammad Ikram, Hafiz Majid Rasheed, Muhammad Ijaz Khan, Taous Khan, Tariq G. Alsahli, Sami I. Alzarea, Musaad Althobaiti, and Abdul Jabbar Shah. 2023. "7-Hydroxy Frullanolide Ameliorates Isoproterenol-Induced Myocardial Injury through Modification of iNOS and Nrf2 Genes" Biomedicines 11, no. 9: 2470. https://doi.org/10.3390/biomedicines11092470






