Structure and Function of Canine SP-C Mimic Proteins in Synthetic Surfactant Lipid Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
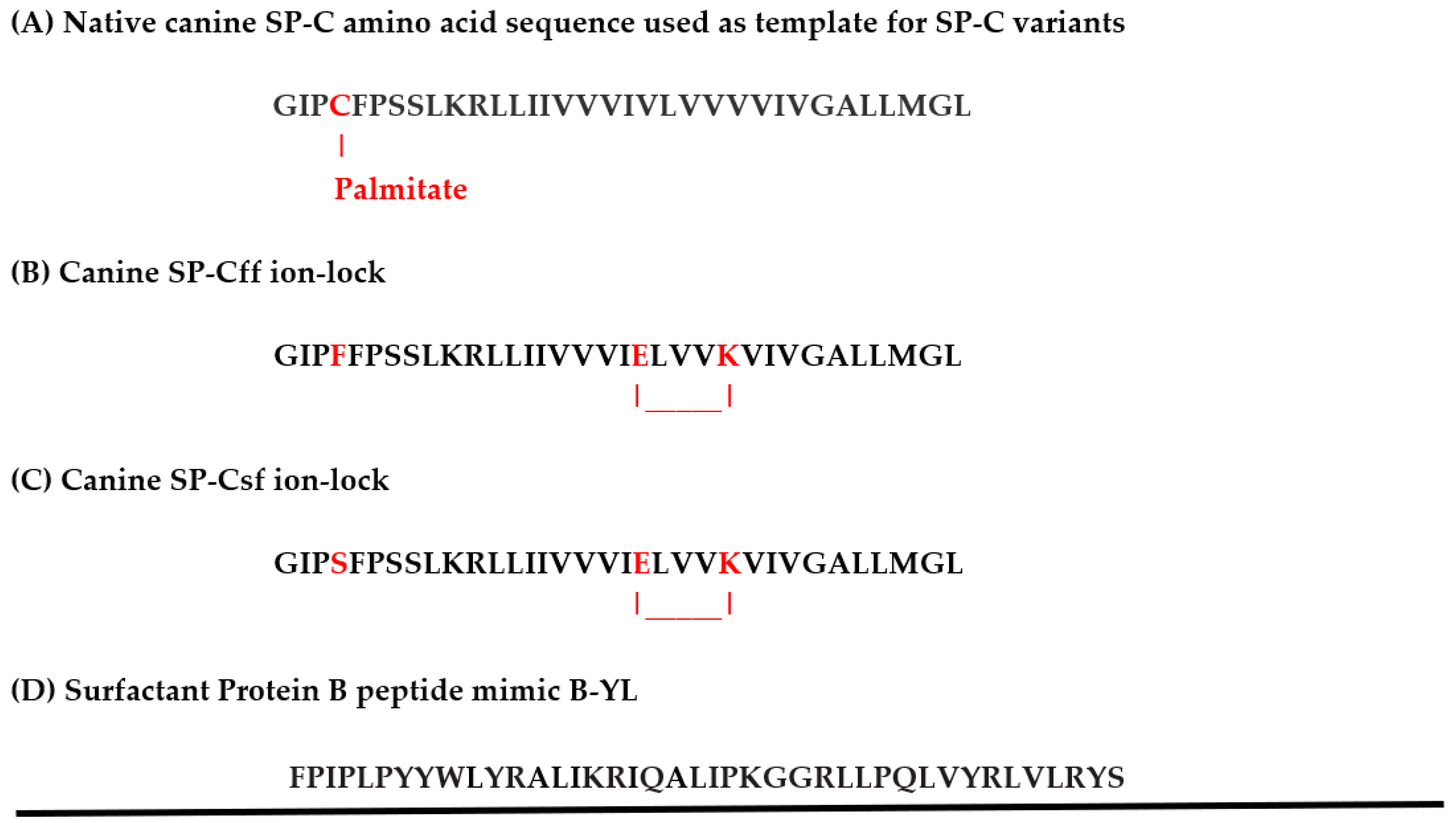
2.2. Canine SP-Cff and SP-Csf Ion-Lock and B-YL Peptide Synthesis
2.3. Surfactant Preparations
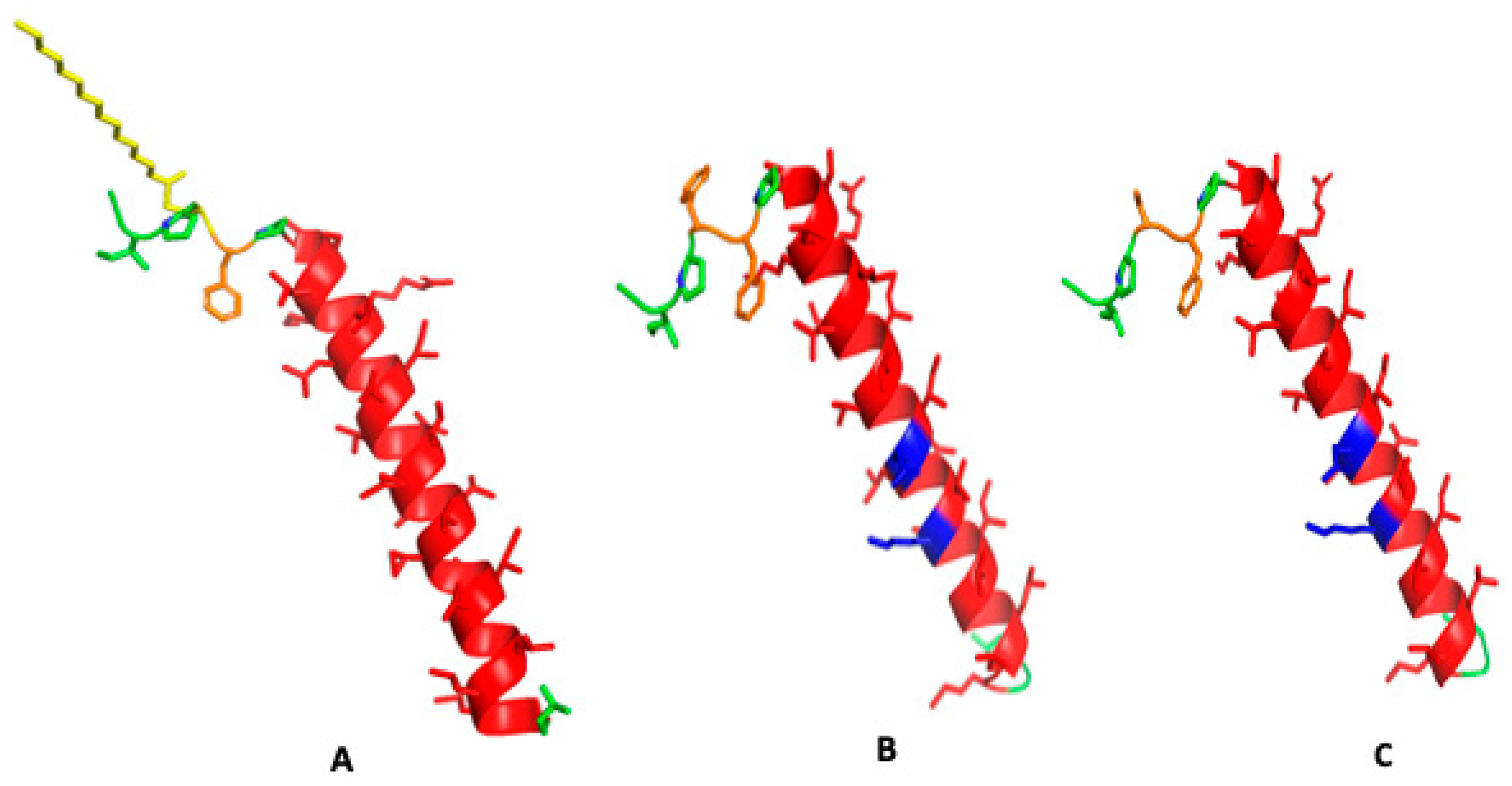
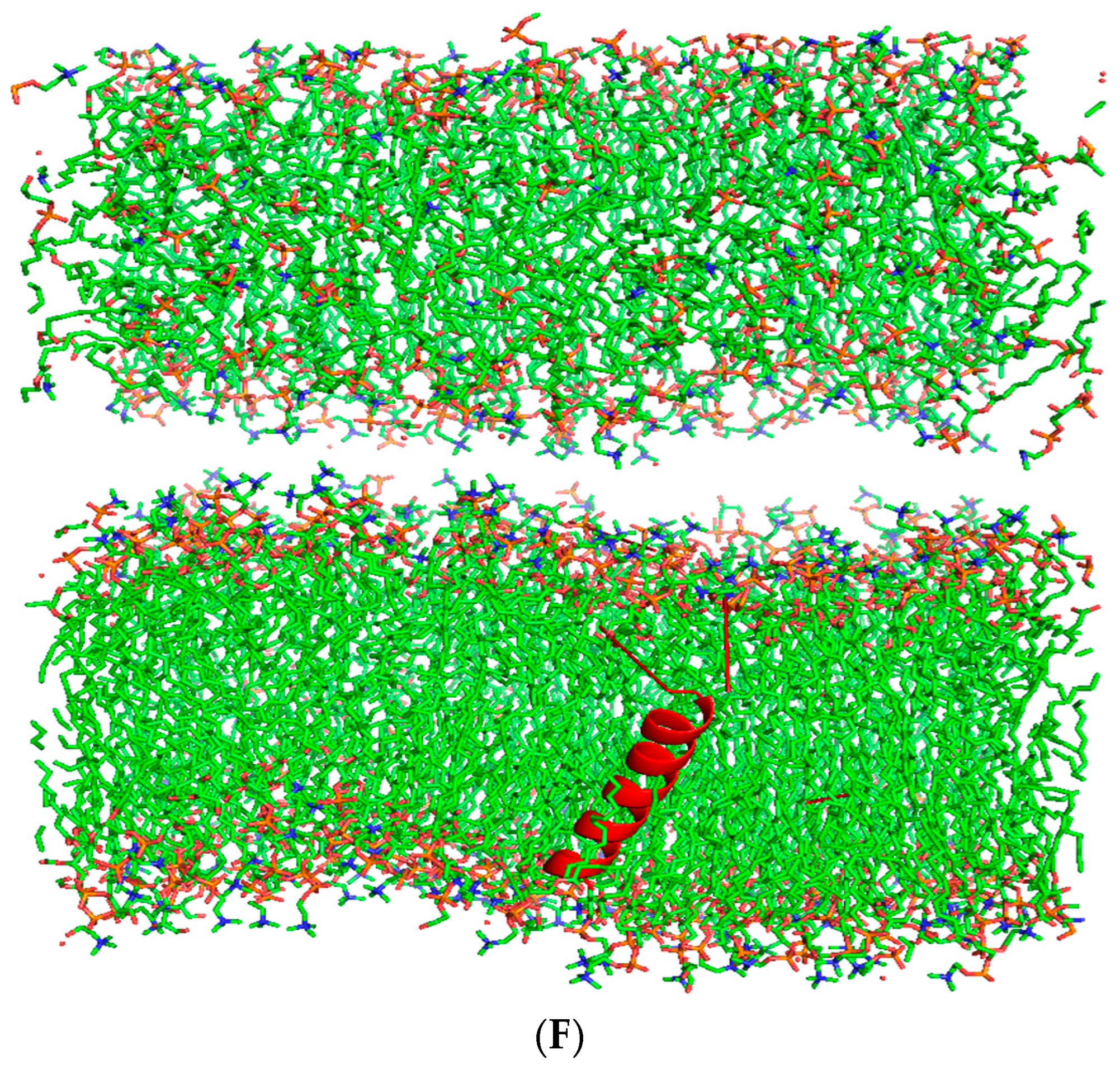
2.4. Structure Prediction—Alpha Fold (AI Modeling)
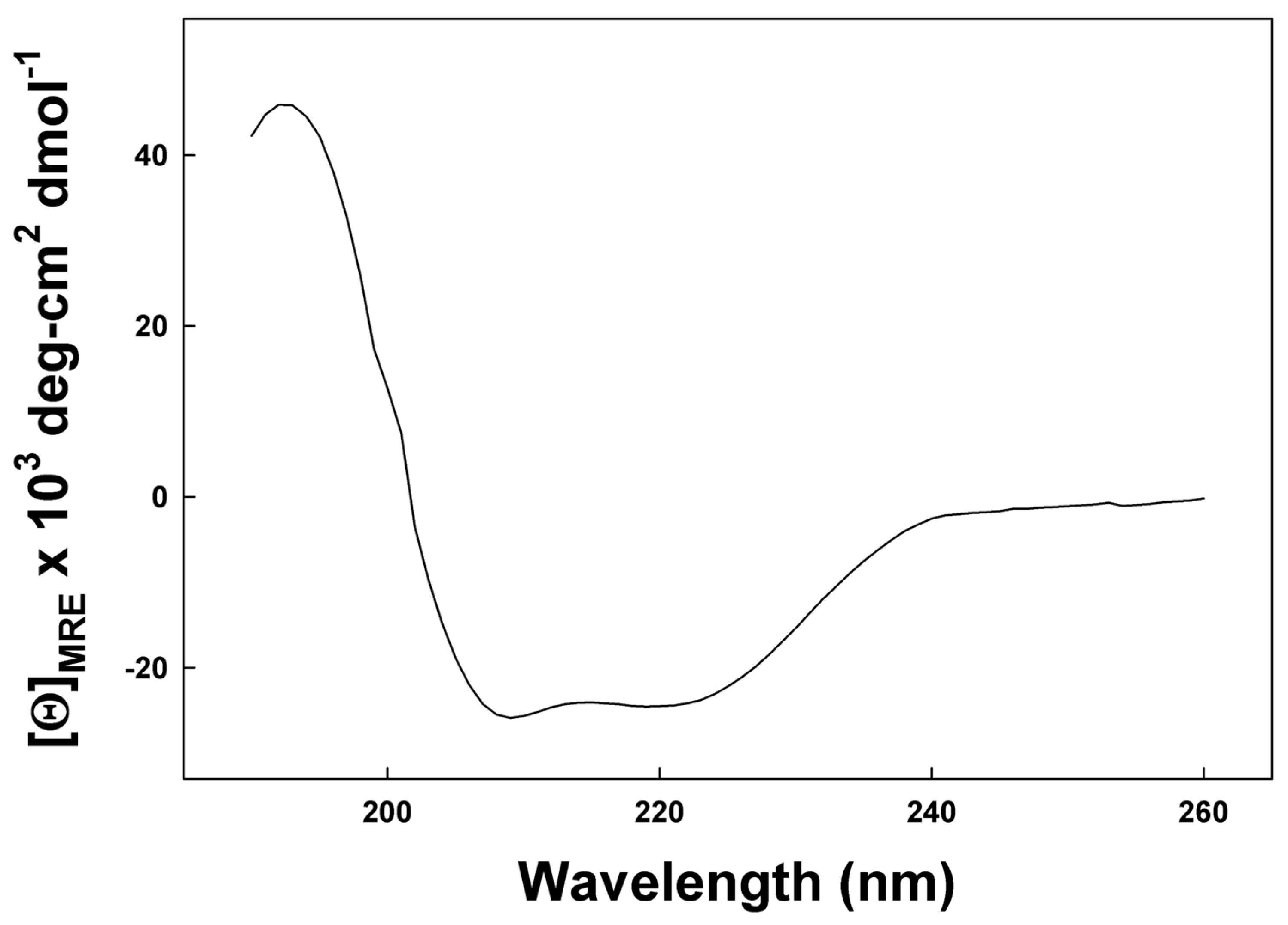
2.5. Circular Dichroic Characterization of SP-Cff ion-Lock Peptide in Membrane Mimic Micelles
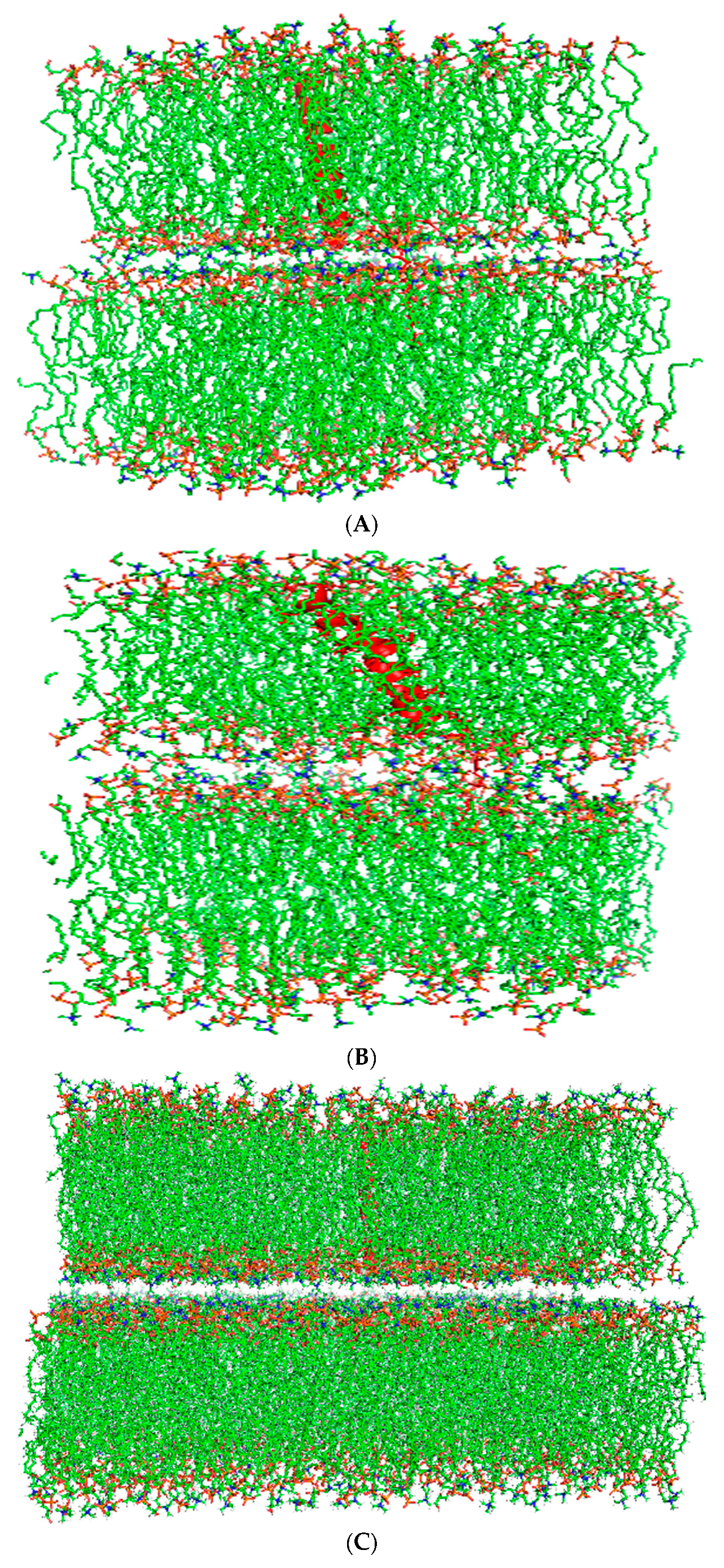
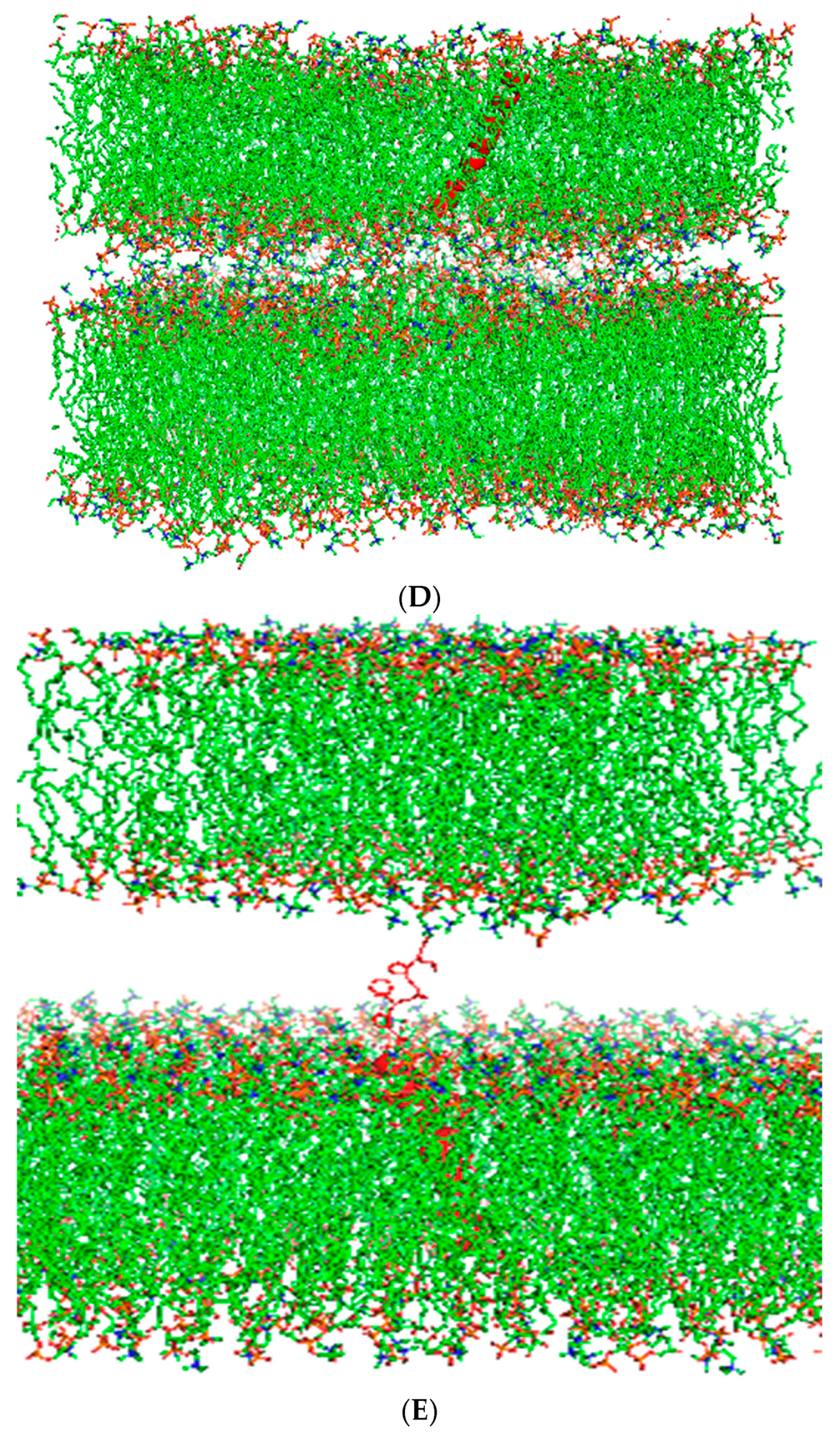
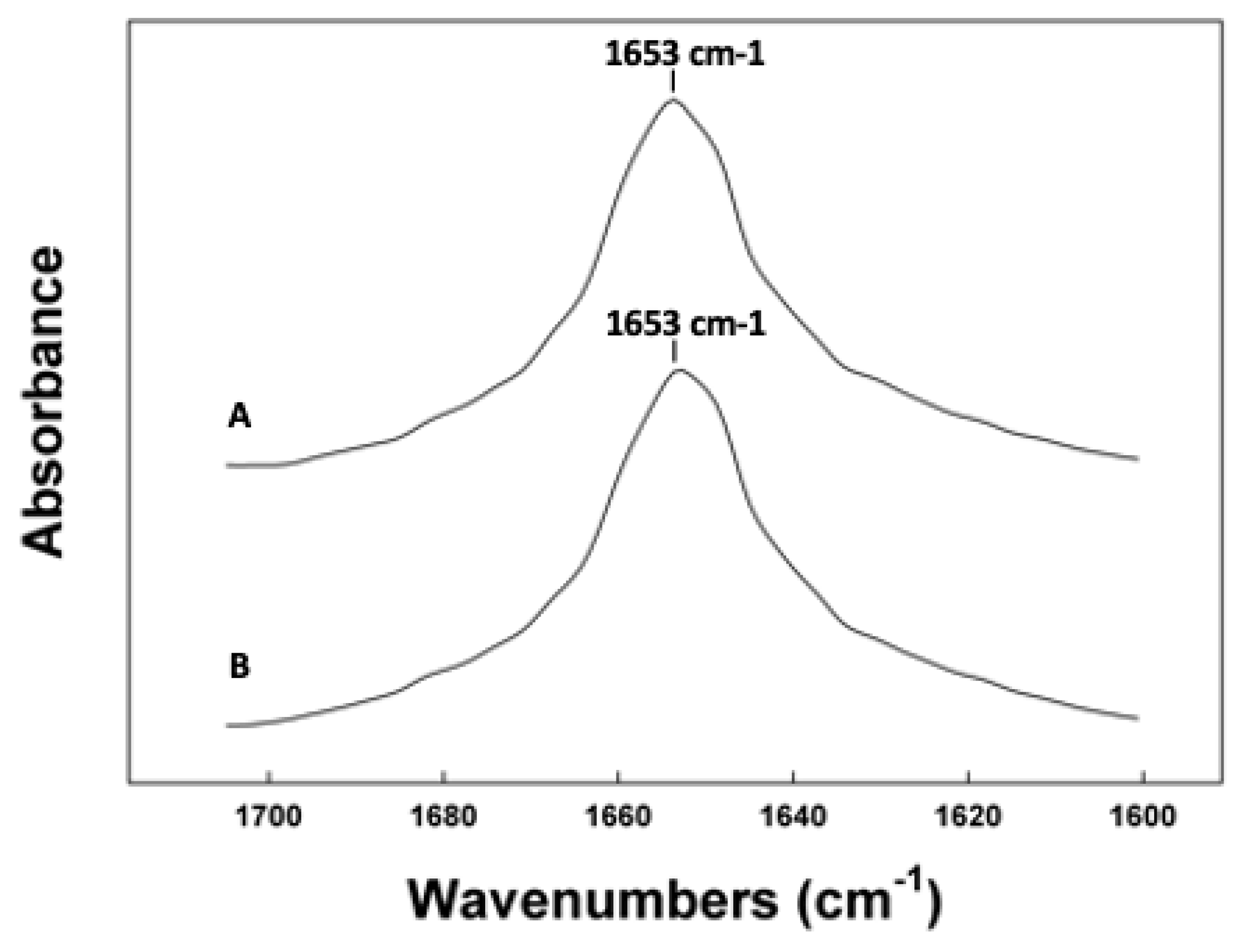
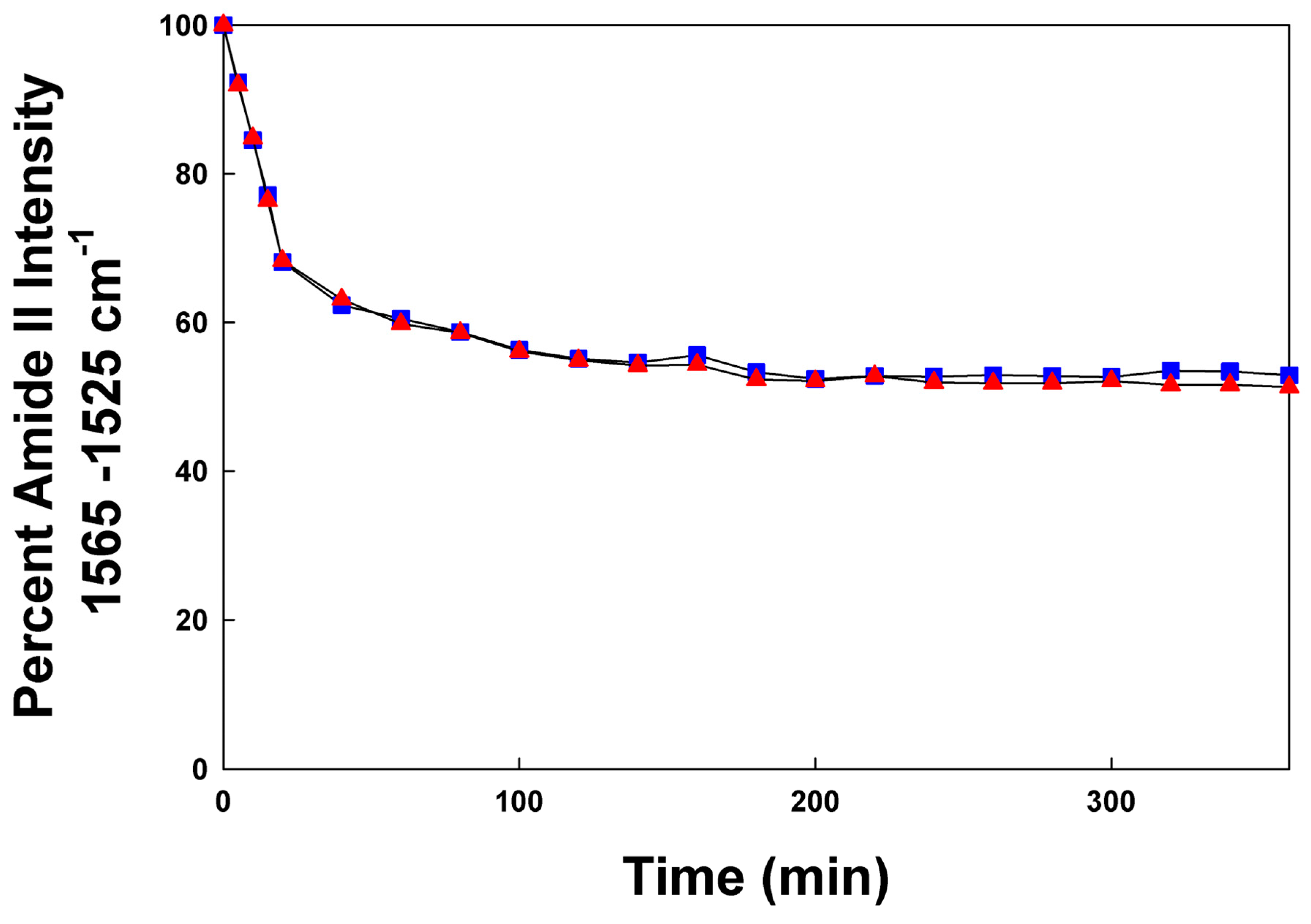
2.6. Secondary Structure Analysis and Orientation of Canine SP-C Ion-Lock Peptide Mimics in Synthetic Surfactant Lipids with FTIR Spectroscopy
2.7. Electron Spin Resonance (ESR) Measurements of Lipid Molecular Order Mediated by Synthetic Surfactant Peptides
2.8. Captive Bubble Surfactometry
3. Results
3.1. Secondary Structure Predictions of Canine SP-C Peptide Mimics
3.2. Experimental Determination of the Secondary Structure of Canine SP-C Ion-Lock Peptide Mimics in Micellar Membrane Mimic Dispersions and Surfactant Lipid Films
3.3. Changes in Lipid Molecular Order Induced by Synthetic Canine SP-C Ion-Lock Peptides
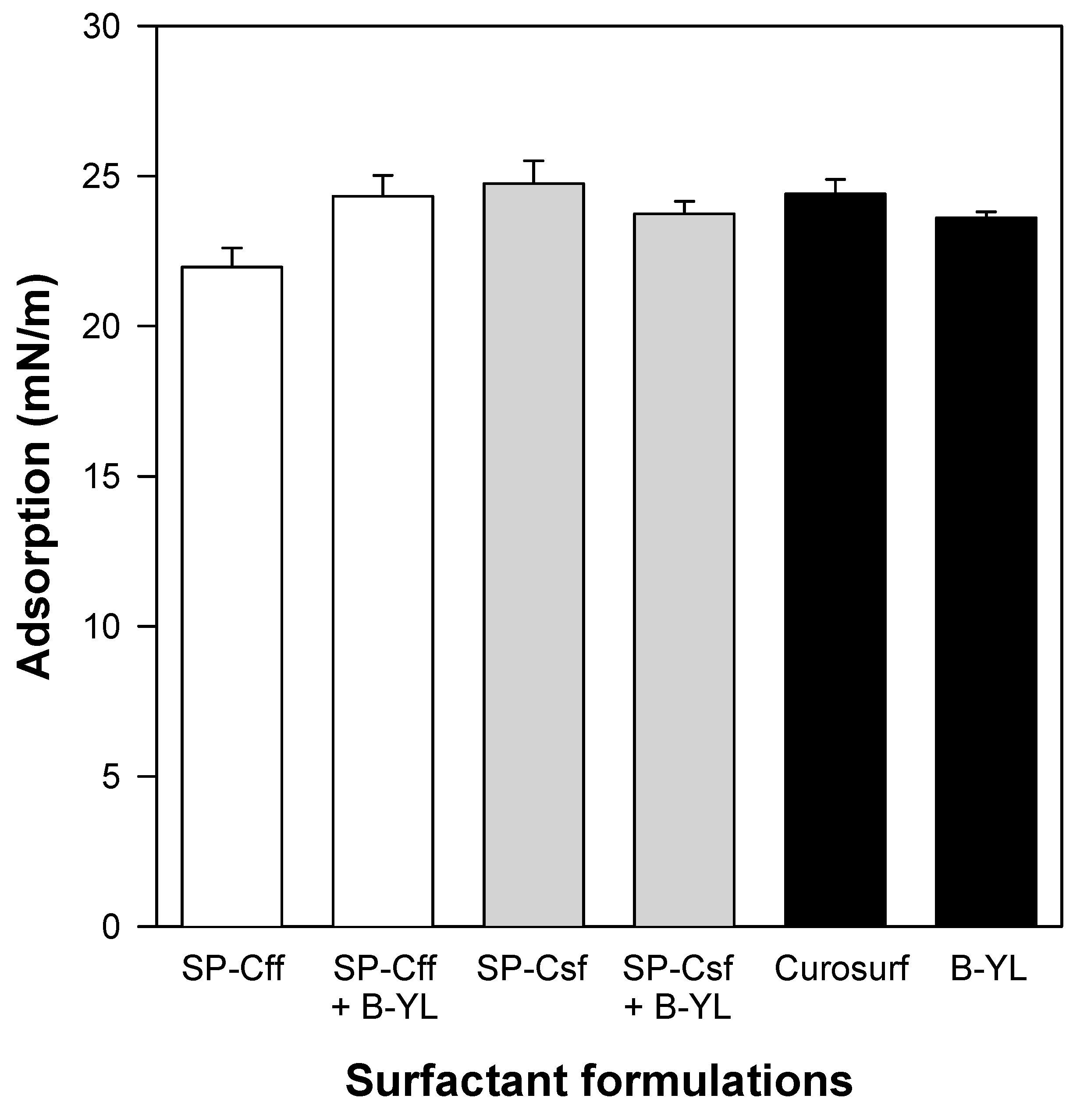
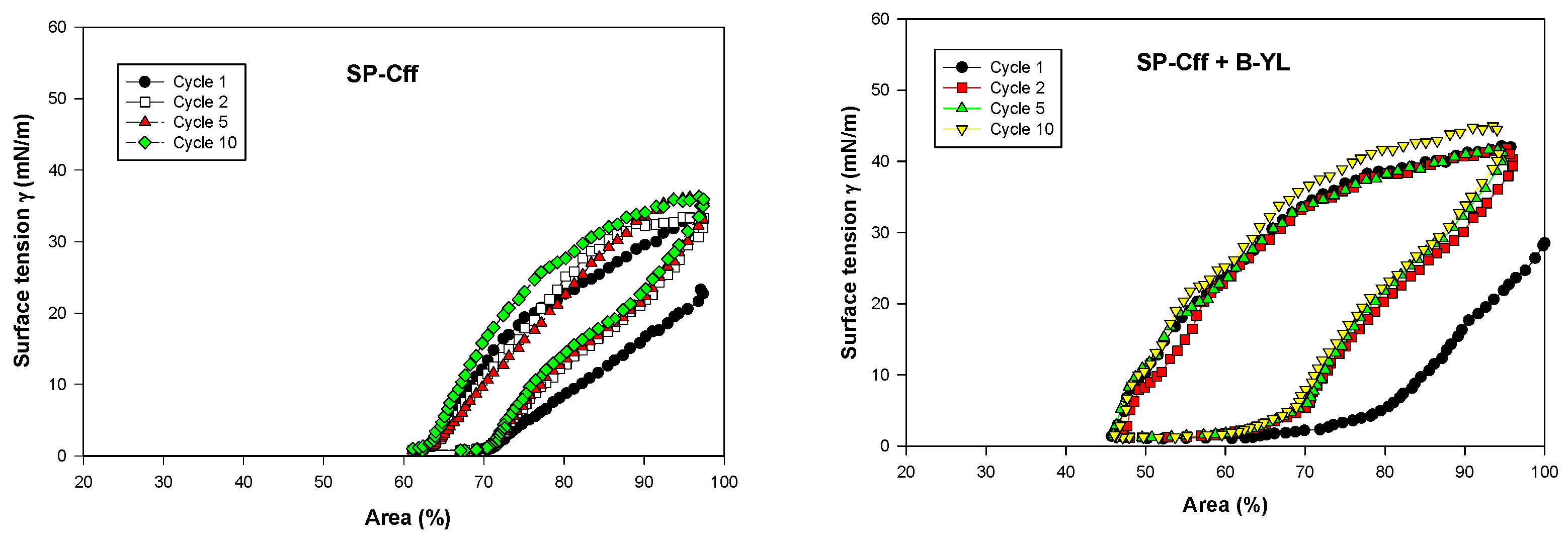
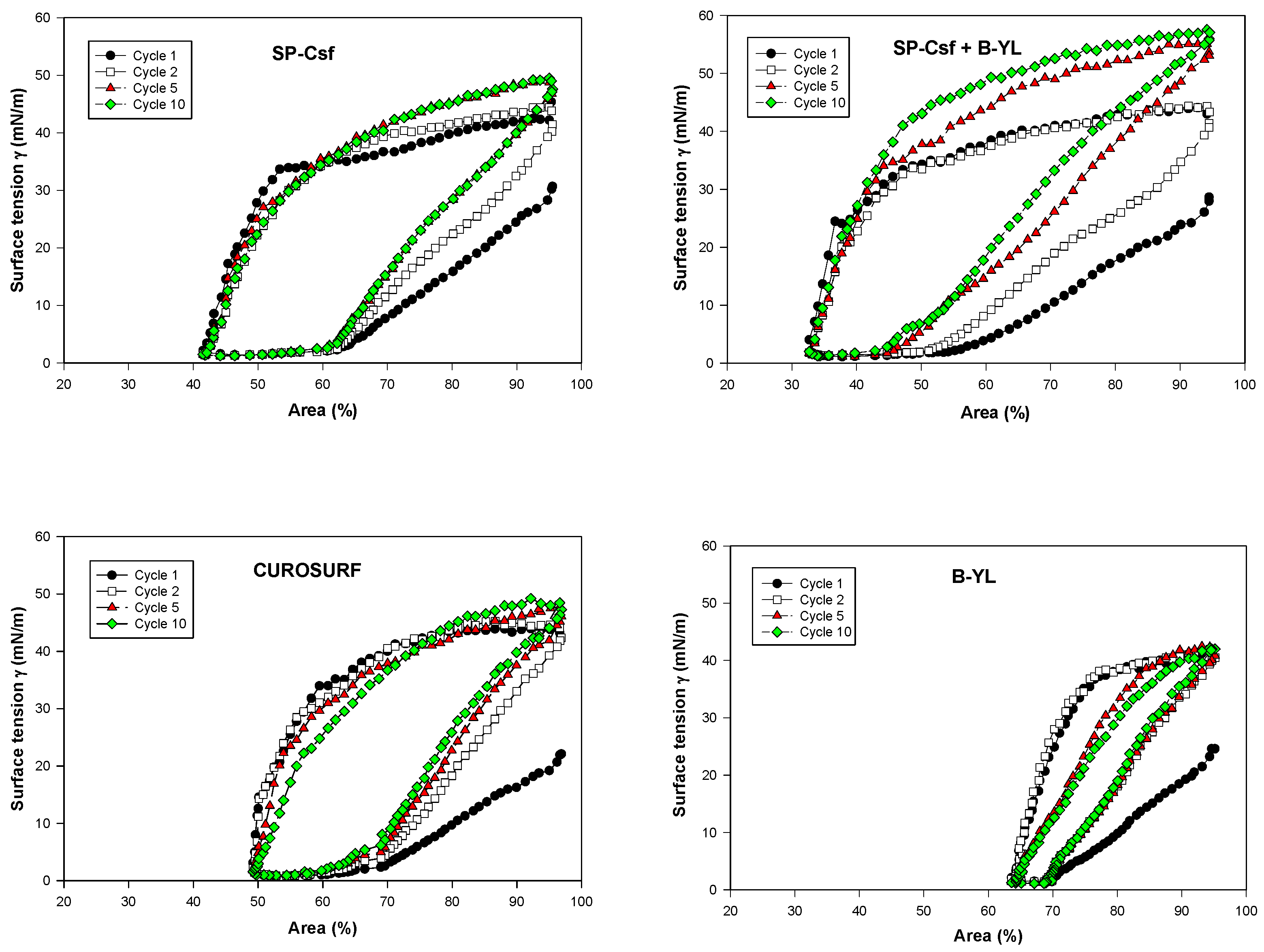
3.4. In Vitro Adsorption and Quasi-Static and Dynamic Surface Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Possmayer, F.; Zuo, Y.Y.; Veldhuizen, R.A.W.; Petersen, N.O. Pulmonary Surfactant: A Mighty Thin Film. Chem. Rev. 2023, 123, 13209–13290. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D. Respiratory distress syndrome in preterm neonates in the era of precision medicine: A modern critical care-based approach. Pediatr. Neonatol. 2021, 62 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef]
- Pérez-Gil, J. A recipe for a good clinical pulmonary surfactant. Biomed. J. 2022, 45, 615–628. [Google Scholar] [CrossRef]
- Milligan, D.W.; Ainsworth, S.B. Animal-derived or synthetic surfactant for the treatment of neonatal respiratory distress syndrome: A review. Acta Paediatr. Suppl. 2001, 90, 25–27. [Google Scholar] [CrossRef]
- Horbar, J.D.; Soll, R.F.; Sutherland, J.M.; Kotagal, U.; Philip, A.G.; Kessler, D.L.; Little, G.A.; Edwards, W.H.; Vidyasagar, D.; Raju, T.N.; et al. A multicenter randomized, placebo-controlled trial of surfactant therapy for respiratory distress syndrome. N. Engl. J. Med. 1989, 320, 959–965. [Google Scholar] [CrossRef]
- Horbar, J.D.; Wright, E.C.; Onstad, L. Decreasing mortality associated with the introduction of surfactant therapy: An observational study of neonates weighing 601 to 1300 grams at birth. The Members of the National Institute of Child Health and Human Development Neonatal Research Network. Pediatrics 1993, 92, 191–196. [Google Scholar] [CrossRef]
- Walther, F.J.; Waring, A.J.; Hernandez-Juviel, J.M.; Gordon, L.M.; Wang, Z.; Jung, C.L.; Ruchala, P.; Clark, A.P.; Smith, W.M.; Sharma, S.; et al. Critical structural and functional roles for the N-terminal insertion sequence in surfactant protein B analogs. PLoS ONE 2010, 5, e8672. [Google Scholar] [CrossRef]
- Walther, F.J.; Gupta, M.; Gordon, L.M.; Waring, A.J. A sulfur-free peptide mimic of surfactant protein B (B-YL) exhibits high in vitro and in vivo surface activities. Gates Open Res. 2018, 2, 13. [Google Scholar] [CrossRef]
- Walther, F.J.; Sharma, S.; Gordon, L.M.; Waring, A.J. Structural and functional stability of the sulfur-free surfactant protein B peptide mimic B-YL in synthetic surfactant lipids. BMC Pulm. Med. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Walther, F.J.; Waring, A.J.; Hernández-Juviel, J.M.; Ruchala, P.; Wang, Z.; Notter, R.H.; Gordon, L.M. Surfactant protein C peptides with salt-bridges (“ion-locks”) promote high surfactant activities by mimicking the α-helix and membrane topography of the native protein. PeerJ 2014, 2, e485. [Google Scholar] [CrossRef] [PubMed]
- Nogee, L.M.; Garnier, G.; Dietz, H.C.; Singer, L.; Murphy, A.M.; deMello, D.E.; Colten, H.R. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J. Clin. Investig. 1994, 93, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Szyperski, T.; Curstedt, T.; Wüthrich, K. The NMR structure of the pulmonary surfactant-associated polypeptide SP-C in an apolar solvent contains a valyl-rich alpha-helix. Biochemistry 1994, 33, 6015–6023. [Google Scholar] [CrossRef]
- Griese, M.; Lorenz, E.; Hengst, M.; Schams, A.; Wesselak, T.; Rauch, D.; Wittmann, T.; Kirchberger, V.; Escribano, A.; Schaible, T.; et al. Surfactant proteins in pediatric interstitial lung disease. Pediatr. Res. 2016, 79, 34–41. [Google Scholar] [CrossRef]
- Nogee, L.M.; Dunbar AE 3rd Wert, S.E.; Askin, F.; Hamvas, A.; Whitsett, J.A. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N. Engl. J. Med. 2001, 344, 573–579. [Google Scholar] [CrossRef]
- von Nahmen, A.; Schenk, M.; Sieber, M.; Amrein, M. The structure of a model pulmonary surfactant as revealed by scanning force microscopy. Biophys. J. 1997, 72, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Takamoto, D.Y.; von Nahmen, A.; Lipp, M.M.; Lee, K.Y.; Waring, A.J.; Zasadzinski, J.A. Effects of lung surfactant proteins, SP-B and SP-C, and palmitic acid on monolayer stability. Biophys. J. 2001, 80, 2262–2272. [Google Scholar] [CrossRef]
- Johansson, J.; Persson, P.; Löwenadler, B.; Robertson, B.; Jörnvall, H.; Curstedt, T. Canine hydrophobic surfactant polypeptide SP-C. A lipopeptide with one thioester-linked palmitoyl group. FEBS Lett. 1991, 281, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Creuwels, L.A.; Demel, R.A.; van Golde, L.M.; Haagsman, H.P. Characterization of a dimeric canine form of surfactant protein C (SP-C). Biochim. Biophys. Acta 1995, 1254, 326–332. [Google Scholar] [CrossRef]
- Notter, R.H.; Wang, Z.; Egan, E.A.; Holm, B.A. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem. Phys. Lipids 2002, 114, 21–34. [Google Scholar] [CrossRef]
- Walther, F.J.; Hernández-Juviel, J.M.; Gordon, L.M.; Waring, A.J.; Stenger, P.; Zasadzinski, J.A. Comparison of three lipid formulations for synthetic surfactant with a surfactant protein B analog. Exp. Lung Res. 2005, 31, 563–579. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B.; Summer, R.S. Surfactant Lipids at the Host-Environment Interface. Metabolic Sensors, Suppressors, and Effectors of Inflammatory Lung Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Holz, O.; Koster, G.; Postle, A.; Olin, A.C.; Hohlfeld, J.M. Exhaled breath particles as a novel tool to study lipid composition of epithelial lining fluid from the distal lung. BMC Pulm. Med. 2023, 23, 423. [Google Scholar] [CrossRef]
- Andrushchenko, V.V.; Vogel, H.J.; Prenner, E.J. Optimization of the hydrochloric acid concentration used for trifluoroacetate removal from synthetic peptides. J. Pept. Sci. 2007, 13, 37–43. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Miles, A.J.; Wien, F.; Lees, J.G.; Rodger, A.; Janes, R.W.; Wallace, B.A. Calibration and standardisation of synchroton radiation circular dichroism and conventional circular dichroism spectrometers. Spectroscopy 2003, 17, 653–661. [Google Scholar] [CrossRef]
- Sreerama, N.; Venyaminov, S.Y.; Woody, R.W. Estimation of the number of alpha-helical and beta-strand segments in proteins using circular dichroism spectroscopy. Protein Sci. 1999, 8, 370–380. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Structural characterization of membrane proteins and peptides by FTIR and ATR-FTIR spectroscopy. Methods Mol. Biol. 2013, 974, 177–218. [Google Scholar] [CrossRef]
- Waring, A.J.; Whitelegge, J.P.; Sharma, S.K.; Gordon, L.M.; Walther, F.J. Emulation of the structure of the Saposin protein fold by a lung surfactant peptide construct of surfactant Protein B. PLoS ONE 2022, 17, e0276787. [Google Scholar] [CrossRef]
- Kauppinen, J.K.; Moffatt, D.J.; Mantsch, H.H.; Cameron, D.G. Fourier self-deconvolution: A method for resolving intrinsically overlapped bands. Appl. Spectrosc. 1981, 35, 271–276. [Google Scholar] [CrossRef]
- Kukol, A.; Adams, P.D.; Rice, L.M.; Brunger, A.T.; Arkin, T.I. Experimentally based orientational refinement of membrane protein models: A structure for the Influenza A M2 H+ channel. J. Mol. Biol. 1999, 286, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Beevers, A.J.; Kukol, A. Secondary structure, orientation, and oligomerization of phospholemman, a cardiac transmembrane protein. Protein Sci. 2006, 15, 1127–1132. [Google Scholar] [CrossRef]
- Fringeli, U.P.; Gunthard, H.H. Infrared membrane spectroscopy. In Membrane Spectroscopy; Ernst, G., Ed.; Springer: Berlin, Germany, 1981; pp. 270–332. [Google Scholar]
- LaFrance, C.P.; Nablet, A.; Prud’homme, R.E.; Pezolet, M. On the relationship between the order parameter <P2(cos Θ)> and the shape of orientation distributions. Can. J. Chem. 1995, 73, 1497–1505. [Google Scholar]
- Gaffney, B.J. Fatty acid chain flexibility in the membranes of normal and transformed fibroblasts. Proc. Natl. Acad. Sci. USA 1975, 72, 664–668. [Google Scholar] [CrossRef]
- Schürch, S.; Bachofen, H.; Goerke, J.; Possmayer, F. A captive bubble method reproduces the in situ behavior of lung surfactant monolayers. J. Appl. Physiol. (1985) 1989, 67, 2389–2396. [Google Scholar] [CrossRef]
- Schürch, S.; Bachofen, H.; Goerke, J.; Green, F. Surface properties of rat pulmonary surfactant studied with the captive bubble method: Adsorption, hysteresis, stability. Biochim. Biophys. Acta 1992, 1103, 127–136. [Google Scholar] [CrossRef]
- Schürch, S.; Bachofen, H.; Possmayer, F. Surface activity in situ, in vivo, and in the captive bubble surfactometer. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Schürch, S.; Green, F.H.; Bachofen, H. Formation and structure of surface films: Captive bubble surfactometry. Biochim. Biophys. Acta 1998, 1408, 180–202. [Google Scholar] [CrossRef]
- Malcolm, J.D.; Elliot, C.D. Interfacial tension from height and diameter of a single profile drop or captive bubble. Can. J. Chem. Eng. 1980, 58, 151–153. [Google Scholar] [CrossRef]
- Schoel, W.M.; Schürch, S.; Goerke, J. The captive bubble method for the evaluation of pulmonary surfactant: Surface tension, area, and volume calculations. Biochim. Biophys. Acta 1994, 1200, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S. Structural biology in the age of AI. Nat. Methods 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Terwilliger, T.C.; Liebschner, D.; Croll, T.I.; Williams, C.J.; McCoy, A.J.; Poon, B.K.; Afonine, P.V.; Oeffner, R.D.; Richardson, J.S.; Read, R.J.; et al. AlphaFold predictions are valuable hypotheses and accelerate but do not replace experimental structure determination. Nat. Methods 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Hernández, P.M.; Arango, C.A.; Kim, S.K.; Jaramillo-Botero, A.; Goddard, W.A., 3rd. Predicted Three-Dimensional Structure of the GCR1 Putative GPCR in Arabidopsis thaliana and Its Binding to Abscisic Acid and Gibberellin A1. J. Agric. Food Chem. 2023, 71, 5770–5782. [Google Scholar] [CrossRef]
- von Nahmen, A.; Post, A.; Galla, H.J.; Sieber, M. The phase behavior of lipid monolayers containing pulmonary surfactant protein C studied by fluorescence light microscopy. Eur. Biophys. J. 1997, 26, 359–369. [Google Scholar] [CrossRef]
- Gustafsson, M.; Thyberg, J.; Näslund, J.; Eliasson, E.; Johansson, J. Amyloid fibril formation by pulmonary surfactant protein C. FEBS Lett. 1999, 464, 138–142. [Google Scholar] [CrossRef]
- Johansson, J.; Nilsson, G.; Strömberg, R.; Robertson, B.; Jörnvall, H.; Curstedt, T. Secondary structure and biophysical activity of synthetic analogues of the pulmonary surfactant polypeptide SP-C. Biochem. J. 1995, 307 Pt 2, 535–541. [Google Scholar] [CrossRef]
- Vandenbussche, G.; Clercx, A.; Clercx, M.; Curstedt, T.; Johansson, J.; Jörnvall, H.; Ruysschaert, J.M. Secondary structure and orientation of the surfactant protein SP-B in a lipid environment. A Fourier transform infrared spectroscopy study. Biochemistry 1992, 31, 9169–9176. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Rios, B.; Taneva, S.; Keough, K.M.; Mautone, A.J.; Mendelsohn, R. External reflection absorption infrared spectroscopy study of lung surfactant proteins SP-B and SP-C in phospholipid monolayers at the air/water interface. Biophys. J. 1995, 69, 2531–2540. [Google Scholar] [CrossRef]
- Marsh, D. Electron Spin Resonance: Spin Labels. In Membrane Spectroscopy, Molecular Biology Biochemistry and Biophysics; Grell, E., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981; Volume 31, pp. 51–142. [Google Scholar]
- Roldan, N.; Pérez-Gil, J.; Morrow, M.R.; García-Álvarez, B. Divide & Conquer: Surfactant Protein SP-C and Cholesterol Modulate Phase Segregation in Lung Surfactant. Biophys. J. 2017, 113, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Dluhy, R.A.; Shanmukh, S.; Leapard, J.B.; Krüger, P.; Baatz, J.E. Deacylated pulmonary surfactant protein SP-C transforms from alpha-helical to amyloid fibril structure via a pH-dependent mechanism: An infrared structural investigation. Biophys. J. 2003, 85, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, D.Y.; Lipp, M.M.; von Nahmen, A.; Lee, K.Y.; Waring, A.J.; Zasadzinski, J.A. Interaction of lung surfactant proteins with anionic phospholipids. Biophys. J. 2001, 81, 153–169. [Google Scholar] [CrossRef]
- Johansson, J.; Some, M.; Linderholm, B.M.; Almlén, A.; Curstedt, T.; Robertson, B. A synthetic surfactant based on a poly-Leu SP-C analog and phospholipids: Effects on tidal volumes and lung gas volumes in ventilated immature newborn rabbits. J. Appl. Physiol. (1985) 2003, 95, 2055–2063. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.M. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim. Biophys. Acta 1999, 1422, 105–185. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]

| Sample | % Conformation | ||||
|---|---|---|---|---|---|
| α helix | β sheet | Turn/Loop | Disordered | Helix Tilt° | |
| Canine SP-Cff ion-lock | 73.2 | 12.1 | 7.9 | 6.8 | 34.3 |
| Canine SP-Csf ion-lock | 72.3 | 8.2 | 7.6 | 11.9 | 33.8 |
| (A) | |||
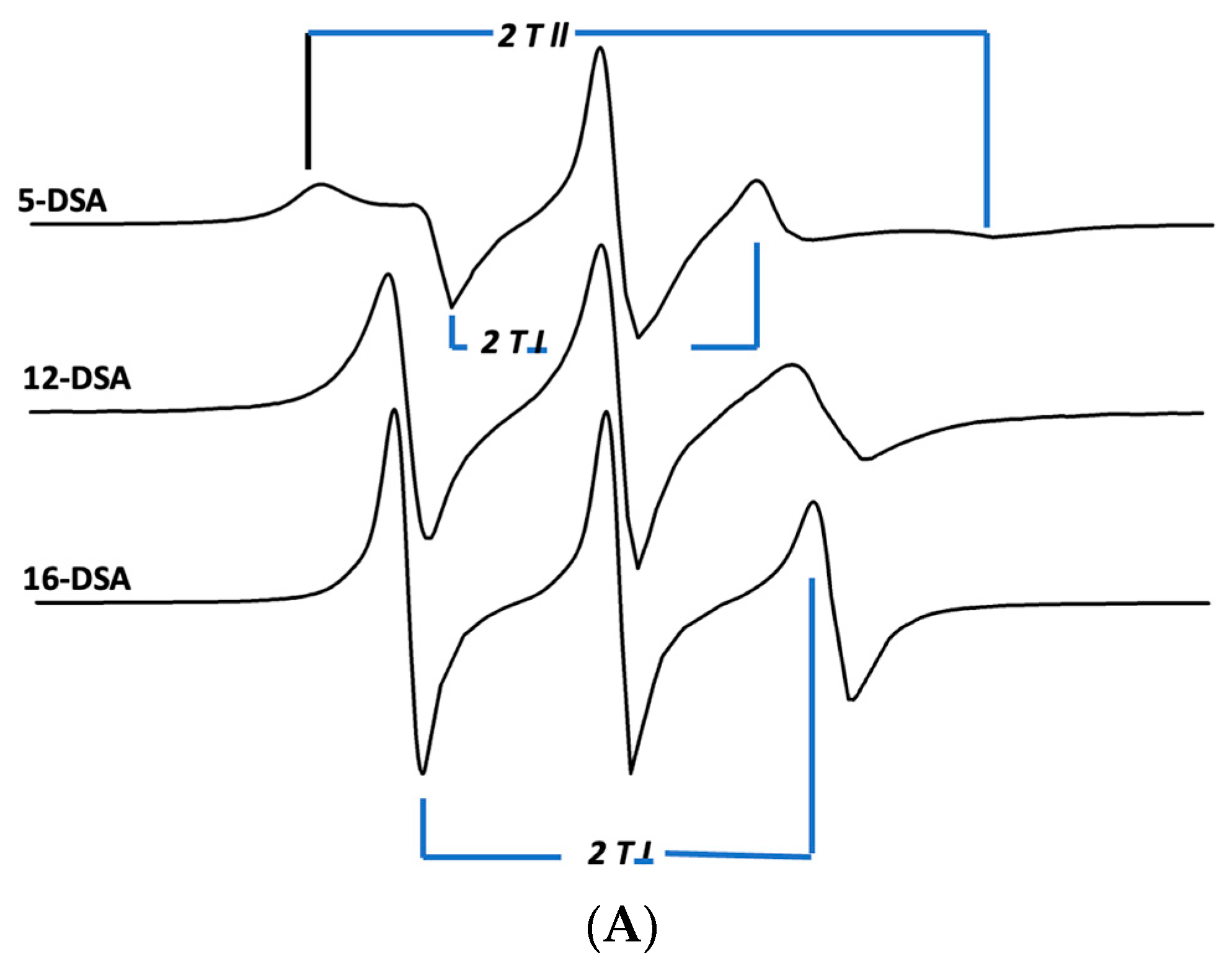
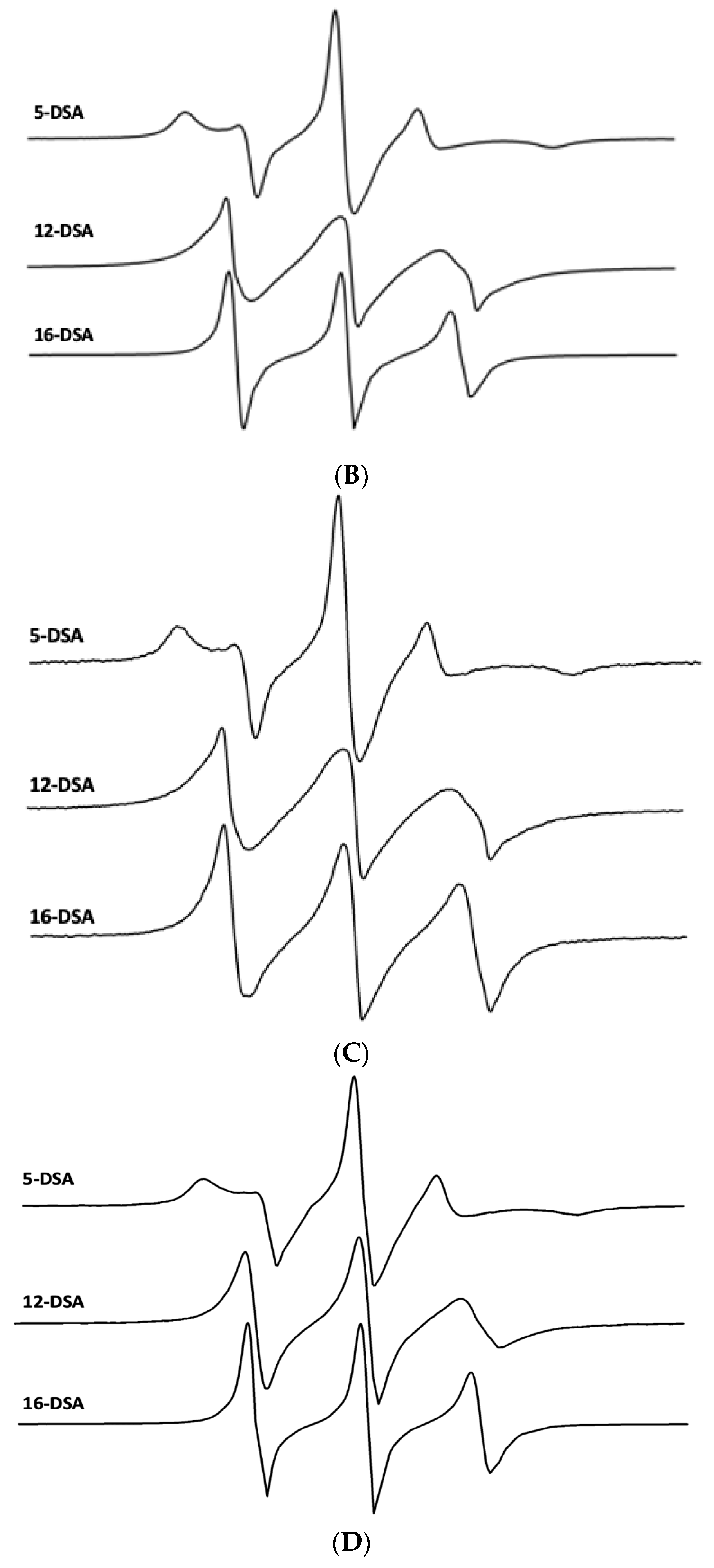
| Sample 5 doxyl-stearate | 2AII | 2Al | S |
| Surfactant Lipids | 46.0 G | 21.0 G | 0.547 |
| Surfactant Lipids 2% SP-Cff ion-lock | 48.0 G | 20.8 G | 0.595 |
| Surfactant Lipids 2% SP-Csf ion-lock | 47.8 G | 20.7 G | 0.593 |
| Surfactant Lipids 4% BYL + 2% SP-Cff ion-lock | 48.3 G | 20.9 G | 0.602 |
| (B) | |||
| Sample 12 doxyl-stearate | 2AII | 2Al | S |
| Surfactant Lipids | 25.2 G | 0.564 | |
| Surfactant Lipids 2% SP-Cff ion-lock | 24.7 G | 0.582 | |
| Surfactant Lipids 2% SP-Cfs ion-lock | 24.3 G | 0.597 | |
| Surfactant Lipids 4% BYL + 2% SP-Cff ion-lock | 24.7 G | 0.582 | |
| (C) | |||
| Sample 16 doxyl-stearate | 2AII | 2Al | S |
| Surfactant Lipids | 26.9 G | 0.501 | |
| Surfactant Lipids 2% SP-Cff ion-lock | 26.2 G | 0.527 | |
| Surfactant Lipids 2% SP-Csf ion-lock | 25.9 G | 0.538 | |
| Surfactant Lipids 4% BYL + 2% SP-Cff ion-lock | 26.0 G | 0.557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walther, F.J.; Waring, A.J. Structure and Function of Canine SP-C Mimic Proteins in Synthetic Surfactant Lipid Dispersions. Biomedicines 2024, 12, 163. https://doi.org/10.3390/biomedicines12010163
Walther FJ, Waring AJ. Structure and Function of Canine SP-C Mimic Proteins in Synthetic Surfactant Lipid Dispersions. Biomedicines. 2024; 12(1):163. https://doi.org/10.3390/biomedicines12010163
Chicago/Turabian StyleWalther, Frans J., and Alan J. Waring. 2024. "Structure and Function of Canine SP-C Mimic Proteins in Synthetic Surfactant Lipid Dispersions" Biomedicines 12, no. 1: 163. https://doi.org/10.3390/biomedicines12010163





