A Brain-Protective Sterol from Soft Coral Inhibits Lipopolysaccharide-Induced Matrix Metalloproteinase-9-Mediated Astrocytic Migration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coral Material
2.3. Extraction and Isolation
2.4. Cell Culture and Treatment
2.5. MMP Gelatin Zymography
2.6. Total RNA Extraction and Real-Time RT-PCR Analysis
2.7. Western Blot Analysis
2.8. Immunofluorescence Stain
2.9. Promoter-Luciferase Reporter Gene Assay
2.10. Cell Migration Assay
2.11. Statistical Analysis
3. Results
3.1. The Effect of Soft Coral-Derived Sterol Extract on LPS-Induced MMP-9 Expression in Brain Astrocytes
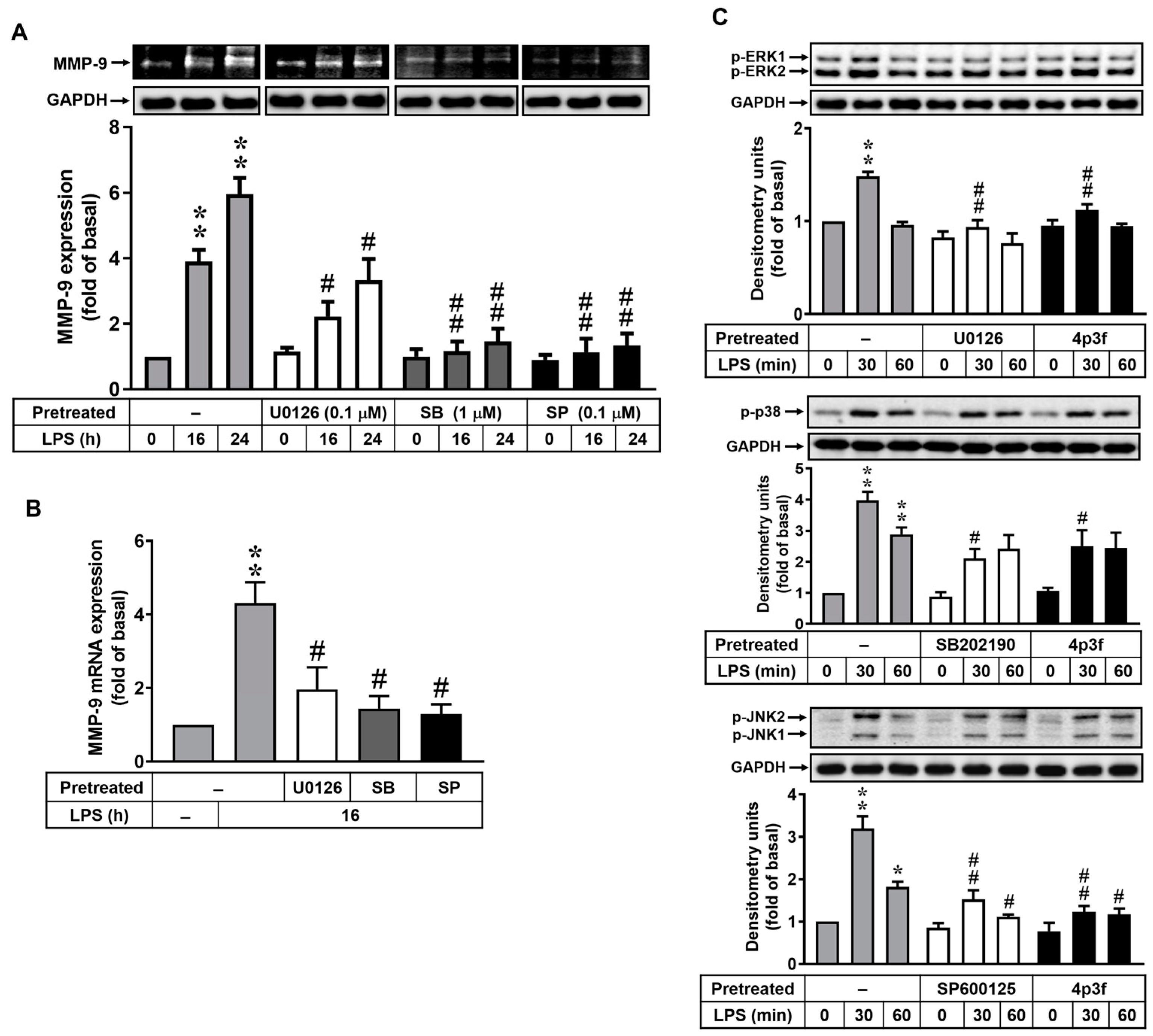
3.2. 4p3f Attenuates LPS-Induced MMP-9 Expression by Blocking the MAPK Signaling Pathways
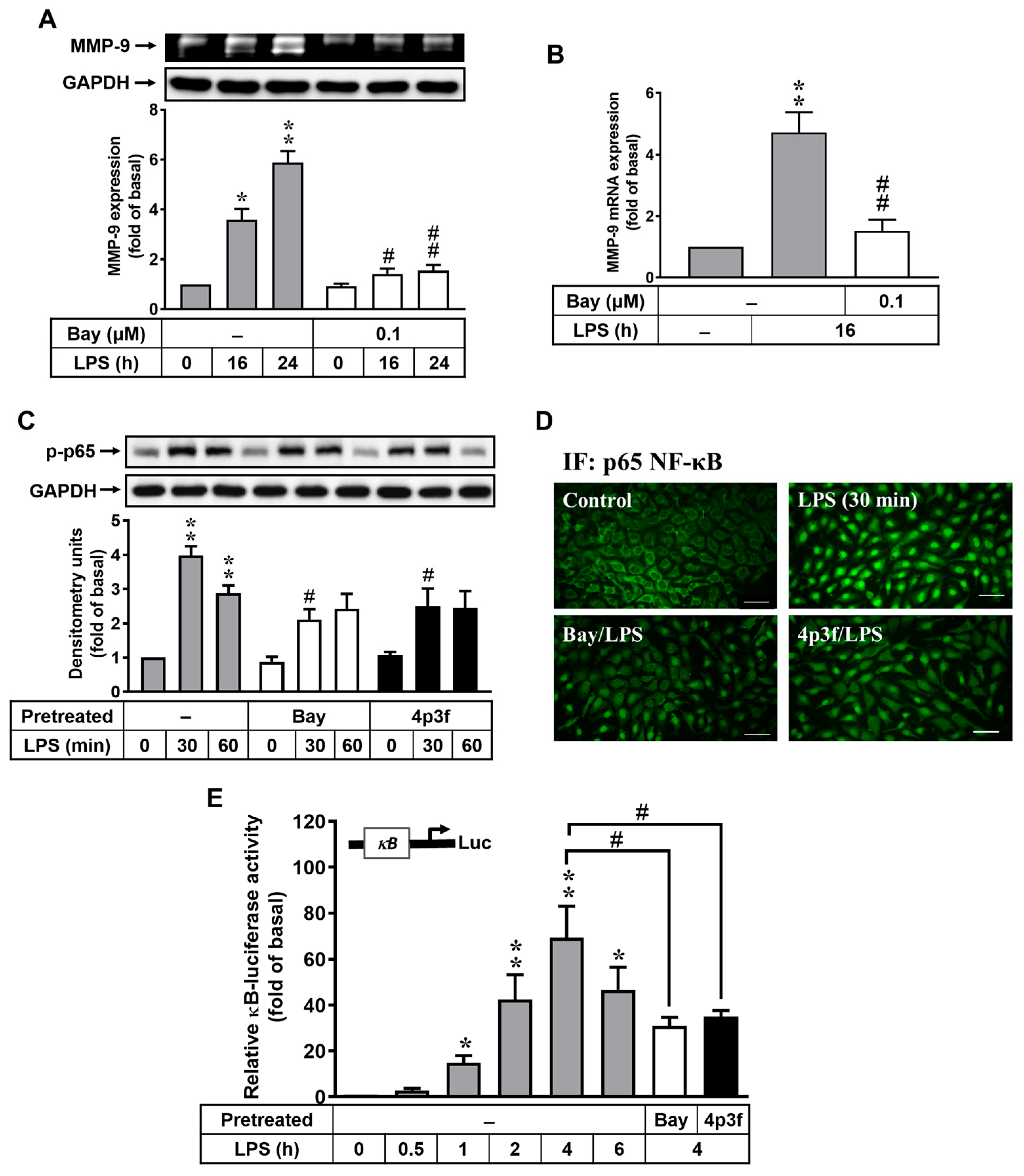
3.3. 4p3f Inhibits LPS-Induced MMP-9 Expression by Reducing NF-κB Activation
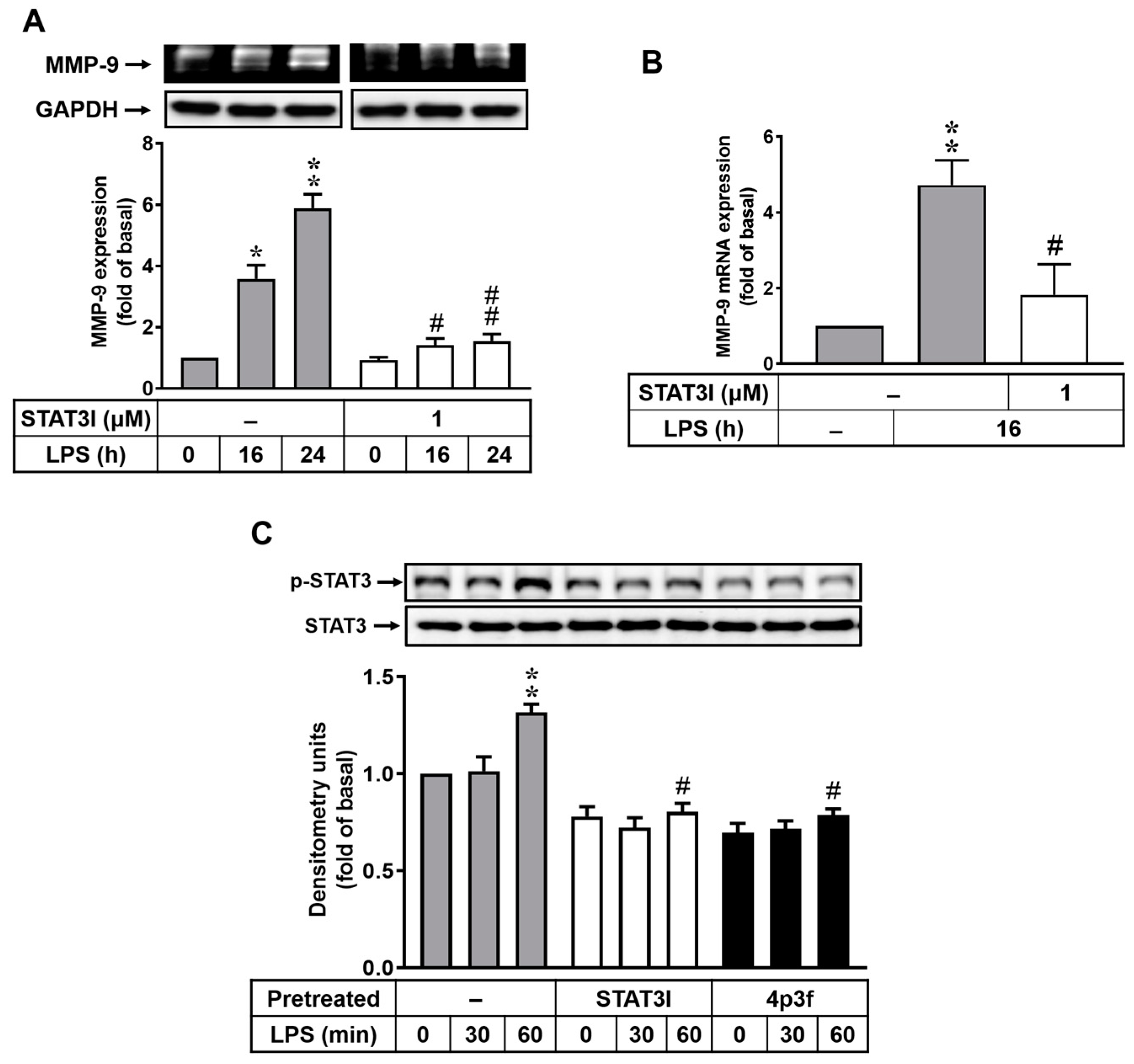
3.4. Effects of 4p3f on LPS-Induced STAT3-Mediated MMP-9 Expression in RBAs
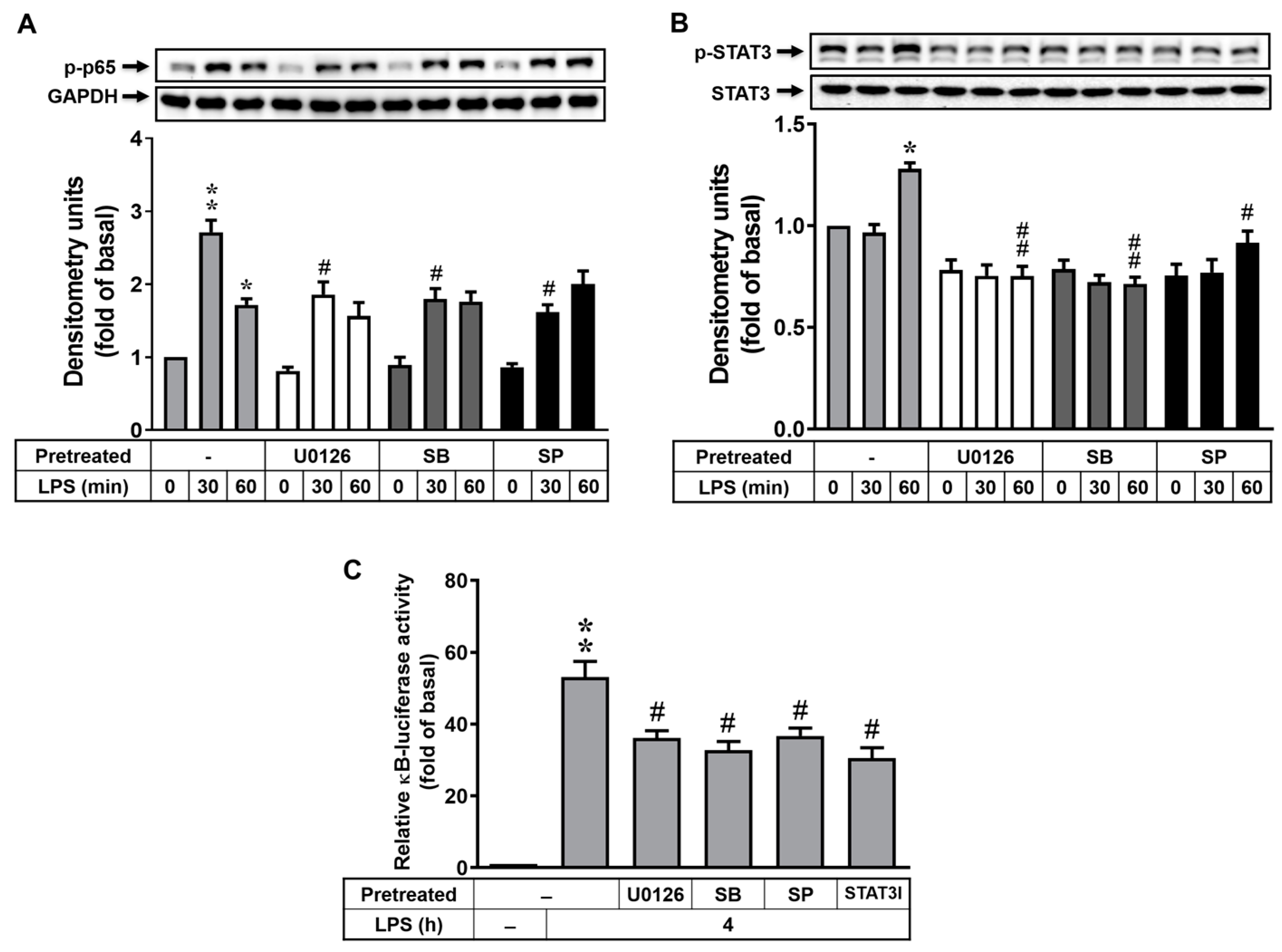
3.5. MAPK-STAT3 Signaling Axis Is Crucial for LPS-Stimulated NF-κB Transcriptional Activity in RBAs
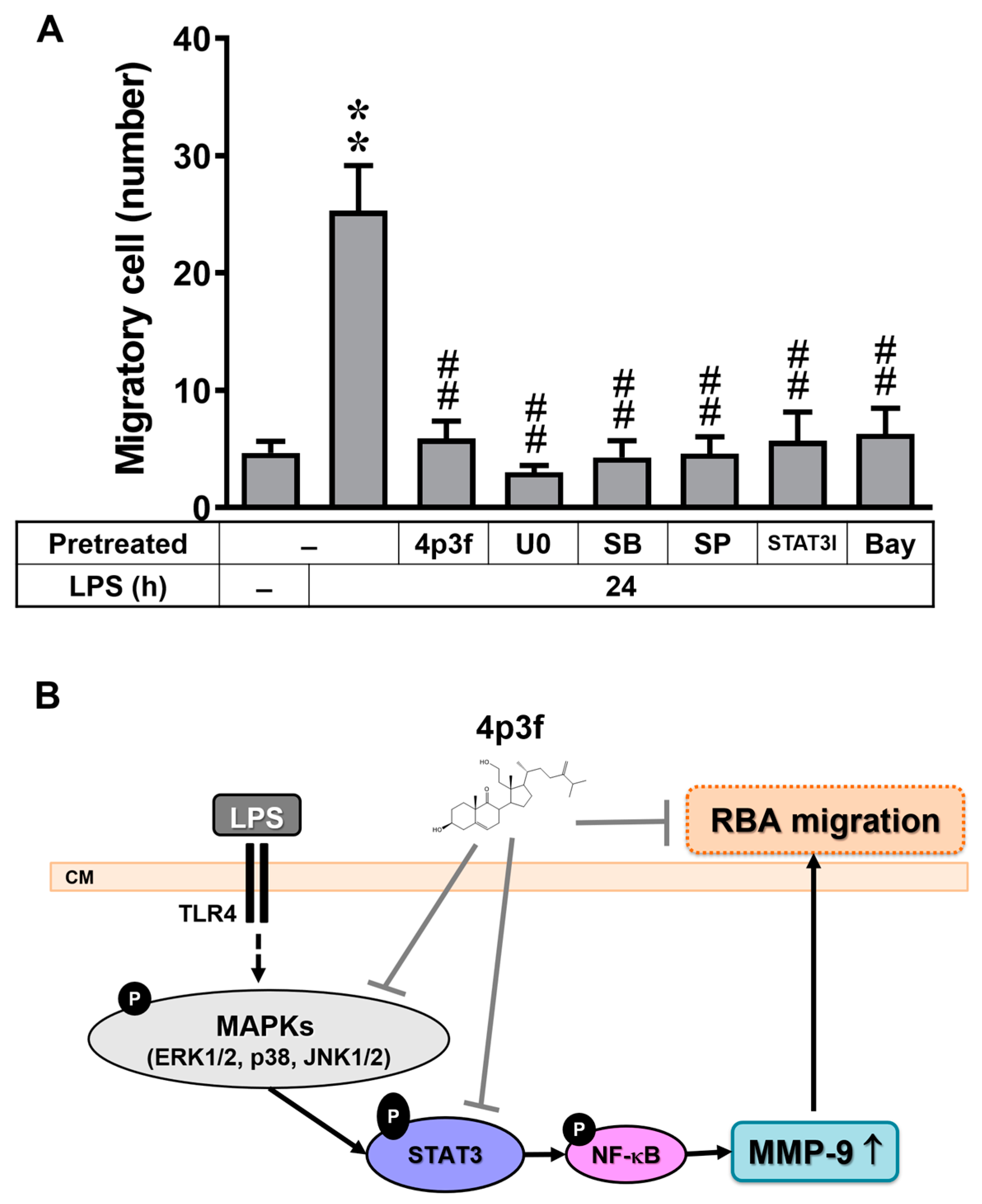
3.6. 4p3f Inhibits LPS-Induced MMP-9-Mediated Astrocytic Migration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef] [PubMed]
- Vivinetto, A.L.; Kim, I.D.; Goldberg, D.C.; Fones, L.; Brown, E.; Tarabykin, V.S.; Hill, C.E.; Cho, S.; Cave, J.W. Zeb2 Is a Regulator of Astrogliosis and Functional Recovery after CNS Injury. Cell Rep. 2020, 31, 107834. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia 2021, 69, 436–472. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.G.; Puvanachandra, N.; Horner, C.H.; Polito, A.; Ostenfeld, T.; Svendsen, C.N.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 1999, 23, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Elston, G.N.; Oga, T.; Fujita, I. Spinogenesis and pruning scales across functional hierarchies. J. Neurosci. 2009, 29, 3271–3275. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Gate, D.; Town, T. CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J. Neuroimmune Pharmacol. 2009, 4, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Cabré, R.; Burgos-Bravo, F.; Avalos, A.M.; Leyton, L. Connexins in Astrocyte Migration. Front. Pharmacol. 2019, 10, 1546. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. S2), 91–114. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Q.; Ma, Y.; Wang, F.; Tan, X.; Song, D.; Hoo, R.L.; Wang, Z.; Ge, X.; Han, H.; et al. An MMP-9 exclusive neutralizing antibody attenuates blood-brain barrier breakdown in mice with stroke and reduces stroke patient-derived MMP-9 activity. Pharmacol. Res. 2023, 190, 106720. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hsiao, L.D.; Yang, C.M. Galangin Inhibits LPS-Induced MMP-9 Expression via Suppressing Protein Kinase-Dependent AP-1 and FoxO1 Activation in Rat Brain Astrocytes. J. Inflamm. Res. 2020, 13, 945–960. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hsiao, L.D.; Tseng, H.C.; Kuo, C.M.; Yang, C.M. Pristimerin Inhibits MMP-9 Expression and Cell Migration Through Attenuating NOX/ROS-Dependent NF-κB Activation in Rat Brain Astrocytes Challenged with LPS. J. Inflamm. Res. 2020, 13, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Liu, P.S.; Tsai, M.M.; Chen, J.L.; Wang, S.J.; Hsieh, H.L. The COX-2-derived PGE(2) autocrine contributes to bradykinin-induced matrix metalloproteinase-9 expression and astrocytic migration via STAT3 signaling. Cell Commun. Signal 2020, 18, 185. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Sica, D.; Musumeci, D. Secosteroids of marine origin. Steroids 2004, 69, 743–756. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lai, K.H.; Kumar, S.; Chen, P.J.; Wu, Y.H.; Lai, C.L.; Hsieh, H.L.; Sung, P.J.; Hwang, T.L. (1)H NMR-Based Isolation of Anti-Inflammatory 9,11-Secosteroids from the Octocoral Sinularia leptoclados. Mar. Drugs 2020, 18, 271. [Google Scholar] [CrossRef]
- Tsai, C.R.; Huang, C.Y.; Chen, B.W.; Tsai, Y.Y.; Shih, S.P.; Hwang, T.L.; Dai, C.F.; Wang, S.Y.; Sheu, J.H. New bioactive steroids from the soft coral Klyxum flaccidum. RSC Adv. 2015, 5, 12546–12554. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Hanh, T.T.H.; Thanh, N.V.; Thao, D.T.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Cytotoxic Steroids from the Vietnamese Soft Coral Sinularia leptoclados. Chem. Pharm. Bull. 2017, 65, 593–597. [Google Scholar] [CrossRef]
- Camacho, C.V.; Choudhari, R.; Gadad, S.S. Long noncoding RNAs and cancer, an overview. Steroids 2018, 133, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Ru, T.; Huan, X.J.; Miao, Z.H.; Guo, Y.W. New cytotoxic ergostane-type sterols from the Chinese soft coral Sinularia sp. Steroids 2019, 149, 108425. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kaminari, A.; Tsilibary, E.C.; Tzinia, A. A New Perspective in Utilizing MMP-9 as a Therapeutic Target for Alzheimer’s Disease and Type 2 Diabetes Mellitus. J. Alzheimers Dis. 2018, 64, 1–16. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Wu, C.Y.; Yang, C.M. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes. Glia 2008, 56, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Papaconstantinou, J. Akt/PKB and p38 MAPK signaling, translational initiation and longevity in Snell dwarf mouse livers. Mech. Ageing Dev. 2004, 125, 785–798. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hsieh, H.L.; Jou, M.J.; Yang, C.M. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J. Neurochem. 2004, 90, 1477–1488. [Google Scholar] [CrossRef]
- Rhee, J.W.; Lee, K.W.; Kim, D.; Lee, Y.; Jeon, O.H.; Kwon, H.J.; Kim, D.S. NF-kappaB-dependent regulation of matrix metalloproteinase-9 gene expression by lipopolysaccharide in a macrophage cell line RAW 264.7. J. Biochem. Mol. Biol. 2007, 40, 88–94. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, D.L.; Bi, X.Y.; Sun, L.; Yu, X.J.; Fang, H.L.; Miao, Y.; Zhao, M.; He, X.; Liu, J.J.; et al. Acetylcholine Inhibits LPS-Induced MMP-9 Production and Cell Migration via the α7 nAChR-JAK2/STAT3 Pathway in RAW264.7 Cells. Cell Physiol. Biochem. 2015, 36, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.H.; Jia, Y.; Guo, F.J.; Chen, J.; Zhang, X.W.; Cui, M.H. Phosphorylation of STAT3 at Tyr705 regulates MMP-9 production in epithelial ovarian cancer. PLoS ONE 2017, 12, e0183622. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hoentjen, F.; Sartor, R.B.; Ozaki, M.; Jobin, C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood 2005, 105, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Chen, J.L.; Liu, P.S.; Tsai, M.M.; Wang, S.J.; Hsieh, H.L. Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-δ-dependent ROS signal. J. Neuroinflammation 2020, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Lin, C.C.; Hsiao, L.D.; Kuo, J.M.; Tseng, H.C.; Yang, C.M. Lipopolysaccharide-Induced Matrix Metalloproteinase-9 Expression Associated with Cell Migration in Rat Brain Astrocytes. Int. J. Mol. Sci. 2019, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow. Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Tan, Z.; Lucke-Wold, B.P.; Logsdon, A.F.; Turner, R.C.; Tan, C.; Li, X.; Hongpaison, J.; Alkon, D.L.; Simpkins, J.W.; Rosen, C.L.; et al. Bryostatin extends tPA time window to 6 h following middle cerebral artery occlusion in aged female rats. Eur. J. Pharmacol. 2015, 764, 404–412. [Google Scholar] [CrossRef]
- Panther, E.J.; Lucke-Wold, B. Subarachnoid hemorrhage: Management considerations for COVID-19. Explor. Neuroprotective Ther. 2022, 2, 65–73. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Jou, T.C.; Jou, M.J.; Chen, J.Y.; Lee, S.Y. Properties of rat brain astrocytes in long-term culture. Taiwan Yi Xue Hui Za Zhi 1985, 84, 865–881. [Google Scholar] [PubMed]
- Huang, C.Y.; Liaw, C.C.; Chen, B.W.; Chen, P.C.; Su, J.H.; Sung, P.J.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Withanolide-based steroids from the cultured soft coral Sinularia brassica. J. Nat. Prod. 2013, 76, 1902–1908. [Google Scholar] [CrossRef]
- Li, S.W.; Chen, W.T.; Yao, L.G.; Guo, Y.W. Two new cytotoxic steroids from the Chinese soft coral Sinularia sp. Steroids 2018, 136, 17–21. [Google Scholar] [CrossRef]
- Huang, C.Y.; Su, J.H.; Liaw, C.C.; Sung, P.J.; Chiang, P.L.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Bioactive Steroids with Methyl Ester Group in the Side Chain from a Reef Soft Coral Sinularia brassica Cultured in a Tank. Mar. Drugs 2017, 15, 280. [Google Scholar] [CrossRef]
- Zhang, N.X.; Tang, X.L.; van Ofwegen, L.; Xue, L.; Song, W.J.; Li, P.L.; Li, G.Q. Cyclopentenone derivatives and polyhydroxylated steroids from the soft coral Sinularia acuta. Chem. Biodivers. 2015, 12, 273–283. [Google Scholar] [CrossRef]
- Vandooren, J.; Van Damme, J.; Opdenakker, G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res. 2014, 214, 193–206. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, E.N. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998, 9, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Yang, D.C. Suppression of MAPKs/NF-κB Activation Induces Intestinal Anti-Inflammatory Action of Ginsenoside Rf in HT-29 and RAW264.7 Cells. Immunol. Investig. 2016, 45, 439–449. [Google Scholar] [CrossRef]
- Ahn, S.C.; Kim, G.Y.; Kim, J.H.; Baik, S.W.; Han, M.K.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Kang, J.H.; Kim, B.H.; et al. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and NF-kappaB. Biochem. Biophys. Res. Commun. 2004, 313, 148–155. [Google Scholar] [CrossRef]
- Li, Y.C.; Kuo, P.C.; Yang, M.L.; Chen, T.Y.; Hwang, T.L.; Chiang, C.C.; Thang, T.D.; Tuan, N.N.; Tzen, J.T.C. Chemical Constituents of the Leaves of Peltophorum pterocarpum and Their Bioactivity. Molecules 2019, 24, 240. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yu, M.C.; Cheng, L.C.; Chu, M.Y.; Huang, T.H.; Yeh, T.S.; Tsai, M.M. Quercetin exerts anti-inflammatory effects via inhibiting tumor necrosis factor-α-induced matrix metalloproteinase-9 expression in normal human gastric epithelial cells. World J. Gastroenterol. 2022, 28, 1139–1158. [Google Scholar] [CrossRef]
- Lin, Y.; Ukaji, T.; Koide, N.; Umezawa, K. Inhibition of Late and Early Phases of Cancer Metastasis by the NF-κB Inhibitor DHMEQ Derived from Microbial Bioactive Metabolite Epoxyquinomicin: A Review. Int. J. Mol. Sci. 2018, 19, 729. [Google Scholar] [CrossRef]
- Lambrou, G.I.; Hatziagapiou, K.; Vlahopoulos, S. Inflammation and tissue homeostasis: The NF-κB system in physiology and malignant progression. Mol. Biol. Rep. 2020, 47, 4047–4063. [Google Scholar] [CrossRef]
- Millot, P.; San, C.; Bennana, E.; Porte, B.; Vignal, N.; Hugon, J.; Paquet, C.; Hosten, B.; Mouton-Liger, F. STAT3 inhibition protects against neuroinflammation and BACE1 upregulation induced by systemic inflammation. Immunol. Lett. 2020, 228, 129–134. [Google Scholar] [CrossRef]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Furube, E.; Morita, S.; Wanaka, A.; Nakashima, T.; Miyata, S. Astrocytic TLR4 expression and LPS-induced nuclear translocation of STAT3 in the sensory circumventricular organs of adult mouse brain. J. Neuroimmunol. 2015, 278, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Swarnkar, S.; Rai, S.; Kumar, V.; Tyagi, N. Astrocyte mediated MMP-9 activation in the synapse dysfunction: An implication in Alzheimer disease. Ther. Targets Neurol. Dis. 2014, 1, e243. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Chandrasekar, V.; Laux, P.; Luch, A.; Dakua, S.P.; Zamboni, P.; Shelar, A.; Yang, Y.; Pandit, V.; Tisato, V.; et al. Micropatterned Neurovascular Interface to Mimic the Blood-Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells 2022, 11, 2801. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-H.; Chen, J.-L.; Chang, C.-H.; Tsai, M.-M.; Tseng, H.-C.; Chang, Y.-C.; Shanmugam, V.; Hsieh, H.-L. A Brain-Protective Sterol from Soft Coral Inhibits Lipopolysaccharide-Induced Matrix Metalloproteinase-9-Mediated Astrocytic Migration. Biomedicines 2024, 12, 226. https://doi.org/10.3390/biomedicines12010226
Lee T-H, Chen J-L, Chang C-H, Tsai M-M, Tseng H-C, Chang Y-C, Shanmugam V, Hsieh H-L. A Brain-Protective Sterol from Soft Coral Inhibits Lipopolysaccharide-Induced Matrix Metalloproteinase-9-Mediated Astrocytic Migration. Biomedicines. 2024; 12(1):226. https://doi.org/10.3390/biomedicines12010226
Chicago/Turabian StyleLee, Tsong-Hai, Jiun-Liang Chen, Chuan-Hsin Chang, Ming-Ming Tsai, Hui-Ching Tseng, Yu-Chia Chang, Velayuthaprabhu Shanmugam, and Hsi-Lung Hsieh. 2024. "A Brain-Protective Sterol from Soft Coral Inhibits Lipopolysaccharide-Induced Matrix Metalloproteinase-9-Mediated Astrocytic Migration" Biomedicines 12, no. 1: 226. https://doi.org/10.3390/biomedicines12010226
APA StyleLee, T.-H., Chen, J.-L., Chang, C.-H., Tsai, M.-M., Tseng, H.-C., Chang, Y.-C., Shanmugam, V., & Hsieh, H.-L. (2024). A Brain-Protective Sterol from Soft Coral Inhibits Lipopolysaccharide-Induced Matrix Metalloproteinase-9-Mediated Astrocytic Migration. Biomedicines, 12(1), 226. https://doi.org/10.3390/biomedicines12010226







