Increased Levels of VCAM-1 in Patients with High Cardiovascular Risk and Obstructive Sleep Apnea Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sleep Study
2.3. Echocardiography
2.4. Measurements of Anthropometric and Biochemical Parameters
2.5. Cardiovascular Risk Stratification Using the Scores
2.6. Statistics
3. Results
3.1. Baseline Characteristics
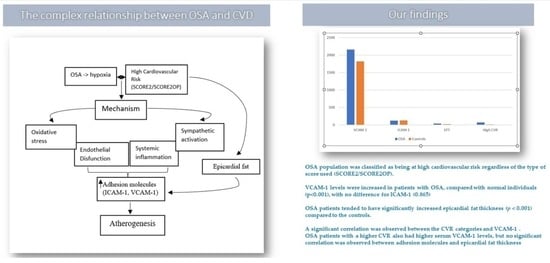
3.2. Association between Serum Biomarkers and Epicardial Fat with CVR Scores
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ursavaş, A.; Karadağ, M.; Rodoplu, E.; Yılmaztepe, A.; Oral, H.B.; Gözü, R.O. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration 2006, 74, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Tietjens, J.; Claman, D.; Kezirian, E.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; D’Ambrosio, C.; Mohsenin, V. Sleep-related breathing disorders and cardiovascular disease. Am. J. Med. 2000, 108, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chetan, I.M.; Gergely-Domokos, B.; Beyer, R.; Tomoaia, R.; Cabau, G.; Vulturar, D.; Chis, A.; Lesan, A.; Vesa, C.S.; Pop, D.; et al. The role of 3D Speckle tracking echocardiography in the diagnosis of obstructive sleep apnea and its severity. Sci. Rep. 2022, 12, 22347. [Google Scholar] [CrossRef] [PubMed]
- Newman, A. Relation of Sleep-disordered Breathing to Cardiovascular Disease Risk Factors: The Sleep Heart Health Study. Am. J. Epidemiol. 2001, 154, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ouwens, D.M.; Sell, H.; Greulich, S.; Eckel, J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell. Mol. Med. 2010, 14, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Fiedorczuk, P.; Polecka, A.; Walasek, M.; Olszewska, E. Potential diagnostic and monitoring biomarkers of obstructive sleep apnea–Umbrella Review of Meta-analyses. J. Clin. Med. 2022, 12, 60. [Google Scholar] [CrossRef]
- Timmerman, I.; Daniel, A.E.; Kroon, J.; van Buul, J.D. Leukocytes crossing the endothelium: A matter of communication. Int. Rev. Cell Mol. Biol. 2016, 322, 281–329. [Google Scholar] [CrossRef]
- Schmidt, C.; Hulthe, J.; Fagerberg, B. Baseline ICAM-1 and VCAM-1 are increased in initially healthy middle-aged men who develop cardiovascular disease during 6.6 years of follow-up. Angiology 2009, 60, 108–114. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.-C.; Connelly, P.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, M.; Liu, Y.; Li, H.; Shang, L.; Xu, T.; Chen, Z.; Wang, F.; Qiao, T.; Li, K. Iron accumulation in macrophages promotes the formation of foam cells and development of atherosclerosis. Cell Biosci. 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Paolillo, S.; Rengo, G.; Formisano, R.; Petraglia, L.; Grieco, F.; D’Amore, C.; Dellegrottaglie, S.; Marciano, C.; Ferrara, N.; et al. Sleep-disordered breathing and epicardial adipose tissue in patients with heart failure. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Fiore, D.; Barbaro, G.; Basciani, S.; Saponara, M.; D’Arcangelo, E.; Ulisse, S.; Moretti, C.; Fabbri, A.; Gnessi, L. Association of epicardial fat thickness with the severity of obstructive sleep apnea in obese patients. Int. J. Cardiol. 2013, 167, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Sun, F.; Wu, D.; Bi, W. Association of epicardial adipose tissues with obstructive sleep apnea and its severity: A meta analysis study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, J.S.; Kim, Y.J.; Lee, I.S.; Kim, J.H.; Choi, S.W.; Choi, S.W.; Jeong, J.O.; Seong, I.W. Effects of statins on the epicardial fat thickness in patients coronary artery stenosis underwent percutaneous coronary intervention: Comparison of atorvastatin with simvastatine/ezetimibe. J. Cardiovasc. Ultrasound 2010, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Is Sleep Apnea Connected to Heart Disease? Sleep Foundation. 2022. Available online: https://www.sleepfoundation.org/sleep-apnea/sleep-apnea-linked-heart-disease#:~:text=It’s%20estimated%20that%20patients%20with,coronary%20heart%20disease%20by%2030%25 (accessed on 4 March 2023).
- Kim, K.H.; Hyun, D.W.; Kim, W.-S.; Yang, J.K.; Kwon, T.G.; Bae, J.H. Carotid intima media thickness is associated with the Framingham risk score in Korean patients with coronary arteriosclerosis—Association between IMT and Framingham Risk Score. Korean Circ. J. 2007, 37, 425. [Google Scholar] [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- SCORE2-OP Working Group and ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef]
- Riha, R.L. Defining obstructive sleep apnoea syndrome: A failure of Semantic Rules. Breathe 2021, 17, 210082. [Google Scholar] [CrossRef]
- Berry, R.; Budhiraja, R.; Gottlieb, D.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA). CMS.Gov Centers for Medicare & Medicaid Services. Available online: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=204 (accessed on 4 March 2023).
- Iacobellis, G.; Assael, F.; Ribaudo, M.C.; Zappaterreno, A.; Alessi, G.; Di Mario, U.; Leonetti, F. Epicardial fat from echocardiography: A new method for visceral adipose tissue prediction. Obes. Res. 2003, 11, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Hedner, J.; Norum, J.; Kraiczi, H.; Carlson, J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 166, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chetan, I.M.; Maierean, A.D.; Domokos Gergely, B.; Cabau, G.; Tomoaia, R.; Chis, A.F.; Albu, A.; Stoia, M.A.; Vesa, S.C.; Blendea, D.; et al. A prospective study of CPAP therapy in relation to cardiovascular outcome in a cohort of Romanian obstructive sleep apnea patients. J. Pers. Med. 2021, 11, 1001. [Google Scholar] [CrossRef] [PubMed]
- Patt, B.T.; Jarjoura, D.; Haddad, D.N.; Sen, C.K.; Roy, S.; Flavahan, N.A.; Khayat, R.N. Endothelial dysfunction in the microcirculation of patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2010, 182, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Lechner, K.; Waldeyer, C.; Shapiro, M.D.; Koenig, W. Inflammation and Cardiovascular Disease: The Future. Eur. Cardiol. 2021, 16, e20. [Google Scholar] [CrossRef] [PubMed]
- Golia, E.; Limongelli, G.; Natale, F.; Fimiani, F.; Maddaloni, V.; Pariggiano, I.; Bianchi, R.; Crisci, M.; D’acierno, L.; Giordano, R.; et al. Inflammation and cardiovascular disease: From pathogenesis to Therapeutic Target. Curr. Atheroscler. Rep. 2014, 16, 435. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; Garcia, L.; Gabrielli, L.; Corbalan, R.; et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Ballantyne, C.M.; Sharrett, A.R.; Smith, L.C.; Davis, C.E.; Gotto, A.M.; Boerwinkle, E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases. Circulation 1997, 96, 4219–4225. [Google Scholar] [CrossRef]
- Ciftci, T.; Kokturk, O.; Bukan, N.; Bilgihan, A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 2004, 28, 87–91. [Google Scholar] [CrossRef]
- Price, D.T.; Loscalzo, J. Cellular adhesion molecules and atherogenesis. Am. J. Med. 1999, 107, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lattimore, J.D.; Wilcox, I.; Nakhla, S.; Langenfeld, M.; Jessup, W.; Celermajer, D.S. Repetitive hypoxia increases lipid loading in human macrophages—A potentially atherogenic effect. Atherosclerosis 2005, 179, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Gholamipour, M.A.; Brühl, A.B.; Sadeghi-Bahmani, D.; Brand, S. Evaluation of blood intercellular adhesion molecule-1 (ICAM-1) level in obstructive sleep apnea: A systematic review and meta-analysis. Medicina 2022, 58, 1499. [Google Scholar] [CrossRef] [PubMed]
- Ohga, E.; Nagase, T.; Tomita, T.; Teramoto, S.; Matsuse, T.; Katayama, H.; Ouchi, Y. Increased levels of circulating ICAM-1, VCAM-1 and L-selectin in obstructive sleep apnea syndrome. J. Appl. Physiol. 1999, 87, 10–14. [Google Scholar] [CrossRef] [PubMed]
- El-Solh, A.A.; Mador, M.J.; Sikka, P.; Dhillon, R.S.; Amsterdam, D.; Grant, B.J. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest 2002, 121, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Luc, G.; Arveiler, D.; Evans, A.; Amouyel, P.; Ferrieres, J.; Bard, J.M.; Elkhalil, L.; Fruchart, J.-C.; Ducimetiere, P. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: The PRIME Study. Atherosclerosis 2003, 170, 169–176. [Google Scholar] [CrossRef]
- Pak, V.M.; Grandner, M.A.; Pack, A.I. Circulating adhesion molecules in sleep apnea and cardiovascular disease. Sleep Med. Rev. 2013, 18, 25–34. [Google Scholar] [CrossRef]
- Mulvihill, N.T.; Foley, J.B.; Murphy, R.T.; Curtin, R.; Crean, P.A.; Walsh, M. Risk stratification in unstable angina and non-Q wave myocardial infarction using soluble cell adhesion molecules. Heart 2001, 85, 623–627. [Google Scholar] [CrossRef]
- Stehouwer, C.D.; Gall, M.A.; Twisk, J.W.; Knudsen, E.; Emeis, J.J.; Parving, H.H. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: Progressive, interrelated, and independently associated with risk of death. Diabetes 2002, 51, 1157–1165. [Google Scholar] [CrossRef]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Quercioli, A.; Mach, F.; Montecucco, F. Inflammation accelerates atherosclerotic processes in obstructive sleep apnea syndrome (OSAS). Sleep Breath 2010, 14, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Edlinger, C.; Lichtenauer, M.; Wernly, B.; Pistulli, R.; Paar, V.; Prodinger, C.; Krizanic, F.; Thieme, M.; Kammler, J.; Jung, C.; et al. Disease-specific characteristics of vascular cell adhesion molecule-1 levels in patients with peripheral artery disease. Heart Vessel. 2019, 34, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Harling, L.; Lambert, J.; Ashrafian, H.; Darzi, A.; Gooderham, N.J.; Athanasiou, T. Pre-operative serum VCAM-1 as a biomarker of atrial fibrillation after coronary artery bypass grafting. J. Cardiothorac. Surg. 2017, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Lino, D.O.C.; Freitas, I.A.; Meneses, G.C.; Martins, A.M.C.; Daher, E.F.; Rocha, J.H.C.; Silva Junior, G.B. Interleukin-6 and adhesion molecules VCAM-1 and ICAM-1 as biomarkers of post-acute myocardial infarction heart failure. Braz. J. Med. Biol. Res. 2019, 52, e8658. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Pischon, T.; Hu, F.B.; Ascherio, A.; Rifai, N.; Rimm, E.B. Soluble intercellular adhesion molecules, soluble vascular cell adhesion molecules, and risk of coronary heart disease. Obesity 2006, 14, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; de la Cruz-Moron, I.; Almeida-Gonzalez, C.; Catalan-Serra, P.; Montserrat, J.M. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: A cohort study. Ann. Int. Med. 2012, 156, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pak, V.M.; Keenan, B.T.; Jackson, N.; Grandner, M.A.; Maislin, G.; Teff, K.; Schwab, R.J.; Arnardottir, E.S.; Júlíusson, S.; Benediktsdottir, B.; et al. Adhesion molecule increases in sleep apnea: Beneficial effect of positive airway pressure and moderation by obesity. Int. J. Obes. 2014, 39, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Ohga, E.; Tomita, T.; Wada, H.; Yamamoto, H.; Nagase, T.; Ouchi, Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J. Appl. Physiol. 2003, 94, 179–184. [Google Scholar] [CrossRef]
- Zou, D.; Celik, Y.; Lindberg, T.; Thunström, E.; Peker, Y. Effect of CPAP treatment on adhesion molecules in coronary artery disease with nonsleepy obstructive sleep apnoea: The RICCADSA randomized controlled trial. Eur. Respir. J. 2020, 56, 4740. [Google Scholar] [CrossRef]
- Chin, K.; Nakamura, T.; Shimizu, K.; Mishima, M.; Nakamura, T.; Miyasaka, M.; Ohi, M. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am. J. Med. 2000, 109, 562–567. [Google Scholar] [CrossRef]
- Htoo, A.K.; Greenberg, H.; Tongia, S.; Chen, G.; Henderson, T.; Wilson, D.; Liu, S.F. Activation of nuclear factor ΚB in obstructive sleep apnea: A pathway leading to systemic inflammation. Sleep Breath. 2006, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, A.S.M.; Winnicki, M.; Lanfranchi, P.; Wolk, R.; Kara, T.; Accurso, V.; Somers, V.K. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002, 105, 2462–2464. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, F.; Asensio-Cruz, M.I.; Cordero-Guevara, J.; Jurado-Gamez, B.; Carmona-Bernal, C.; Gonzalez-Martinez, M.; Troncoso, M.F.; Sanchez-Lopez, V.; Arellano-Orden, E.; Garcia-Sanchez, M.I.; et al. Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: A randomized controlled trial. Sleep 2019, 42, zsz145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Shui, W.; Zhang, Z.; Li, J.; Ma, J. Different clinical parameters inform epicardial fat thickness in pre- and post-menopausal women with obstructive sleep apnea. BMC Women’s Health 2021, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulos, K.; Alhanatis, E.; Pampoukas, K.; Georgiopoulos, G.; Zourla, A.; Panoutsopoulos, A.; Kallianos, A.; Velentza, L.; Zarogoulidis, P.; Trakada, G. CPAP therapy induces favorable short-term changes in epicardial fat thickness and vascular and metabolic markers in apparently healthy subjects with obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep Breath. 2015, 20, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Çetin, S.; Vural, M.G.; Gündüz, H.; Akdemir, R.; Fırat, H. Epicardial fat thickness regression with continuous positive airway pressure therapy in patients with obstructive sleep apnea: Assessment by two-dimensional echocardiography. Wien. Klin. Wochenschr. 2016, 128, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Melek, B.H.; Arepalli, C.D.; Hartlage, G.-R.; Chen, Z.; Kim, S.; Stillman, A.E.; Raggi, P. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women. J. Am. Coll. Cardiol. 2013, 61, 1956–1961. [Google Scholar] [CrossRef]
- Sengul, C.; Ozveren, O. Epicardial adipose tissue: A review of physiology, pathophysiology, and clinical applications. Anadolu Kardiyol. Derg. 2013, 13, 261–265. [Google Scholar] [CrossRef]
- Gaborit, B.; Sengenes, C.; Ancel, P.; Jacquier, A.; Dutour, A. Role of epicardial adipose tissue in health and disease: A matter of fat? Compr. Physiol. 2017, 7, 1051–1082. [Google Scholar]
- Liu, B.; Li, Y.; Du, J.; She, Q.; Deng, S. EPICARDIAL adipose tissue in patients with obstructive sleep apnea: A systematic review and meta-analysis. Cardiovasc. Innov. Appl. 2020, 5, 81–88. [Google Scholar] [CrossRef]
- Derin, S.; Altun, I.; Koseoglu, S.; Sahin, C.; Yilmaz, M.; Akin, F.; Sahan, M. Association of epicardial fat thickness with clinical and polysomnographic parameters in non-obese obstructive sleep apnoea patients. J. Laryngol. Otol. 2018, 132, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Aitken-Buck, H.M.; Moharram, M.; Babakr, A.A.; Reijers, R.; Van Hout, I.; Fomison-Nurse, I.C.; Sugunesegran, R.; Bhagwat, K.; Davis, P.J.; Bunton, R.W.; et al. Relationship between epicardial adipose tissue thickness and epicardial adipocyte size with increasing body mass index. Adipocyte 2019, 8, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Archontogeorgis, K.; Voulgaris, A.; Nena, E.; Strempela, M.; Karailidou, P.; Tzouvelekis, A.; Mouemin, T.; Xanthoudaki, M.; Steiropoulos, S.; Froudarakis, M.E.; et al. Cardiovascular risk assessment in a cohort of newly diagnosed patients with obstructive sleep apnea syndrome. Cardiol. Res. Pract. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Colangelo, L.A.; Bielinski, S.J.; Larson, N.B.; Ding, J.; Allen, N.B.; Michos, E.D.; Shah, S.J.; Lloyd-Jones, D.M. Circulating vascular cell adhesion molecule-1 and incident heart failure: The multi-ethnic study of atherosclerosis (Mesa). J. Am. Heart Assoc. 2020, 9, e019390. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef]


| CVR | SCORE2 | SCORE2-OP | |
|---|---|---|---|
| <50 Years | 50–69 Years | ≥70 Years | |
| Low | <2.5% | <5% | <7.5% |
| Intermediate | 2.5% to <7.5% | 5% to <10% | 7.5% to <15% |
| High | ≥7.5% | ≥10% | ≥15% |
| Variable | Patients (n = 80) | Controls (n = 37) | p-Value |
|---|---|---|---|
| Age years | 60 (51; 67) | 55 (42; 62) | 0.008 |
| Sex female, n, % | 34 (42.5%) | 18 (48.6%) | 0.673 |
| BMI kg -m2 | 34 (30.8; 40.2) | 29 (28.5; 30.1) | <0.001 |
| Additional cardiovascular risk factors: | |||
| -Arterial hypertension, n, % | 63 (91.3%) | 22 (59.5%) | <0.001 |
| -Non-HDL Cholesterol, mg/dL | 101.5 (82.5; 125.2) | 101.0 (89.0; 123.0) | 0.74 |
| -Glycemia, mg/dL | 109.0 (100.0; 123.0) | 90.0 (85.0; 103.0) | <0.001 |
| -Triglycerides, mg/dL | 120.0 (95.0; 154.0) | 98.0 (89.0; 120.0) | 0.012 |
| -Smoking, n, % | 27 (33.8%) | 17 (45.9%) | 0.289 |
| Results of the sleep study: | |||
| AHI h−1 | 28.6 (18.3; 42.1) | 2.8 (1.2; 3.3) | <0.001 |
| ODI h−1 | 29.2 (21.9; 42.9) | 3.5 (2.0; 6.2) | <0.001 |
| Echocardiographic parameters | |||
| LVEF, % | 55 (50; 55) | 55 (55; 63) | 0.001 |
| Epicardial fat, mm | 6 (4.7; 7.0) | 3 (1.5; 5.0) | <0.001 |
| Serum parameters | |||
| ICAM-1, ng/mL | 120 (96.5; 139.5) | 126 (83; 163) | 0.865 |
| VCAM-1, ng/mL | 2160 (1896.5; 2407.5) | 1820 (1625; 1906) | <0.001 |
| CRP, mg/L | 1.0 (0.60; 2.0) | 0.20 (0.20; 0.30) | <0.001 |
| SCORE2; SCORE2-OP | |||
| Risk prediction, % | 15.5 (10; 22.7) | 7 (5; 13.5) | <0.001 |
| Risk classification | |||
| Low/intermediate risk | 12 (15%) | 25 (67.6%) | <0.001 |
| High risk | 68 (85%) | 12 (32.4%) | <0.001 |
| Parameters | Low/Intermediate Risk | High Risk | p-Value |
|---|---|---|---|
| ICAM-1, ng/mL | 133.9 (92.6; 152.8) | 119 (98.7; 138) | 0.509 |
| VCAM-1, ng/mL | 1871 (1623; 2184) | 2187 (1923.5; 2410) | 0.03 |
| CRP, mg/L | 1.50 (0.70; 6.55) | 1.0 (0.60; 2.0) | 0.27 |
| Non-HDL cholesterol, mg/dL | 103.5 (100.25; 120.0) | 100.5 (78.0; 128.0) | 0.58 |
| Glycemia, mg/dL | 100.5 (93.5; 111.5) | 110.0 (101.0; 128.0) | 0.08 |
| EFT, mm | 6.1 (4.5; 8.1) | 6 (5; 7) | 0.589 |
| AHI h−1 | 35.1 (20.4; 68.7) | 29 (20.5; 42.0) | 0.25 |
| ODI h−1 | 34.5 (18.7; 74.4) | 30.2 (22; 44.7) | 0.45 |
| Variables | OR | 95% CI for OR | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| VCAM-1 > 1904 ng/mL | 6.533 | 1.307 | 32.662 | 0.022 |
| Age | 1.112 | 1.026 | 1.205 | 0.009 |
| Sex: male | 0.315 | 0.054 | 1.846 | 0.200 |
| Epicardial | Fat Thickness | |||
|---|---|---|---|---|
| OSA Patients | Controls | |||
| r | p | r | p | |
| ICAM-1 | 0.19 | 0.86 | 0.22 | 0.18 |
| VCAM-1 | 0.20 | 0.06 | -0.98 | 0.50 |
| Variables; AUC | Pairwise | Comparison | 95% CI | p-Value |
|---|---|---|---|---|
| ICAM-1; 0.563 | ICAM-1 | AHI | 0.0215 to 0.356 | 0.060 |
| VCAM-1; 0.696 | ODI | 0.0298 to 0.360 | 0.059 | |
| AHI; 0.604 | CRP | −0.0350 to 0.327 | 0.11 | |
| ODI; 0.568 | VCAM-1 | AHI | −0.123 to 0.128 | 0.97 |
| CRP; 0.599 | ODI | −0.117 to 0.134 | 0.89 | |
| CRP | −0.0811 to 0.162 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chetan, I.-M.; Vesa, Ș.C.; Domokos Gergely, B.; Beyer, R.S.; Tomoaia, R.; Cabau, G.; Vulturar, D.M.; Pop, D.; Todea, D. Increased Levels of VCAM-1 in Patients with High Cardiovascular Risk and Obstructive Sleep Apnea Syndrome. Biomedicines 2024, 12, 48. https://doi.org/10.3390/biomedicines12010048
Chetan I-M, Vesa ȘC, Domokos Gergely B, Beyer RS, Tomoaia R, Cabau G, Vulturar DM, Pop D, Todea D. Increased Levels of VCAM-1 in Patients with High Cardiovascular Risk and Obstructive Sleep Apnea Syndrome. Biomedicines. 2024; 12(1):48. https://doi.org/10.3390/biomedicines12010048
Chicago/Turabian StyleChetan, Ioana-Maria, Ștefan Cristian Vesa, Bianca Domokos Gergely, Ruxandra Stefana Beyer, Raluca Tomoaia, Georgiana Cabau, Damiana Maria Vulturar, Dana Pop, and Doina Todea. 2024. "Increased Levels of VCAM-1 in Patients with High Cardiovascular Risk and Obstructive Sleep Apnea Syndrome" Biomedicines 12, no. 1: 48. https://doi.org/10.3390/biomedicines12010048