Abstract
Background: New-onset postoperative arrhythmia (PA) has previously been described as a pivotal risk factor for postoperative morbidity and mortality after visceral surgery. However, there is a lack of data concerning liver surgery. The incidence and impact of new-onset postoperative arrhythmia after liver surgery was, therefore, analyzed in a monocentric study. Methods: In total, n = 460 patients (221 female, 239 male) who underwent liver surgery between January 2012 and April 2020 without any prior arrhythmia in their medical history were included in this retrospective analysis. Clinical monitoring started with the induction of anesthesia and was terminated with discharge from the intensive care unit (ICU) or intermediate care unit (IMC). Follow-up included documentation of complications during the hospital stay, as well as long-term survival analysis. Results: Postoperative arrhythmia after liver surgery was observed in 25 patients, corresponding to an incidence of 5.4%. The occurrence of arrhythmia was significantly associated with intraoperative complications (p < 0.05), liver fibrosis/cirrhosis (p < 0.05), bile fistula/bile leakage/bilioma (p < 0.05), and organ failure (p < 0.01). Survival analysis showed a significantly poorer overall survival of patients who developed postoperative arrhythmia after liver surgery (p < 0.001). Conclusions: New-onset postoperative arrhythmia after liver surgery has an incidence of only 5.4% but is significantly associated with higher postoperative morbidity and poorer overall survival.
1. Introduction
Postoperative arrhythmia (PA) is an underrecognized complication after visceral surgery. Recently, we analyzed the occurrence of PA after surgical procedures involving the esophagus, stomach and pancreas with a retrospective study design and showed an incidence of 8.3% of the total patients, with a vast difference between the surgical procedures. Moreover, PA was significantly associated with in-house mortality and severe postoperative complications [1]. These findings were in line with those of a subsequent study of PA after surgery on the lower gastrointestinal tract: While the incidence was distinctively lower, the impact remained severe [2].
Among the existing studies concerning PA after visceral surgery [3,4], most have focused on esophageal surgery [5,6,7], presumably because it is performed partly intrathoracically and has a high rate of postoperative morbidity and mortality.
Comparable to esophageal surgery, major liver surgery also results in high rates of postoperative morbidity and mortality. Some general risk factors are already well described, but data concerning PA and general liver surgery have not yet been published. Only the relevance of PA after liver transplantation has been described in some studies [8,9,10].
Liver surgery does not have a thoracic component, but the anatomical proximity and the functional dependence between the heart and liver necessitate an analysis of PA after liver surgery. Additionally, PA has often been associated with postoperative complications (e.g., infection, organ failure, mortality) [1,2] that regularly occur after liver surgery.
In the present study, our aim was to evaluate the incidence and impact of PA after liver surgery and identify associated risk factors.
We present this article in accordance with the STROBE reporting checklist.
2. Materials and Methods
2.1. Patient Cohort
For this study, we collected data from n = 528 patients who underwent liver surgery in the period from January 2012 to April 2020 in the Department of General, Visceral, and Pediatric Surgery, University Medical Centre Göttingen, Germany. Patients with a preexisting cardiac arrhythmia (n = 58) or a previously implanted pacemaker (n = 16) were excluded from further analysis. We included only patients who went to the ICU/IMC after surgery and were monitored for at least 24 h.
Overall, data from n = 460 patients were included in the present study. The data included age, sex, type of surgical procedure, preexisting conditions (e.g., comorbidities), intraoperative complications (e.g., iatrogenic injury or blood transfusion requirement after strong bleeding), main diagnoses, postoperative complications, duration of ICU stay, and in-house mortality, as well as general mortality. Preexisting cardiac illness was classified as present if patients had a myocardial infarction, coronary heart disease, heart failure, or a valvular heart disease in the past. The classification of the extent of liver resection was based on that of Reddy et al. [11]: Operations in which fewer than four liver segments were resected were considered minor resections (vs. four or more segments in major resections). HCC and intrahepatic CCC were partly grouped as “primary liver cancer”. Other tumor entities infiltrating the liver without any origin of liver parenchyma (gallbladder carcinoma, perihilar cholangiocarcinoma, or distal bile duct carcinoma) were grouped as ‘liver-infiltrating malignant tumors’. Regarding the definition of organ failure as a possible postoperative complication, a subdivision into liver, lung, and kidney failure was made. According to the “50–50 Criteria” [12], postoperative liver failure correlates with a prothrombin time below 50% on the fifth postoperative day and, at the same time, a serum bilirubin level of more than 50 μmol/L (equals 2.94 mg/dL). Lung failure was defined as moderate or severe acute respiratory distress syndrome (ARDS) with a Horowitz quotient or oxygenation index of 200 mmHg or less according to the Berlin definition [13]. Finally, acute kidney failure was determined based on the KDIGO guidelines [14]. In order to identify patients with sepsis, first, the qSOFA score was calculated as the sum of the following (fulfilled) clinical criteria [15]:
- Respiratory frequency: ≥22/min;
- Systolic blood pressure: <100 mmHg;
- CNS: reduced vigilance or altered mental status (GCS score);
If at least two of these criteria applied to a patient, septic organ dysfunction was suspected, and the simultaneous presence of infections (documented by a doctor in the patient file) and organ failure were checked as further evidence.
For all patients who had developed PA, we conducted follow-up screening for permanent arrhythmia and thromboembolic events. We contacted the patients, their family doctors, and, if available, their cardiologists. A standardized survey was used (Supplementary Figure S1 shows the translated version of the German survey). Overall survival (OS) was analyzed for all included patients if the data were available. Existing medical records and published obituaries served as proof of death. The minimum survival time (in months) was calculated as the difference between the day of surgery and the day of death. If the death of a patient could not be traced, the last patient visit at the University Medical Center Göttingen (e.g., a presentation in the outpatient clinic or the end of another inpatient stay) was used as the point in time up to which the minimum survival could be calculated. Survival time analyses were subsequently carried out.
2.2. Statistical Analysis
All statistical analyses were performed using the statistical programming environment “R” (version 4.1.2.; R Foundation for Statistical Computing, Vienna, Austria). Descriptive data are summarized in tables with absolute and relative frequencies (n/number of cases) for categorical variables. For continuous variables, mean values (including the standard deviation), medians (with interquartile range), maxima, and minima are reported in tables or in the text. The significance level was set to 0.05 for all analyses.
Pairwise comparisons between nominally scaled variables were conducted using the chi-square test or Fisher’s exact test (Fisher–Yates test) if the expected number of events in one field of the four-field table was less than 5. For ordinal variables, two-group comparisons were conducted using the Mann–Whitney U test (Wilcoxon rank sum test). Means for interval-scaled data from two patient groups were compared using two-tailed t-tests.
The effects of different diagnosis groups on the occurrence of PA were assessed using one-way ANOVA and Tukey’s post hoc test to compare all possible group combinations.
Multivariable logistic regression models were fit to the data to model the occurrence of PA and in-hospital mortality. The relationship between each independent variable and the occurrence of PA in-hospital mortality was examined in advance (univariable analyses), and variables showing no associations (p > 0.05) with in-hospital mortality were excluded from the multivariable logistic regression modeling. The selection of which variables were ultimately relevant predictors was carried out using a step-down procedure in the logistic regression model fitting. This greedily minimized the Akaike information criterion (AIC) by excluding variables (in a stepwise procedure) to reduce the prediction error.
From the final model, the odds ratios are presented graphically with 95% confidence intervals (CIs).
Survival curves were estimated and are presented graphically using the Kaplan–Meier estimator. Using the log-rank test, the survival curves of patients with malignant and benign diseases and those of patients with different types of liver tumors were compared.
3. Results
3.1. Demographic Data
Overall, n = 460 patients who underwent liver surgery were included, among whom n = 239 were male (52%) and n = 221 were female (48%). The mean (±SD) age of the subjects was 60.9 ± 13.6 years (range: 19–87 years). Surgical procedures included n = 163 hemihepatectomies on the right, n = 64 hemihepatectomies on the left, n = 43 segmentectomies, n = 41 right trisegmentectomies, n = 46 multivisceral resections, n = 47 bisegmentectomies (mainly left lateral and right posterior sectorectomies), n = 31 resections of other segment combinations, n = 12 atypical liver resections or wedge resections, n = 11 “in situ split” liver resections (with subsequent trisegmentectomy or hemihepatectomy), and n = 2 left trisegmentectomies. A total of 387 patients (84%) were diagnosed with malignant neoplasia. Specific diagnoses, comorbidities, perioperative complications, and other individual parameters are listed in Table 1.

Table 1.
Descriptive values of the recorded parameters in all included patients undergoing liver surgery and in patients with PA.
3.2. Incidence, Time Point, and Type of PA
In total, 25 of 460 patients developed PA (5.4%) after liver surgery, with a wide range between the different surgical procedures (Table 1). The difference in incidence between the surgical procedures, however, remained statistically nonsignificant. Table 1 shows all surgical procedures and the occurrence of PA in the respective groups.
The detected types of PA were mostly tachycardic arterial fibrillation (n = 14, 56%), asystole (n = 5, 20%), and ventricular tachycardia (n = 3, 12%). Only one patient developed bradycardic arterial fibrillation (4%). In n = 3 cases, PA led to cardiopulmonary resuscitation (12%). The mean time interval between liver surgery and the occurrence of PA was 186.1 h (median (Mdn) = 132; interquartile range = 40–312; Min = 0; Max = 672).
3.3. PA and Associated Factors
The incidence of PA differed significantly based on the diagnosis (F(3456) = 7.72, p < 0.001); Tukey post hoc analysis revealed a significantly higher incidence of PA in patients with primary liver cancer (HCC/intrahepatic CCC) than in patients with liver metastases. Additionally, comparing primary liver cancer patients to those with benign lesions, the latter showed a significantly lower incidence of PA (p < 0.05).
Patients with liver-infiltrating malignant tumors showed a significantly higher rate of PA than those with benign lesions and patients with liver metastases (Table 2).

Table 2.
List of all significant differences between the diagnosis groups regarding the incidence of PA in Tukey post hoc analysis with the median, 95% confidence interval, and p-values (asterisks indicate the level of significance: p < 0.05 *, p < 0.01 **, p < 0.001 ***). Liver-infiltrating malignant tumors (without origin of liver parenchyma): tumors such as gallbladder carcinoma, perihilar cholangiocarcinoma or distal bile duct carcinoma.
To evaluate the impact of the extent of liver tissue resection per se, we pooled all cases into major and minor liver resections. Here, no significant differences in the occurrence of PA were observed between the two groups.
Further univariate tests were carried out to examine associations between the different variables listed in Table 1 and the occurrence of postoperative cardiac arrhythmia.
All variables that were significantly associated with the occurrence of PA were included in a logistic regression model, and those with the greatest explanatory power were extracted using logistic regression analysis. Here, intraoperative complications, liver fibrosis/cirrhosis, organ failure, ascites (defined as 500 > mL/24 h drainage after 3 postoperative days), and bile fistula were identified as significantly associated factors. Figure 1 shows the odds ratios with 95% confidence intervals of the variables in the stepwise logistic regression model for the postoperative development of arrhythmia.
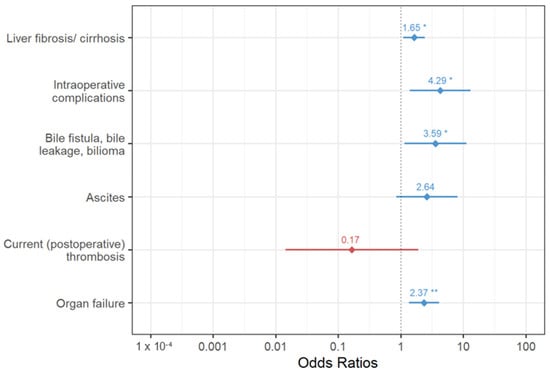
Figure 1.
Odds ratios with 95% confidence intervals of variables in the final logistic regression model for the postoperative development of arrhythmia (asterisks indicate the level of significance: p < 0.05 *, p < 0.01 **).
In the further course after liver surgery, the occurrence of PA was also significantly associated with a longer stay in the ICU/IMC (Mdn: 19 days versus 5 days; p < 0.01). The mean ICU/IMC stay for all patients was 9.8 days (Mdn = 5 days).
3.4. PA and In-House Mortality
In total, n = 20 (4.3%) patients died after liver surgery during their inpatient stay. To test the association between clinical variables and in-house mortality, univariate tests were performed. Significant associations were detected between in-house mortality and new-onset PA, organ failure, sepsis, infection, electrolyte disorder, ascites, revision surgery, bile fistula/bile leakage/bilioma, CHA2DS2-VASc score ≥ 4, current (postoperative) thrombosis, anastomosis/stump insufficiency, age, extent of liver resection, and intraoperative complications (see Table 3).

Table 3.
Descriptive values of the recorded parameters of all included patients undergoing liver surgery and those of patients who died during their hospital stay.
A forest plot (Figure 2) is used to present the results of the stepwise logistic regression model for in-house mortality with their odds ratios, 95% confidence intervals, and p-values.
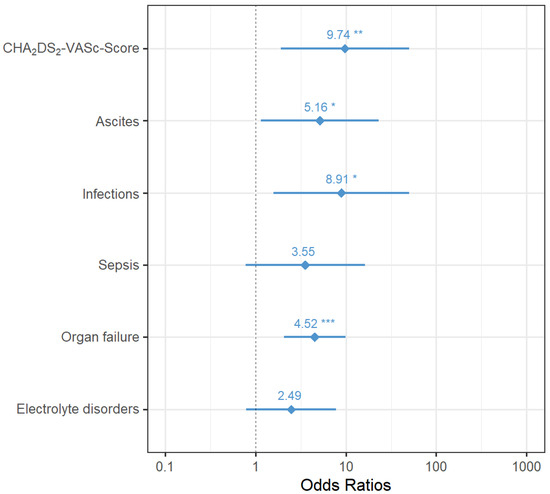
Figure 2.
Odds ratios with 95% confidence intervals of variables in the final logistic regression model for postoperative in-house mortality (asterisks indicate the level of significance: p < 0.05 *, p < 0.01 **, p < 0.001 ***).
3.5. Long-Term Overall Survival (OS)
Altogether, the follow-up data of 387 of 460 patients (84.1% of all patients) could be obtained, and n = 73 were lost to follow-up, as there was no access to any information after their discharge. The median survival time of all patients was 54 months (IQR = 43–69.1), and the median follow-up time was 41.3 months (IQR = 36.8–46.5).
The survival of n = 329 patients with liver surgery for malignant disease was compared with the survival of n = 58 patients with a benign underlying disease. In the postoperative period up to a maximum of 118.6 months after liver surgery, a total of n = 8 (13.8%) of the patients with a benign underlying disease died, and almost half of the patients with a malignant disease died; n = 149 (45.3%). In a direct comparison, the survival of patients with a benign underlying disease (Mdn = NA, 95% CI [85.3; NA]) was longer than the survival of patients with a malignant disease (Mdn = 45.3, 95% CI [39.3; 59.2]). A log-rank test was performed and showed significant differences between the survival distributions of both patient groups; χ2 (1) = 14.1, p < 0.001.
Moreover, a comparison was made between patients diagnosed with hepatocellular carcinoma (HCC), cholangiocellular carcinoma (CCC), and colorectal liver metastases (CRLMs). Among the 53 patients with HCC, 43 (81.1%) could be tracked, in addition to 60 of 72 patients (83.3%) with CCC and 169 of 200 patients (84.5%) with CRLM. The median overall survival of patients with HCC was 69.1 months (95% CI [31.4; NA]), compared to 47 months (95% CI [39.5; 60.3]) for patients with CRLM. Thus, the two diagnosis groups, CRLM and HCC, both survived significantly longer than patients with CCC (Mdn = 33.3, 95% CI [14.9; 61.2]; p < 0.05). All other comparisons between these diagnosis groups were not significant.
Patients without PA survived significantly longer than those with PA after liver surgery (median 56.8 months vs. 11.3 months, p < 0.001). The difference between the survival curves of patients with vs. without PA shown in Figure 3 was significant; χ2(1) = 26.9, p < 0.001.
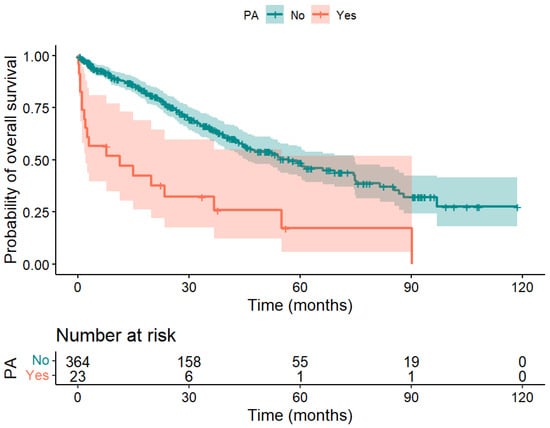
Figure 3.
Kaplan–Meier curve of patients with PA (red) and without PA (blue) after liver surgery. A significant difference in overall survival was obtained; χ2(1) = 26.9, p < 0.001.
4. Discussion
New onset of cardiac arrhythmia is typically considered a cardiac-surgery-related phenomenon. Little attention has been given to its incidence and impact in noncardiac surgery, although its relevance is known [16]. This may be due to a poor understanding of the pathophysiological process. Chung et al. postulated an inflammatory response triggering disorganized electrical activity within atrial myocytes [17]. These data are in line with our previous data showing a significant association of PA after surgery on the upper and lower gastrointestinal tract with postoperative complications (e.g., infections, sepsis, and organic failure). On the other hand, reduced organ perfusion based on PA is under discussion, leading to the debate of whether PA precedes complications or if it is just a symptom.
Subramani et al. recently published a systematic review and meta-regression analysis of observational studies confirming postoperative atrial fibrillation as a risk factor for postoperative cardiac complications, stroke, and higher mortality. Advanced age, male sex, preoperative hypertension, diabetes mellitus, and cardiac disease were identified as important risk factors for perioperative atrial fibrillation. Faced with an aging and comorbid surgical population, the need for risk stratification and close monitoring increases [18].
In visceral surgery, the type of surgery appears to have an impact on the incidence of PA and may be associated with the intensity of trauma. Interestingly, in liver surgery, the incidence of PA is not associated with the extent of parenchymal removal. Data on liver surgery and PA are rare. Such data are typically collected in liver transplantation, showing arrhythmias as the main cardiovascular disease complication within 30 days after liver transplantation [19,20]. A poorer postoperative outcome in pretransplant arterial fibrillation patients was shown by Dangl et al., who analyzed 45,357 patients who underwent orthotopic liver transplantation [21]. The prevalence of preexisting atrial fibrillation and/or incidence of AF following liver transplantation was analyzed by Chokesuwattanaskul et al. in a meta-analysis enrolling 38,586 liver transplant patients, also indicating a poorer clinical outcome [22]. Rivas et al. published data on 857 patients, which revealed a new onset of postoperative atrial fibrillation (POAF) in 10.4% of patients. While POAF was not associated with in-hospital mortality, the one-year mortality was increased [23].
Posttransplant reperfusion causing hemodynamic stress can result in hemodynamic instability along with arrhythmia [24,25,26]. Reperfusion effects, however, were not attributed to the analyzed patients of the present study, as the Pringle maneuver was not applied. Nevertheless, these findings may confirm the significant association in the present study between PA and intraoperative complications/transfusions of erythrocyte concentrates, as this also represents a process of reperfusion.
The association of PA and preoperative conditions such as older age and preexisting multiple cardiovascular diseases confirms the abovementioned data from Subramani et al. It is of further importance that the diagnosis of primary liver cancer is associated with an increased risk of PA compared to that in cases of liver metastases from colorectal cancer. It can be hypothesized that underlying circumstances leading to primary cancer in the liver are also a trigger for the new onset of PA.
A central finding in the present study is that PA is significantly associated with a poorer OS. This trend was shown in liver transplant patients as outlined above. In patients undergoing surgery for solid tumors, this finding has not yet been published. It is very notable that the occurrence of PA is independently associated with a poorer prognosis. Here, we used a Cox regression model, and both variables, PA and dignity, were significantly associated with poorer survival—each of them as independent variables. The association of PA and poorer survival is of interest, as, e.g., atrial fibrillation itself is not associated with poorer prognosis in the general population. It could be speculated that the occurrence of PA after surgery for liver tumors reveals a previously undetected cardiac disease. On the other hand, fluid management during and after the surgical procedure may have an effect on cardiac function and integrity. As intraoperative low-volume therapy and postoperative high-volume therapy are cornerstones of liver surgery, these volume loads may also trigger a previously undetected heart frailty.
Due to the retrospective nature of this study, no causalities may be discovered here. However, whether as a cause or just as an indicator, PA after liver surgery is significantly associated with postoperative complications and poorer overall survival. Therefore, its occurrence is an important clinical finding, which should be recognized and raise awareness for a possibly critical situation of the patient.
Whether a poorer prognosis of patients with PA is due to the development of undetected heart disease or the result of a rather complicated postoperative course remains unknown.
5. Conclusions
To the best of our knowledge, we are the first to report the association between liver surgery and postoperative arrhythmia. Despite its low incidence of 5.4% after liver surgery (compared to other types of visceral surgery), PA is associated with a set of postoperative complications, longer ICU/IMC stay, and poorer overall survival. Due to the retrospective character of this analysis, no conclusion regarding causalities can be stated. Further analyses, especially those regarding causalities, remain subjects for a prospectively designed study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12020271/s1, Figure S1: Questionnaire used for the follow-up screening for permanent arrhythmia and thromboembolic events in patients with new-onset PA.
Author Contributions
Conceptualization, F.R., T.T., M.G., T.P., A.A. and J.G.; Methodology, D.E. and A.L.; Validation, A.L.; Formal Analysis, D.E. and M.B.; Investigation, F.R., D.E., T.T., A.L., M.B. and J.G.; Data Curation, F.R., D.E., A.F.M., M.S.H., T.P. and A.A.; Writing—Original Draft Preparation, F.R.; Writing—Review and Editing, T.T., M.B., M.G., T.P., A.A. and J.G.; Supervision, M.G. and J.G.; Project Administration, A.A. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of University Medical Center Göttingen (study number 14/2/19, approval on 29 August 2019), which waived the need for informed consent due to its retrospective character.
Informed Consent Statement
The local ethics committee waived the need for informed consent due to its retrospective character.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ruhlmann, F.; Tichelbacker, T.; Mackert, A.F.; Engelhardt, D.; Leha, A.; Bernhardt, M.; Ghadimi, M.; Perl, T.; Azizian, A.; Gaedcke, J. Incidence, Associated Risk Factors, and Outcomes of Postoperative Arrhythmia after Upper Gastrointestinal Surgery. JAMA Netw. Open 2022, 5, e2223225. [Google Scholar] [CrossRef]
- Ruhlmann, F.; Hedicke, M.S.; Engelhardt, D.; Mackert, A.F.; Tichelbacker, T.; Leha, A.; Bernhardt, M.; Ghadimi, M.; Perl, T.; Azizian, A.; et al. Incidence and impact of new-onset postoperative arrhythmia after surgery of the lower gastrointestinal tract. Sci. Rep. 2023, 13, 1284. [Google Scholar] [CrossRef]
- Chebbout, R.; Heywood, E.G.; Drake, T.M.; Wild, J.R.L.; Lee, J.; Wilson, M.; Lee, M.J. A systematic review of the incidence of and risk factors for postoperative atrial fibrillation following general surgery. Anaesthesia 2018, 73, 490–498. [Google Scholar] [CrossRef]
- Bender, J.S. Supraventricular tachyarrhythmias in the surgical intensive care unit: An under-recognized event. Am. Surg. 1996, 62, 73–75. [Google Scholar]
- Rao, V.P.; Addae-Boateng, E.; Barua, A.; Martin-Ucar, A.E.; Duffy, J.P. Age and neo-adjuvant chemotherapy increase the risk of atrial fibrillation following oesophagectomy. Eur. J. Cardiothorac. Surg. 2012, 42, 438–443. [Google Scholar] [CrossRef]
- Mc Cormack, O.; Zaborowski, A.; King, S.; Healy, L.; Daly, C.; O’Farrell, N.; Donohoe, C.L.; Ravi, N.; Reynolds, J.V. New-onset atrial fibrillation post-surgery for esophageal and junctional cancer: Incidence, management, and impact on short- and long-term outcomes. Ann. Surg. 2014, 260, 772–778; discussion 778. [Google Scholar] [CrossRef]
- Schizas, D.; Kosmopoulos, M.; Giannopoulos, S.; Giannopoulos, S.; Kokkinidis, D.G.; Karampetsou, N.; Papanastasiou, C.A.; Rouvelas, I.; Liakakos, T. Meta-analysis of risk factors and complications associated with atrial fibrillation after oesophagectomy. Br. J. Surg. 2019, 106, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Koshy, A.N.; Enyati, A.; Weinberg, L.; Han, H.C.; Horrigan, M.; Gow, P.J.; Ko, J.; Thijs, V.; Testro, A.; Lim, H.S.; et al. Postoperative Atrial Fibrillation and Long-Term Risk of Stroke in Patients Undergoing Liver Transplantation. Stroke 2021, 52, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Xia, V.W.; Worapot, A.; Huang, S.; Dhillon, A.; Gudzenko, V.; Backon, A.; Agopian, V.G.; Aksoy, O.; Vorobiof, G.; Busuttil, R.W.; et al. Postoperative atrial fibrillation in liver transplantation. Am. J. Transplant. 2015, 15, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Rachwan, R.J.; Kutkut, I.; Hathaway, T.J.; Timsina, L.R.; Kubal, C.A.; Lacerda, M.A.; Ghabril, M.S.; Bourdillon, P.D.; Mangus, R.S. Postoperative Atrial Fibrillation and Flutter in Liver Transplantation: An Important Predictor of Early and Late Morbidity and Mortality. Liver Transpl. 2020, 26, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Barbas, A.S.; Turley, R.S.; Steel, J.L.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Clary, B.M. A standard definition of major hepatectomy: Resection of four or more liver segments. Hpb 2011, 13, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; Belghiti, J.; Farges, O.; Ogata, S.; Sauvanet, A.; Delefosse, D.; Durand, F. The “50-50 criteria” on postoperative day 5: An accurate predictor of liver failure and death after hepatectomy. Ann. Surg. 2005, 242, 824–828; discussion 828–829. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Lamb, E.J.; Levey, A.S.; Stevens, P.E. The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: Evolution not revolution. Clin. Chem. 2013, 59, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bhave, P.D.; Goldman, L.E.; Vittinghoff, E.; Maselli, J.; Auerbach, A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am. Heart J. 2012, 164, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Subramani, Y.; El Tohamy, O.; Jalali, D.; Nagappa, M.; Yang, H.; Fayad, A. Incidence, Risk Factors, and Outcomes of Perioperative Atrial Fibrillation following Noncardiothoracic Surgery: A Systematic Review and Meta-Regression Analysis of Observational Studies. Anesthesiol. Res. Pract. 2021, 2021, 5527199. [Google Scholar] [CrossRef]
- Aghaulor, B.; VanWagner, L.B. Cardiac and Pulmonary Vascular Risk Stratification in Liver Transplantation. Clin. Liver Dis. 2021, 25, 157–177. [Google Scholar] [CrossRef]
- Patel, S.S.; Lin, F.P.; Rodriguez, V.A.; Bhati, C.; John, B.V.; Pence, T.; Siddiqui, M.B.; Sima, A.P.; Abbate, A.; Reichman, T.; et al. The relationship between coronary artery disease and cardiovascular events early after liver transplantation. Liver Int. 2019, 39, 1363–1371. [Google Scholar] [CrossRef]
- Dangl, M.; Grant, J.K.; Vincent, L.; Ebner, B.; Maning, J.; Olorunfemi, O.; Zablah, G.; Sancassani, R.; Colombo, R. The association of pre-transplant atrial fibrillation with in-hospital outcomes in patients undergoing orthotopic liver transplantation: A propensity score matching analysis. J. Card. Surg. 2022, 37, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Chokesuwattanaskul, R.; Thongprayoon, C.; Bathini, T.; Ungprasert, P.; Sharma, K.; Wijarnpreecha, K.; Pachariyanon, P.; Cheungpasitporn, W. Liver transplantation and atrial fibrillation: A meta-analysis. World J. Hepatol. 2018, 10, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Rivas, E.; Sasaki, K.; Liang, C.; Wang, J.; Quintini, C.; Maheshwari, K.; Turan, A.; Fares, M.; Cywinski, J.B. New-Onset Atrial Fibrillation in Patients Undergoing Liver Transplantation: Retrospective Analysis of Risk Factors and Outcomes. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Brems, J.J.; Takiff, H.; McHutchison, J.; Collins, D.; Biermann, L.A.; Pockros, P. Systemic versus nonsystemic reperfusion of the transplanted liver. Transplantation 1993, 55, 527–529. [Google Scholar] [CrossRef]
- Raval, Z.; Harinstein, M.E.; Skaro, A.I.; Erdogan, A.; DeWolf, A.M.; Shah, S.J.; Fix, O.K.; Kay, N.; Abecassis, M.I.; Gheorghiade, M.; et al. Cardiovascular risk assessment of the liver transplant candidate. J. Am. Coll. Cardiol. 2011, 58, 223–231. [Google Scholar] [CrossRef]
- Shi, X.Y.; Xu, Z.D.; Xu, H.T.; Jiang, J.J.; Liu, G. Cardiac arrest after graft reperfusion during liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2006, 5, 185–189. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).