100 Years since the Discovery of Insulin, from Its Discovery to the Insulins of the Future
Abstract
:1. History of Diabetes
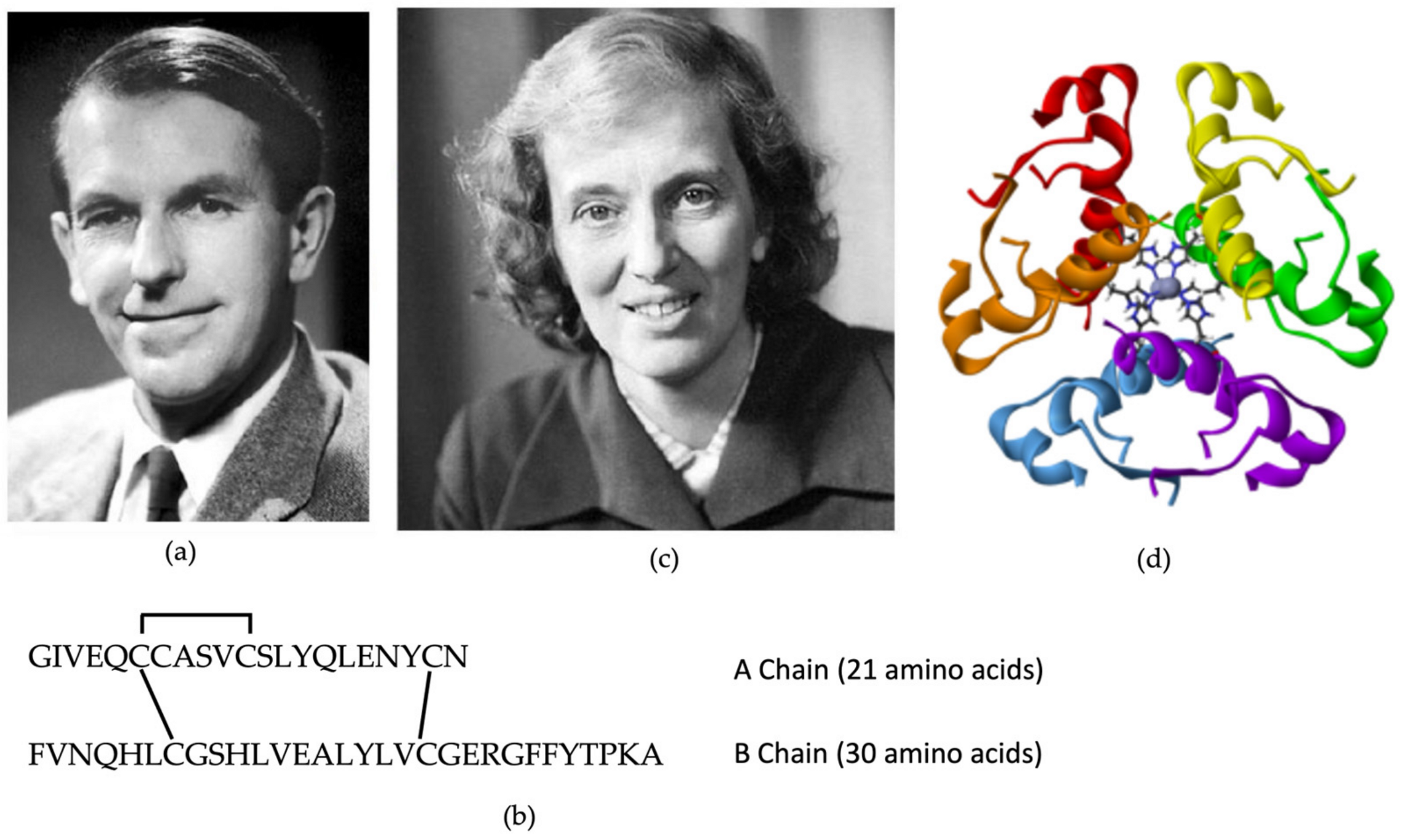
2. The Discovery of Insulin
Insulin Arrives in Europe
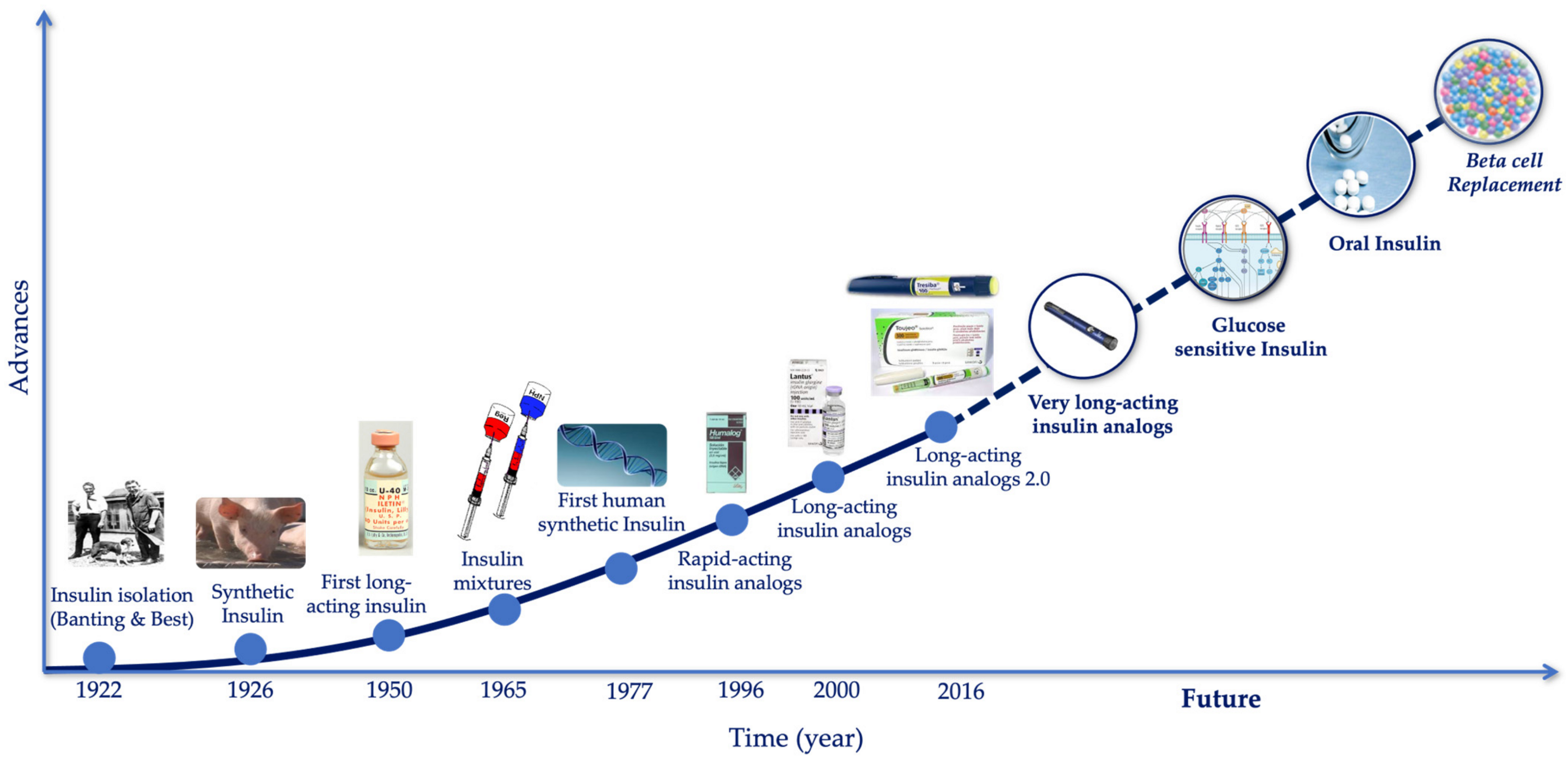
3. The Evolution of the Treatment of Diabetes
3.1. The Evolution of Insulin Therapy
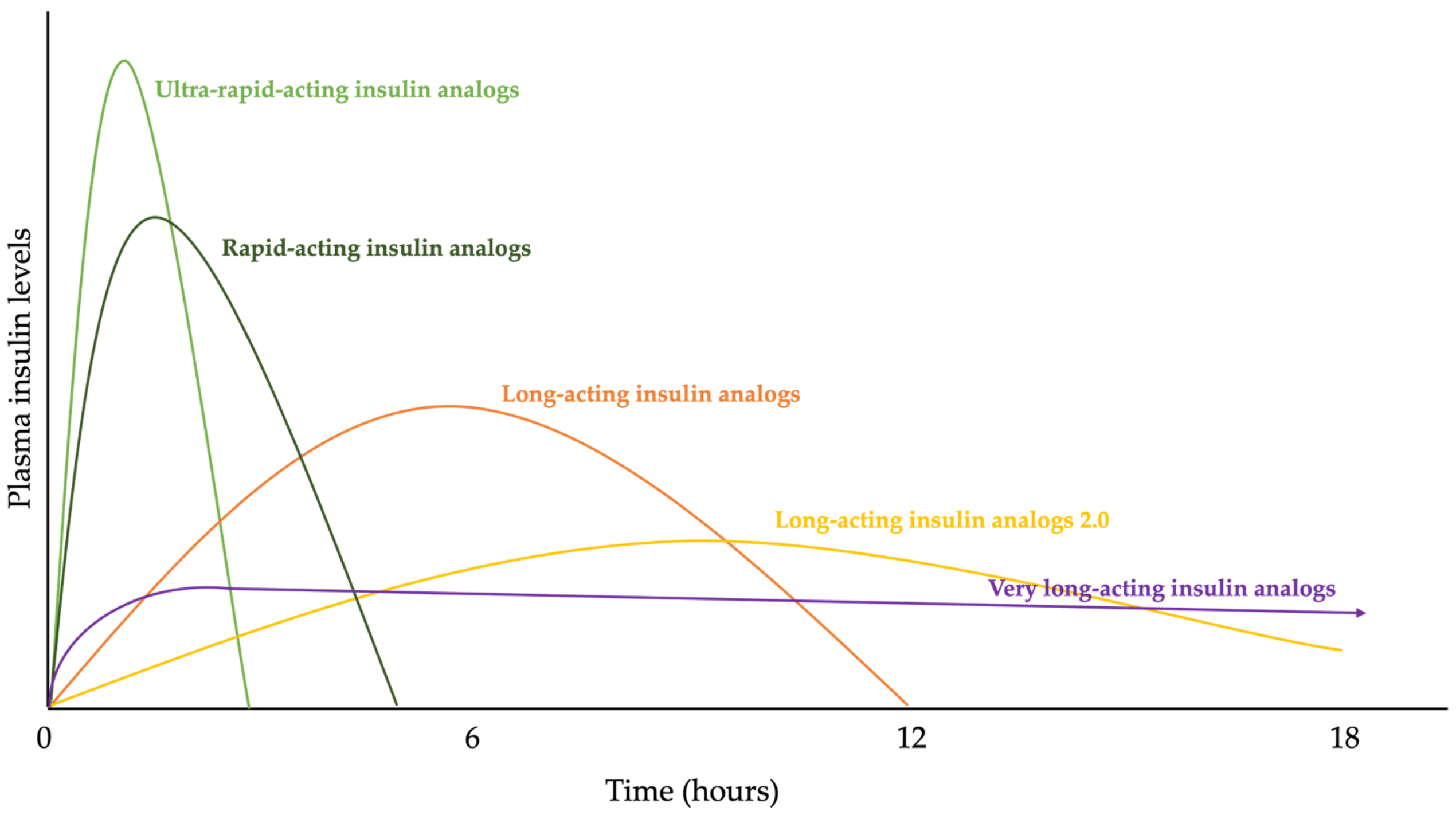
3.2. The Evolution of Insulin Therapy, More Than Insulin Analogues
3.2.1. Syringes
3.2.2. Insulin Pens
3.2.3. Continuous Subcutaneous Insulin Infusion-CSII
4. The Future of Insulin Therapy
4.1. Once-Weekly Basal Insulin Analogue
4.2. Glucose Sensitivity
4.3. Non-Injectable Insulins
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- King, K.M.; Rubin, G. A History of Diabetes- from Antiquity to Discovering Insulin. Br. J. Nurs. 2003, 12, 1091–1095. [Google Scholar] [CrossRef]
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the History of Diabetes Mellitus: The Main Contributors. World J. Diabetes 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Gemmill, C.L. The Greek Concept of Diabetes. Bull. N. Y. Acad. Med. 1972, 48, 1033. [Google Scholar]
- Chevreul, M.E. Note Sur Le Sucre Du Diabète. Ann. Chem. 1815, 95, 319–320. [Google Scholar]
- De Leiva-Hidalgo, A.; De Leiva-Pérez, A. I-European Research, the Cradle of the Discovery of the Antidiabetic Hormone: The Pioneer Roles and the Relevance of Oskar Minkowski and Eugène Gley. Acta Diabetol. 1976, 59, 1635–1651. [Google Scholar] [CrossRef] [PubMed]
- De Leiva-Hidalgo, A.; De Leiva-Pérez, A. Pancreatic Extracts for the Treatment of Diabetes (1889–1914): Acomatol. Am. J. Ther. 2020, 27, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- The Discovery and Early Development of Insulin. Available online: https://insulin.library.utoronto.ca/ (accessed on 30 January 2024).
- Karamitsos, D.T. The Story of Insulin Discovery. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. S1), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Bliss, M. Banting: A Biography; University of Toronto Press: Toronto, ON, Canada, 1993; ISBN 9780802073860. [Google Scholar]
- Barron, M. The Relation of the Islets of Langerhans with Special Reference to Cases of Pancreatic Lithiasis. Surg. Gynecol. Obs. 1920, 31, 437–448. [Google Scholar]
- Tan, S.Y.; Merchant, J. Frederick Banting (1891–1941): Discoverer of Insulin. Singap. Med. J. 2017, 58, 2–3. [Google Scholar] [CrossRef]
- Hegele, R.A.; Maltman, G.M. Insulin’s Centenary: The Birth of an Idea. Lancet Diabetes Endocrinol. 2020, 8, 971–977. [Google Scholar] [CrossRef]
- Marshall, S.M. Celebrating 100 Years of Insulin. Diabetologia 2021, 64, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Collip, J.B. The Original Method as Used for the Isolation of Insulin in Semipure Form for the Treatment of the First Clinical Cases. J. Biol. Chem. 1923, 55, 40–41. [Google Scholar]
- Banting, F.G.; Barron, M.; Best, C.H. The Internal Secretion of the Pancreas. J. Lab. Clin. Med. 1922, 7, 465–480. [Google Scholar]
- Macleod, J.J.R. Insulin and the Steps Taken to Secure an Effective Preparation. Can. Med. Assoc. J. 1922, 12, 899–900. [Google Scholar]
- Poole, D.C.; Pittman, R.N.; Musch, T.I.; Østergaard, L. August Krogh: Physiology Genius and Compassionate Humanitarian. J. Physiol. 2020, 598, 4423–4424. [Google Scholar] [CrossRef]
- Poulsen, J.E. The Impact of August Krogh on the Insulin Treatment of Diabetes and Our Present Status. Acta Med. Scand. Suppl. 1975, 578, 7–14. [Google Scholar] [CrossRef]
- Ahmad, K. Insulin Sources and Types: A Review of Insulin in Terms of Its Mode on Diabetes Mellitus. J. Tradit. Chin. Med. 2014, 34, 234–237. [Google Scholar] [CrossRef]
- John Jacob Abel (1875–1938). Available online: https://www.historiadelamedicina.org/abel.html (accessed on 3 January 2024).
- Hagedorn, H.C.; Jensen, B.N.; Krarup, N.B.; Wodstrup, I. Protamine Insulinate. J. Am. Med. Assoc. 1936, 106, 177–180. [Google Scholar] [CrossRef]
- Fisher, A.M.; Scott, D.A. Zinc Content of Bovine Pancreas. Biochem. J. 1935, 29, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, M.; Lacey, A.H.; Fisher, A.M. NPH Insulin. Can. Med. Assoc. J. 1951, 65, 20–23. [Google Scholar] [PubMed]
- Clarki, B.F.; Munro, J.F.; Duncan, L.J.P. Time-Action Studies and Clinical Trial of Actrapid and Crystal II Novo Insulins. Br. Med. J. 1965, 2, 265–267. [Google Scholar] [CrossRef]
- Schlichtkrull, J.; Munck, O.; Jersild, M. Insulin Rapitard and Insulin Actrapid. Acta Med. Scand. 1965, 177, 103–113. [Google Scholar] [CrossRef]
- SANGER, F.; THOMPSON, E.O. The Amino-Acid Sequence in the Glycyl Chain of Insulin. II. The Investigation of Peptides from Enzymic Hydrolysates. Biochem. J. 1953, 53, 366–374. [Google Scholar] [CrossRef]
- Glusker, J.P. Dorothy Crowfoot Hodgkin (1910–1994). Protein Sci. 1994, 3, 2465–2469. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.N.; Chang, A.C.Y.; Boyer, H.W.; Helling, R.B. Construction of Biologically Functional Bacterial Plasmids In Vitro. Proc. Natl. Acad. Sci. USA 1973, 70, 3240–3244. [Google Scholar] [CrossRef] [PubMed]
- Villa-Komaroff, L.; Efstratiadis, A.; Broome, S.; Lomedico, P.; Tizard, R.; Naber, S.P.; Chick, W.L.; Gilbert, W. A Bacterial Clone Synthesizing Proinsulin. Proc. Natl. Acad. Sci. USA 1978, 75, 3727–3731. [Google Scholar] [CrossRef] [PubMed]
- Borgoño, C.A.; Zinman, B. Insulins: Past, Present, and Future. Endocrinol. Metab. Clin. N. Am. 2012, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Baghban Taraghdari, Z.; Imani, R.; Mohabatpour, F. A Review on Bioengineering Approaches to Insulin Delivery: A Pharmaceutical and Engineering Perspective. Macromol. Biosci. 2019, 19, e1800458. [Google Scholar] [CrossRef] [PubMed]
- Bolli, G.B.; Cheng, A.Y.Y.; Owens, D.R. Insulin: Evolution of Insulin Formulations and Their Application in Clinical Practice over 100 Years. Acta Diabetol. 2022, 59, 1129–1144. [Google Scholar] [CrossRef]
- Home, P. The Evolution of Insulin Therapy. Diabetes Res. Clin. Pract. 2021, 175, 108816. [Google Scholar] [CrossRef]
- Danne, T.; Bolinder, J. New Insulins and Insulin Therapy. Int. J. Clin. Pract. Suppl. 2011, 65, 26–30. [Google Scholar] [CrossRef]
- Wilson, L.M.; Castle, J.R. Recent Advances in Insulin Therapy. Diabetes Technol. Ther. 2020, 22, 929–936. [Google Scholar] [CrossRef]
- Jung, H.N.; Cho, Y.K.; Min, S.H.; Kim, H.S.; Kim, Y.J.; Park, J.Y.; Lee, W.J.; Jung, C.H. Free versus Fixed-Ratio Combination of Basal Insulin and GLP-1 Receptor Agonists in Type 2 Diabetes Uncontrolled with GLP-1 Receptor Agonists: A Systematic Review and Indirect Treatment Comparison. Front. Endocrinol. 2022, 13, 870722. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Trujillo, J.M. Basal Insulin Use With GLP-1 Receptor Agonists. Diabetes Spectr. 2016, 29, 152–160. [Google Scholar] [CrossRef] [PubMed]
- GLP-1 Receptor Agonist and Basal Insulin Co-Therapy in Type 2 Diabetes: Clinical Evidence and Practicalities of Use—PCDS. Available online: https://www.pcdsociety.org/resources/details/glp-1-receptor-agonist-and-basal-insulin-co-therapy-in-type-2-diabetes-clinical-evidence-and-practicalities-of-use (accessed on 15 February 2024).
- Kesavadev, J.; Saboo, B.; Krishna, M.B.; Krishnan, G. Evolution of Insulin Delivery Devices: From Syringes, Pens, and Pumps to DIY Artificial Pancreas. Diabetes Ther. 2020, 11, 1251–1269. [Google Scholar] [CrossRef]
- Shah, R.B.; Patel, M.; Maahs, D.M.; Shah, V.N. Insulin Delivery Methods: Past, Present and Future. Int. J. Pharm. Investig. 2016, 6, 1. [Google Scholar] [CrossRef]
- Pincock, S. Colin Murdoch. Lancet 2008, 371, 1994. [Google Scholar] [CrossRef]
- Rai, I.; Wanjari, A.; Acharya, S. Recent Advances in Insulin Delivery Devices and Modes of Insulin Therapy. J. Pharm. Res. Int. 2021, 33, 358–367. [Google Scholar] [CrossRef]
- Alsaleh, F.M.; Smith, F.J.; Keady, S.; Taylor, K.M.G. Insulin Pumps: From Inception to the Present and toward the Future. J. Clin. Pharm. Ther. 2010, 35, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ontario, H.Q. Continuous Monitoring of Glucose for Type 1 Diabetes: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2018, 18, 1. [Google Scholar]
- Beck, R.W.; Bergenstal, R.M.; Laffel, L.M.; Pickup, J.C. Advances in Technology for Management of Type 1 Diabetes. Lancet 2019, 394, 1265–1273. [Google Scholar] [CrossRef]
- Warshauer, J.T.; Bluestone, J.A.; Anderson, M.S. New Frontiers in the Treatment of Type 1 Diabetes. Cell Metab. 2020, 31, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Faulds, E.R.; Zappe, J.; Dungan, K.M. Real-World Implications of Hybrid Close Loop (HCl) Insulin Delivery System. Endocr. Pract. 2019, 25, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Insulin|FDA. Available online: https://www.fda.gov/consumers/free-publications-women/insulin (accessed on 15 February 2024).
- Rosenstock, J.; Bain, S.C.; Gowda, A.; Jódar, E.; Liang, B.; Lingvay, I.; Nishida, T.; Trevisan, R.; Mosenzon, O. Weekly Icodec versus Daily Glargine U100 in Type 2 Diabetes without Previous Insulin. N. Engl. J. Med. 2023, 389, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Bajaj, H.S.; Janež, A.; Silver, R.; Begtrup, K.; Hansen, M.V.; Jia, T.; Goldenberg, R. Once-Weekly Insulin for Type 2 Diabetes without Previous Insulin Treatment. N. Engl. J. Med. 2020, 383, 2107–2116. [Google Scholar] [CrossRef]
- Hoeg-Jensen, T. Review: Glucose-Sensitive Insulin. Mol. Metab. 2021, 46, 101107. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A. A Glucose-Controlled Insulin-Delivery System: Semisynthetic Insulin Bound to Lectin. Science 1979, 206, 1190–1191. [Google Scholar] [CrossRef]
- Nkonge, K.M.; Nkonge, D.K.; Nkonge, T.N. Insulin Therapy for the Management of Diabetes Mellitus: A Narrative Review of Innovative Treatment Strategies. Diabetes Ther. 2023, 14, 1801–1831. [Google Scholar] [CrossRef]
- Mohanty, R.R.; Das, S. Inhaled Insulin—Current Direction of Insulin Research. J. Clin. Diagn. Res. 2017, 11, OE01–OE02. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Recent Advancements in Oral Administration of Insulin-Loaded Liposomal Drug Delivery Systems for Diabetes Mellitus. Int. J. Pharm. 2018, 549, 201–217. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, Microcapsules and Microspheres: A Review of Recent Developments and Prospects for Oral Delivery of Insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Halberg, I.B.; Lyby, K.; Wassermann, K.; Heise, T.; Zijlstra, E.; Plum-Mörschel, L. Efficacy and Safety of Oral Basal Insulin versus Subcutaneous Insulin Glargine in Type 2 Diabetes: A Randomised, Double-Blind, Phase 2 Trial. Lancet Diabetes Endocrinol. 2019, 7, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Abramson, A.; Caffarel-Salvador, E.; Khang, M.; Dellal, D.; Silverstein, D.; Gao, Y.; Frederiksen, M.R.; Vegge, A.; Hubálek, F.; Water, J.J.; et al. An Ingestible Self-Orienting System for Oral Delivery of Macromolecules. Science 2019, 363, 611–615. [Google Scholar] [CrossRef] [PubMed]




| Type of Insulin | Brand Name | Other Names | |
|---|---|---|---|
| PRANDIAL | Rapid-Acting | Admelog | insulin lispro injection |
| Afrezza (inhalation powder) | regular human insulin | ||
| Apidra | insulin glulisine | ||
| Fiasp | insulin aspart | ||
| Humalog | insulin lispro | ||
| Novolog | insulin aspart | ||
| BASAL | Intermediate-Acting | Humulin N | NPH human insulin |
| Novolin N | |||
| Short-Acting | Humulin R | regular human insulin | |
| Novolin R | |||
| Long-Acting | Basaglar KwikPen | insulin glargine | |
| Lantus | |||
| Toujeo | |||
| Levemir | insulin detemir | ||
| Tresiba FlexTouch | insulin degludec | ||
| MIXTURES | Intermediate- and Rapid-Acting | Humalog Mix 75/25 | 75% insulin lispro protamine suspension |
| 25% insulin lispro injection | |||
| Humalog 70/30 | 70% human insulin isophane suspension | ||
| 30% human insulin injection | |||
| Humalog Mix 50/50 | 50% insulin lispro protamine suspension | ||
| 50% insulin lispro injection | |||
| NovoLog Mix 70/30 | 70% insulin aspart protamine suspension | ||
| 30% insulin aspart injection | |||
| Long- and Rapid-Acting | Ryzodeg 70/30 FlexTouch | 70% insulin degludec | |
| 30% insulin aspart | |||
| Intermediate- and Short-Acting | Humulin 70/30 | 70% NPH human insulin | |
| 30% regular human insulin injection | |||
| Novolin 70/30 | 70% NPH Human Insulin | ||
| 30% Regular Human Insulin Injection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, C.; Delgado, E. 100 Years since the Discovery of Insulin, from Its Discovery to the Insulins of the Future. Biomedicines 2024, 12, 533. https://doi.org/10.3390/biomedicines12030533
Lambert C, Delgado E. 100 Years since the Discovery of Insulin, from Its Discovery to the Insulins of the Future. Biomedicines. 2024; 12(3):533. https://doi.org/10.3390/biomedicines12030533
Chicago/Turabian StyleLambert, Carmen, and Elias Delgado. 2024. "100 Years since the Discovery of Insulin, from Its Discovery to the Insulins of the Future" Biomedicines 12, no. 3: 533. https://doi.org/10.3390/biomedicines12030533






