Proteomic Profiling of SGLT-2 Inhibitor Canagliflozin in a Swine Model of Chronic Myocardial Ischemia
Abstract
1. Introduction
2. Methods
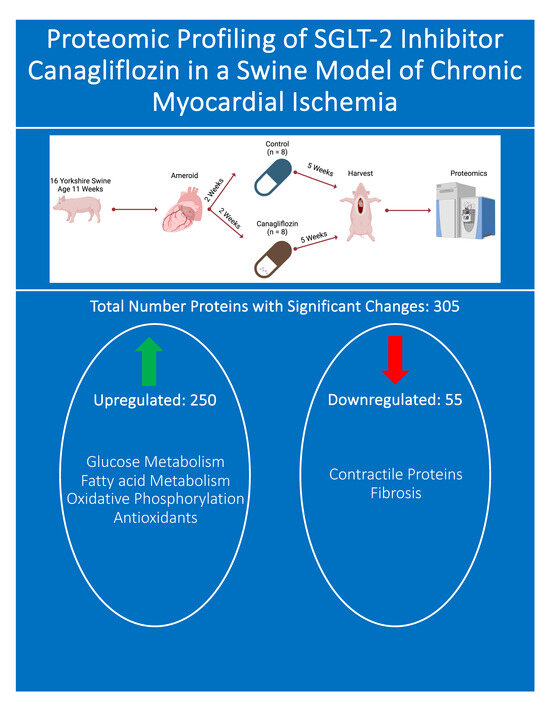
2.1. Animal Model
2.2. Humane Animal Care
2.3. Ameroid Constrictor
2.4. Tissue Harvest
2.5. Proteomic Analysis
2.6. Immunoblotting
2.7. Statistics
3. Results
3.1. Cardiac Index
3.2. Total Proteomics and Pathway Analysis
3.3. Metabolism
3.4. Myocardial Contractility
3.5. Oxidative Phosphorylation
3.6. Antioxidants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassaletta, A.D.; Chu, L.M.; Sellke, F.W. Therapeutic neovascularization for coronary disease: Current state and future prospects. Basic Res. Cardiol. 2011, 106, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Capewell, S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: Public health versus clinical care. Annu. Rev. Public Health 2011, 32, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK576405/ (accessed on 17 January 2024).
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Lachin, J.M.; Inzucchi, S.E. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2016, 374, 1094. [Google Scholar] [CrossRef]
- Pennig, J.; Scherrer, P.; Gissler, M.C.; Anto-Michel, N.; Hoppe, N.; Füner, L.; Härdtner, C.; Stachon, P.; Wolf, D.; Hilgendorf, I.; et al. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Sci. Rep. 2019, 9, 17937. [Google Scholar] [CrossRef]
- Lim, V.G.; Bell, R.M.; Arjun, S.; Kolatsi-Joannou, M.; Long, D.A.; Yellon, D.M. SGLT2 Inhibitor, Canagliflozin, Attenuates Myocardial Infarction in the Diabetic and Nondiabetic Heart. JACC Basic Transl. Sci. 2019, 4, 15–26. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Theodorakis, M.J. Sodium-glucose Cotransporter 2 Inhibitors (SGLT2i): Their Role in Cardiometabolic Risk Management. Curr. Pharm. Des. 2017, 23, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wei, Y.; Li, D.; Pu, J.; Ding, H.; Zhang, X. Mechanisms of SGLT2 Inhibitors in Heart Failure and Their Clinical Value. J. Cardiovasc. Pharmacol. 2023, 81, 4. [Google Scholar] [CrossRef]
- Dyck, J.R.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: Evidence for potential off-target effects. J. Mol. Cell Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632. [Google Scholar] [CrossRef]
- Al Thani, N.A.; Hasan, M.; Yalcin, H.C. Use of Animal Models for Investigating Cardioprotective Roles of SGLT2 Inhibitors. J. Cardiovasc. Transl. Res. 2023, 16, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Sabra, M.; Harris, D.D.; Malhotra, A.; Aboulgheit, A.; Stanley, M.; Abid, M.R.; Sellke, F.W. Canagliflozin Improves Myocardial Perfusion, Fibrosis, and Function in a Swine Model of Chronic Myocardial Ischemia. J. Am. Heart Assoc. 2023, 12, e028623. [Google Scholar] [CrossRef]
- Harris, D.D.; Sabe, S.A.; Xu, C.M.; Sabra, M.; Broadwin, M.; Malhotra, A.; Li, J.W.; Abid, M.R.; Sellke, F.W. Sodium-glucose co-transporter 2 inhibitor canagliflozin modulates myocardial metabolism and inflammation in a swine model for chronic myocardial ischemia. Surgery 2023, 175, 265–270. [Google Scholar] [CrossRef]
- Banerjee, D.; Sabe, S.A.; Xing, H.; Xu, C.; Sabra, M.; Harris, D.D.; Broadwin, M.; Abid, M.R.; Usheva, A.; Feng, J.; et al. Canagliflozin improves coronary microvascular vasodilation and increases absolute blood flow to the myocardium independent of angiogenesis. J. Thorac. Cardiovasc. Surg. 2023, 166, e535–e550. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Sabra, M.; Harris, D.D.; Broadwin, M.; Bellam, K.G.; Banerjee, D.; Usheva, A.; Abid, M.R.; Sellke, F.W. Effects of canagliflozin on myocardial microvascular density, oxidative stress, and proteomic profile. J. Mol. Cell Cardiol. Plus 2023, 6, 100052. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Stanley, W.C. Glucose Metabolism in the Ischemic Heart. Circulation 1997, 95, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Ng, S.M.; Neubauer, S.; Rider, O.J. Myocardial Metabolism in Heart Failure. Curr. Heart Fail. Rep. 2023, 20, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Margreiter, R.; Grimm, M.; Hagenbuchner, J.; Ausserlechner, M.J. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants 2019, 8, 454. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Bilić Ćurčić, I.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors—Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Tsai, K.F.; Chen, Y.L.; Chiou, T.T.Y.; Chu, T.H.; Li, L.C.; Ng, H.Y.; Lee, W.C.; Lee, C.T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium-glucose cotransporter inhibitors and oxidative stress: An update. J. Cell Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The Pathogenesis of Cardiac Fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Querejeta, R.; López, B.; González, A.; Sánchez, E.; Larman, M.; Martínez Ubago, J.L.; Díez, J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis. Circulation 2004, 110, 1263–1268. [Google Scholar] [CrossRef]
- Gupta, M.P. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J. Mol. Cell Cardiol. 2007, 43, 388–403. [Google Scholar] [CrossRef]
- Vander Roest, A.S.; Liu, C.; Morck, M.M.; Kooiker, K.B.; Jung, G.; Song, D.; Dawood, A.; Jhingran, A.; Pardon, G.; Ranjbarvaziri, S.; et al. Hypertrophic cardiomyopathy β-cardiac myosin mutation (P710R) leads to hypercontractility by disrupting super relaxed state. Proc. Natl. Acad. Sci. USA 2021, 118, e2025030118. [Google Scholar] [CrossRef]
| Antibody Name | Company | Catalog Number |
|---|---|---|
| Citrate synthase | Cell Signaling | 14309 |
| CPT1a | Abcam | ab128568 |
| Lactate dehydrogenase A | Cell Signaling | 2012 |
| GAPDH | Cell Signaling | 5174 |
| Myosin heavy chain 7 | Proteintech | 22280-1-AP |
| Vinculin | Cell Signaling | 13901 |
| Mouse secondary antibody | Cell Signaling | 7076 |
| Rabbit secondary antibody | Cell Signaling | 7074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, D.D.; Sabe, S.A.; Broadwin, M.; Stone, C.; Xu, C.; Hu, J.; Kanuparthy, M.; Abid, M.R.; Sellke, F.W. Proteomic Profiling of SGLT-2 Inhibitor Canagliflozin in a Swine Model of Chronic Myocardial Ischemia. Biomedicines 2024, 12, 588. https://doi.org/10.3390/biomedicines12030588
Harris DD, Sabe SA, Broadwin M, Stone C, Xu C, Hu J, Kanuparthy M, Abid MR, Sellke FW. Proteomic Profiling of SGLT-2 Inhibitor Canagliflozin in a Swine Model of Chronic Myocardial Ischemia. Biomedicines. 2024; 12(3):588. https://doi.org/10.3390/biomedicines12030588
Chicago/Turabian StyleHarris, Dwight D., Sharif A. Sabe, Mark Broadwin, Christopher Stone, Cynthia Xu, Jiayu Hu, Meghamsh Kanuparthy, M. Ruhul Abid, and Frank W. Sellke. 2024. "Proteomic Profiling of SGLT-2 Inhibitor Canagliflozin in a Swine Model of Chronic Myocardial Ischemia" Biomedicines 12, no. 3: 588. https://doi.org/10.3390/biomedicines12030588