Abstract
Background: The perioperative outcomes of a partial hepatectomy for hepatocellular carcinoma (HCC) have improved. However, high recurrence rates after a curative hepatectomy for HCC is still an issue. This study aimed to analyze the difference between various recurrence patterns. Methods: We retrospectively reviewed 754 patients with HCC who underwent a curative hepatectomy between January 2012 and March 2021. Patients with recurrent events were categorized into three types: regional recurrence (type I), multiple intrahepatic recurrence (type II), or presence of any distant metastasis (type III). Results: The median follow-up period was 51.2 months. Regarding recurrence, 375 (49.7%) patients developed recurrence, with 244 (32.4%), 51 (6.8%), and 80 (10.6%) patients having type I, II, and III recurrence, respectively. Type III recurrence appeared to be more common in male patients and those with major liver resection, vascular invasion, a large tumor size (>5 cm), a higher tumor grade, and higher levels of AST and AFP (p < 0.05). Patients who had distant metastasis at recurrence had the shortest recurrence time and the worst overall survival (p < 0.001 and p < 0.001). Conclusions: our study demonstrated that recurrence with distant metastasis occurred earliest and had the worst outcome compared to regional or multiple intrahepatic recurrences.
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant disease and the second leading cause of cancer deaths worldwide []. Although the perioperative mortality and short-term outcomes of HCC after a curative hepatectomy have improved, HCC recurrence is still a challenge [,]. There is no definite guideline for the management of recurrent HCC. However, either repeated hepatectomy or salvage liver transplantation are good surgical strategies for the management of selected patients with intrahepatic recurrence. Moreover, systemic immunotherapies combined with target therapies have also shown promising outcomes in a comparative open-label randomized study []. The multi-omics study demonstrated the different patterns between the multicentric origin and intrahepatic metastases. It also proved that tumor aggressiveness and biology were important factors that influenced treatment outcomes []. Therefore, treatment should be based on the location, size, and number of lesions in cases of cancer recurrence. Systemic therapies should also be considered in patients with an advanced-stage disease or those beyond the up-to-seven criteria []. In the real world, because of the limitations of ablation in terms of tumor size, tumor number, and sink effect, surgical resection still remains the main first-line treatment for HCC. Regarding liver transplantation, the scarcity of grafts is the main challenge.
Numerous studies have focused on pathologic, radiologic, and biologic data, and many predicting systems have been developed [,,]. The treatment design should be delicately discussed by a multidisciplinary team to incorporate ablation, trans-arterial chemoembolization (TACE), and surgery. However, the pattern of recurrence is still hard to predict using clinical parameters. The appropriate surgical margin is also another topic of debate. In most of the literature, recurrence within 2 years after a curative hepatectomy is defined as early recurrence [,]. Most of the reviewed literature focuses on the width of the safety margin to prevent early recurrence. However, we previously demonstrated that narrow surgical margins have comparable outcomes to those of wider surgical margins []. Anatomical hepatectomies were more prevalent in eastern countries; nevertheless, their adoption remains controversial due to surgeon-related confounding factors and challenges associated with establishing a precise definition. Moreover, patients’ outcomes could be better in cases with smaller tumors [,]. Although minimal invasive surgery offers superior short-term outcomes when compared with open surgery, its oncologic outcomes need to be carefully examined []. However, the clinical association between the recurrence pattern and the anatomic and pathologic variables are rarely studied. Furthermore, the relationship between recurrence and long-term outcomes was need to be discussed.
In the current study, we investigate and analyze different types of recurrence in patients with HCC after a curative hepatectomy to demonstrate the importance of tumor biology.
2. Materials and Methods
2.1. Study Population
We retrospectively review patients with HCC who were treated with curative hepatectomies by the same surgical team at Chang Gung Memorial Hospital (CGMH) and TuCheng Municipal Hospital (built and operated by Chang Gung Medical Foundation). A total of 764 patients were reviewed between January 2012 and March 2021. This study was approved by the Institutional Review Boards (CGMH IRB No: 201801550B0) of Chang Gung Memorial Hospital (CGMH). All surgical specimens were sent to and checked by qualified pathologists for final diagnosis. Tumor staging was based on the 8th edition of the AJCC TNM staging system for HCC []. The exclusion criteria were cases of combined hepatocellular-cholangiocarcinoma, recurrent HCC, non-curative intent hepatectomies, the presence of distant metastases, preoperative anticancer treatment, or patients with a history of other malignant diseases. Regarding in-hospital mortality, 10 (1.3%) patients who developed perioperative mortality 30 days postoperatively were also excluded from our study cohort. The operative methods included laparotomy, minimally invasive surgery, or hybrid based on the IWATE criteria and the surgeon’s preference. Overall, 754 patients were enrolled in the current study. The patients’ clinical and pathological variables are summarized in Table 1.

Table 1.
Demographic data of 754 HCC patients, along with a comparison of recurrence patterns.
2.2. Definition of Recurrence and Recurrent Pattern
All patients underwent blood test and triphasic computed tomography (CT) of the liver within 1 month after liver resection. Regular cancer follow-up including liver imaging, liver function test, and serum α-fetoprotein (AFP) levels were surveyed every 3 months in the first 2 years and every 4–6 months thereafter. When recurrent event was suspected, serially delicate studies and restaging were conducted. Definite recurrence was defined by the presence of typically dynamic imaging findings, elevated serum AFP levels, or biopsy. Treatment was started based on the suggestions of a multi-modality liver cancer team. Patients were followed up until 31 March 2023.
All cases of recurrence were reviewed and discussed by our cancer team. In the current study, we divided all recurrent events into three types: Type I recurrence was defined as a solitary intrahepatic lesion, which could be considered re-resection technically. Type II recurrence was defined as multiple intrahepatic metastases, while type III recurrence referred to cases that presented with any distant metastasis such as lung, bone, or lymph node metastasis.
2.3. Statistical Analysis
Student’s t test was used to compare continuous variables, while Pearson’s χ2 test was applied to analyze categorical variables. Survival curves of different types of recurrence and overall survival were drawn using the Kaplan–Meier method and compared using log-rank test. Risk factor for recurrence was surveyed using Cox regression analysis. All significant factors in the univariate analysis were included in the multivariate analysis. Results from the multivariate analysis were demonstrated as hazard ratios (HR) and 95% confidence intervals (CI). Statistical analysis was performed using SPSS statistics version 21.0 (IBM Corp., Somers, NY, USA). Two-tailed p-values < 0.05 were considered statistically significant.
3. Results
3.1. Patients’ Clinical and Pathological Variables
Overall, 754 patients were included in this study. The patients’ demographic data were as follows: 76.7% were male, 26.5% had diabetes, 59.3% had HBV, 26.7% had HCV, and most were Child A patients. The mean age of the study cohort was 61.9 ± 11.0 years. A total of 375 (49.7%) patients had recurrence, while 379 (50.3%) patients were disease-free. The median follow-up duration was 51.2 ± 31.7 months and 31.5% of patients had cancer-specific or liver-related mortality during the long-term follow-up (Figure 1). The mean tumor size was 4.5 ± 3.2 cm, while in 212 (28.1%) patients, the tumor size was more than 5 cm (Table 1). The overall survival rates for patients who were disease-free and those with recurrence were 96.0% and 58.2% at 5 years and 89.5% and 35.2% at 10 years, respectively (p < 0.05). Overall, 104 (13.8%), 299 (39.7%), 227 (30.1%), 42 (5.6%), and 82 (10.9%) patients were defined as stage Ia, Ib, II, IIIa, and IIIb, respectively. The 5 years DFS rate was 64.0, 51.2, 38.6, 16.8, and 29.0%, respectively (Figure 1). The odd ratios analysis focused on host, surgical, and pathological factors which showed male gender, cirrhosis, vascular invasion, and satellite lesions were the independent factors for HCC recurrence after resection (Table 2).
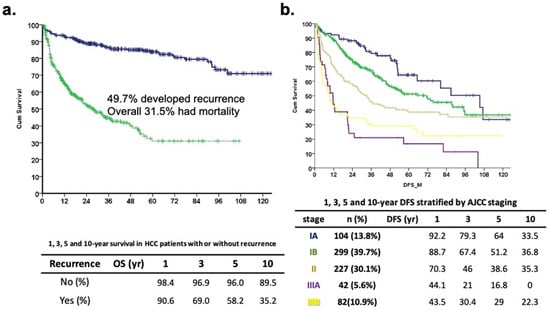
Figure 1.
Long-term outcome of study cohort. (a) Disease-specific survival curve (blue line) and disease-free survival curve (green line) of 754 HCC patients. The 1-, 3-, 5-, and 10-year disease-specific survival was 98.4, 96.9, 96.0, and 89.5 months in no recurrence group and 90.6, 69.0, 58.2, and 35.2 months in recurrence patients, respectively (p < 0.001). (b) Disease-free survival curve stratified by AJCC staging (p < 0.001).

Table 2.
Risk factor analysis between recurrence and non-recurrence patients after curative hepatectomy.
3.2. Comparison of Recurrence Patterns
Regarding recurrence, 244 patients (32.4%) who had solitary intrahepatic lesions that could be considered re-resection technically were classified as having regional recurrence (Type I). There were 51 (6.8%) and 80 (10.6%) patients with type II and type III recurrence, which implied the presence of multiple intrahepatic lesions and distant metastasis, respectively (Table 1). Type III recurrence appeared to be more common in male patients and those who had major liver resection, vascular invasion, a large tumor size (>5 cm), a higher tumor grade, and higher levels of AST and AFP (Table 1, p < 0.05). Furthermore, 139 (57.0%), 23 (45.1%), and 40 (50.0%) patients had cirrhotic livers in type I, II, and III recurrence groups, respectively (p = 0.001). Moreover, type III recurrence was associated with AJCC stage III (p < 0.001). The recurrence time after resection was 24.8 ± 23.0, 15.0 ± 14.1, and 10.9 ± 12.8 months for type I, II, and III recurrence, respectively (Figure 2 p < 0.001 by ANOVA). The overall survival time was 110.30 ± 3.14, 34.52 ± 7.10, and 30.02 ± 8.35 months for type I, II, and III recurrence, respectively (p < 0.001).
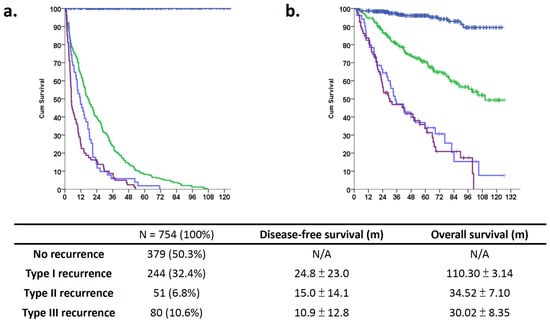
Figure 2.
Long-term outcomes of different recurrence patterns among patients with hepatocellular carcinoma who underwent curative hepatectomy. Different long-term outcomes of patients with HCC stratified according to different recurrence patterns: blue line for no recurrence, green line for intrahepatic single, light blue for multiple intrahepatic, and purple for multiple distant metastasis. (a) The life table shows the recurrence-free survival curve for patients without recurrence and those with different types of recurrence. The recurrence time after resection was 24.8 ± 23.0, 15.0 ± 14.1, and 10.9 ± 12.8 months for type I, II, and III recurrences, respectively (p < 0.001 according to ANOVA). (b) Kaplan–Meier analysis shows that the 5-year overall survival differ among the recurrence groups. The overall survival time was 110.30 ± 3.14, 34.52 ± 7.10, and 30.02 ± 8.35 months for type I, II, and III recurrence patterns, respectively (p < 0.001).
3.3. Analysis of Disease-Free Survival and Overall Survival
Of the 754 patients, 375 (49.73%) had recurrent events and had worse overall survival outcomes compared to those without recurrence (Figure 2). Patients without cancer recurrence had better long-term outcomes compared to those who had recurrence (Figure 1, p < 0.001). After COX regression multivariate analysis, the independent prognostic factors for DFS were male sex, diabetes, higher ICG-R15, large tumor size, vascular invasion, and cirrhosis (Table 3). The recurrence patterns analysis for OS analysis demonstrated that diabetes, the presence of grade III or more surgical complications, higher initial tumor staging, and an aggressive recurrence pattern were significant risk factors for a poor oncological outcome (Table 4). In summary, aggressive tumor biological factors were associated with early recurrences and the presence of distant metastases.

Table 3.
Clinicopathologic Factors and Disease-Free Survival in 754 Patients with HCC.

Table 4.
Clinicopathologic data of 754 patients with HCC in univariate and multivariate regression analysis in relation to OS.
3.4. Higher Tumor Grade Was Associated with Higher Distant Metastasis Risks
The tumor grade was analyzed with the recurrence pattern and we completed statistical analyses with two tiers and four tiers (G1 + G2 vs. G3 + G4 and G1–G4). The results are summarized in Table 5. The higher tumor grade had a higher distant metastasis risk. Most of the literature has mentioned that the tumor grade was dichotomized as a lower and higher grade and was associated with a poor outcome []. The higher tumor grade also had higher tumor genome instability. The copy number aberration analysis showed an increased chromosome imbalance from the normal tissue. The higher chromosome imbalance was an independent factor in the HCC outcome []. The impact of tumor biology characters included the size, number, satellite lesion, rupture, vascular invasion, and tumor grade; however, the tumor grade is not listed in the AJCC staging system.

Table 5.
The impact of histology on recurrence pattern.
4. Discussion
Recurrence is consistently a crucial concern in curative treatment for HCC, prompting studies aimed at predicting the likelihood of recurrence. The ERASL model that included over 3000 cases showed that the male sex, ALBI grade, microscopic invasion, AFP, tumor size, and number of lesions could be incorporated to predict the possibility at a cut-off value; furthermore, the model indicated that risk factors could be stratified from low- to high-risk groups [,,]. Higher serologic factors, such as AFP and des-γ-carboxyprothrombin (DCP), tumor volume, multiple nodules, microvascular invasion, and poorly differentiated types have been assumed to be aggressive tumor factors. Moreover, a multicenter study validated the value of the ADV score in Korea and Japan [,]. Although anatomical hepatectomy has been proposed to improve the clinical outcome of HCC, it is more popular in eastern countries []. A positive resection margin should be prevented as much as possible because of higher recurrence rates; however, there is no clear difference in the long-term outcomes between wide and narrow margins [,]. Postoperative higher-grade complications and blood transfusions related to poor surgical events should also be prevented [,]. However, only a few papers have analyzed the impact of different recurrence patterns on long-term outcomes.
A salvage hepatectomy was assumed to be a standard treatment option for local recurrence after radiofrequency ablation; nevertheless, it was shown to have a minimal benefit in cases of recurrence associated with aggressive tumors [,]. Furthermore, although repeated surgical resection is an important strategy to improve patients’ outcomes, the rate of extrahepatic metastases has been reported to be 2.4–18.0% in some literature reviews [,,,]. Extrahepatic recurrence occurs more commonly in cases of intermediate and advanced HCC, and the lungs and bones are common sites of metastasis []. Our study’s findings show that type III recurrence usually occurs within the first year after resection. To improve the treatment outcome, an aggressive hepatectomy should be considered for early-stage HCC with good tumor biology, while systemic treatment should be considered for cases of advanced HCC with worse tumor biology.
This study demonstrated that distant metastases could be encountered after curative hepatectomies and tended to occur earlier than other recurrence patterns. Moreover, 10.6% of patients with type III recurrence and 6.8% of those with type II recurrence tended to develop a systemic disease earlier and had poor survival outcomes. Therefore, neoadjuvant or adjuvant therapy should be considered in patients with these aggressive tumor statuses. The administration of sorafenib in a phase III adjuvant therapy trial did not lead to a significant difference in recurrence-free survival []. However, a meta-analysis of data from 2655 patients who underwent adjuvant therapy revealed that the administration of sorafenib effectively prolonged overall survival and reduced the recurrence rate []. Following the favorable outcomes observed with atezolizumab plus bevacizumab in the adjuvant context, as reported at the 2023 AACR conference, the overall understanding of the molecular pathogenesis of HCC has advanced, revealing alterations in tumor drivers leading to genomic instability. This knowledge of molecular and immune subclasses could serve as a means to assess tumor aggressiveness []. The neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio have been reported as independent prognostic factors in cases of combined treatment; however, immune escape and resistance to anti-PD-1 therapy through β-catenin activation have also been reported [,].
Our study has some limitation including its retrospective design and small patient numbers. Selection bias was also inevitable and could have affected the statistical results. All the patients in our study were disease-free after a curative hepatectomy. The role of adjuvant therapy could not be determined in this cohort, as it had not been initiated.
5. Conclusions
In conclusion, our study demonstrated that recurrence with distant metastasis after a partial hepatectomy was a poor independent prognostic factor; moreover, it appears to occur earlier and has a worse outcome compared to other patterns of recurrence. Neoadjuvant or adjuvant systemic therapies could be considered in selected patients. More prospective and well-designed trials are also needed to improve patients’ outcomes.
Author Contributions
H.-Y.H. conducted the study and drafted the manuscript. M.-C.Y. and H.-Y.H. designed the study and revised the manuscript. J.-H.T. and S.-E.L. designed the study. J.-H.T. collected clinical data, while S.-E.L. assessed the pathological pattern and scoring independently. C.-W.L. and T.-H.W. contributed their idea and conducted statistical analyses. S.-F.H., C.-W.H. and S.-W.H. draw the figures and established tables. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from Chang Gung Medical Foundation in Taiwan, CMRPVVJ0023 and CMRPVVL0172 for Yu, M.-C.
Institutional Review Board Statement
This study was approved by the Institutional Review Boards (IRB 201801550B0) of Chang Gung Memorial Hospital (CGMH), Linkou Branch.
Informed Consent Statement
The need for informed consent was waived by the IRB because of the retrospective nature of the clinical analysis.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to all our colleagues in the Department of Cancer Center, Department of Pathology, and Graduate Institute of Clinical Medical Sciences, Chang Gung University, for their technical assistance. We are also grateful to Yi-Ping Liu for assisting in data retrieval and processing. This study was supported by Chang Gung Medical Foundation.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Erridge, S.; Pucher, P.H.; Markar, S.R.; Malietzis, G.; Athanasiou, T.; Darzi, A.; Sodergren, M.H.; Jiao, L.R. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1433–1442. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Yu, M.C.; Lee, C.W.; Tsai, H.I.; Sung, C.M.; Chen, C.W.; Huang, S.W.; Lin, C.Y.; Jeng, W.J.; Lee, W.C.; et al. RAM score is an effective predictor for early mortality and recurrence after hepatectomy for hepatocellular carcinoma. BMC Cancer 2017, 17, 742. [Google Scholar] [CrossRef]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Zheng, X.Q.; Yang, R.; Ye, G.J.; Chen, X.H.; Feng, P.X. Resolving and Tuning Mechanical Anisotropy in Black Phosphorus via Nanomechanical Multimode Resonance Spectromicroscopy. Nano Lett. 2016, 16, 5394–5400. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. A Novel Treatment Strategy for Patients with Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer 2021, 10, 539–544. [Google Scholar] [CrossRef]
- Schumm, W.R.; Crawford, D.W. Difficulties with Methodology in Social Science Research with Controversial Issues Regarding Human Sexuality. Linacre Q. 2023, 90, 194–216. [Google Scholar] [CrossRef] [PubMed]
- Wroński, M.; Samochowiec, J.; Pełka-Wysiecka, J.; Liśkiewicz, P.; Bieńkowski, P.; Misiak, B. Deficit Symptomatology of Schizophrenia Is Associated with Attenuated Taste Identification: Findings from a Cross-Sectional Study. Brain Sci. 2022, 12, 1520. [Google Scholar] [CrossRef]
- Huang, C.W.; Wu, T.H.; Hsu, H.Y.; Pan, K.T.; Lee, C.W.; Chong, S.W.; Huang, S.F.; Lin, S.E.; Yu, M.C.; Chen, S.M. Reappraisal of the Role of Alkaline Phosphatase in Hepatocellular Carcinoma. J. Pers. Med. 2022, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Song, Y.; Zhang, J.; Dai, L.; Tang, C.; Li, M.; Liao, W.; Zhou, Y.; Xu, Y.; Zhang, Y.Y.; et al. Preoperative Gadoxetic Acid-Enhanced MRI Based Nomogram Improves Prediction of Early HCC Recurrence After Ablation Therapy. Front. Oncol. 2021, 11, 649682. [Google Scholar] [CrossRef]
- Marasco, G.; Colecchia, A.; Colli, A.; Ravaioli, F.; Casazza, G.; Reggiani, M.L.; Cucchetti, A.; Cescon, M.; Festi, D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J. Hepatol. 2019, 70, 440–448. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lai, Y.; Hung, H.C.; Lee, J.C.; Wang, Y.C.; Wu, T.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Chan, K.M.; et al. Recurrence Patterns after Hepatectomy with Very Narrow Resection Margins for Hepatocellular Carcinoma. Front. Surg. 2022, 9, 926728. [Google Scholar] [CrossRef]
- Sato, N.; Marubashi, S. What is the optimal surgical treatment for hepatocellular carcinoma beyond the debate between anatomical versus non-anatomical resection? Surg. Today 2022, 52, 871–880. [Google Scholar] [CrossRef]
- Dai, X.M.; Xiang, Z.Q.; Wang, Q.; Li, H.J.; Zhu, Z. Oncological outcomes of anatomic versus non-anatomic resections for small hepatocellular carcinoma: Systematic review and meta-analysis of propensity-score matched studies. World J. Surg. Oncol. 2022, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Struecker, B.; Raschzok, N.; Krenzien, F.; Haber, P.; Wabitsch, S.; Waldbaur, C.; Touet, E.M.; Eichelberg, A.C.; Atanasov, G.; et al. Minimal-invasive versus open hepatectomy for hepatocellular carcinoma: Comparison of postoperative outcomes and long-term survivals using propensity score matching analysis. Surg. Oncol. 2018, 27, 751–758. [Google Scholar] [CrossRef]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef]
- Schlageter, M.; Terracciano, L.M.; D’Angelo, S.; Sorrentino, P. Histopathology of hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 15955–15964. [Google Scholar] [CrossRef]
- Yu, M.C.; Lee, C.W.; Lee, Y.S.; Lian, J.H.; Tsai, C.L.; Liu, Y.P.; Wu, C.H.; Tsai, C.N. Prediction of early-stage hepatocellular carcinoma using OncoScan chromosomal copy number aberration data. World J. Gastroenterol. 2017, 23, 7818–7829. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Zhong, J.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.; Tada, T.; Chong, C.C.; Xiang, B.D.; Li, L.Q.; et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Xu, X.; Dong, S.; Yang, M.; Li, L.; Zhao, S.; Duan, Z.; Jia, J.; Wei, L.; Zhuang, H. Consensus on the tertiary prevention of primary liver cancer. Hepatol. Int. 2023, 17, 1057–1071. [Google Scholar] [CrossRef]
- Milana, F.; Polidoro, M.A.; Famularo, S.; Lleo, A.; Boldorini, R.; Donadon, M.; Torzilli, G. Surgical Strategies for Recurrent Hepatocellular Carcinoma after Resection: A Review of Current Evidence. Cancers 2023, 15, 508. [Google Scholar] [CrossRef]
- Park, G.C.; Hwang, S.; Park, Y.H.; Choi, J.U.; Korean Liver Cancer Study Group. Validation of prognostic impact of ADV score for resection of hepatocellular carcinoma: Analysis using Korea Liver Cancer Registry Database. Ann. Surg. Treat. Res. 2020, 98, 235–246. [Google Scholar] [CrossRef]
- Hwang, S.; Joh, J.W.; Wang, H.J.; Kim, D.G.; Kim, K.S.; Suh, K.S.; Kim, S.H.; Yu, H.C.; Cho, C.K.; Lee, Y.J.; et al. Prognostic Prediction Models for Resection of Large Hepatocellular Carcinoma: A Korean Multicenter Study. World J. Surg. 2018, 42, 2579–2591. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Kim, T.S.; Ahn, K.S.; Kim, Y.H.; Kang, K.J. Effect of anatomical liver resection for hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Nara, S.; Shimada, K.; Sakamoto, Y.; Esaki, M.; Kishi, Y.; Kosuge, T.; Ojima, H. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: Evidence from 570 hepatectomies. Surgery 2012, 151, 526–536. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, J.R.; Han, P.S.; Zhang, Y.M. Comparison of survival and post-operation outcomes for minimally invasive versus open hepatectomy in hepatocellular carcinoma: A systematic review and meta-analysis of case-matched studies. Front. Oncol. 2022, 12, 1021804. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Aoki, T.; Inoue, Y.; Kaneko, J.; Sakamoto, Y.; Sugawara, Y.; Hasegawa, K.; Kokudo, N. Outcome of salvage hepatic resection for recurrent hepatocellular carcinoma after radiofrequency ablation therapy. Surgery 2015, 157, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Huang, M.; Liao, J.; Lin, S.; Yu, P.; Yang, J.; Cai, Y.; Zhu, S.; Xu, L.; Peng, Z.; et al. Insufficient Radiofrequency Ablation Promotes Hepatocellular Carcinoma Metastasis Through N6-Methyladenosine mRNA Methylation-Dependent Mechanism. Hepatology 2021, 74, 1339–1356. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Wei, X.F.; Che, P.; Yin, K.L.; Liu, L. Nomograms Incorporating the CNLC Staging System Predict the Outcome of Hepatocellular Carcinoma After Curative Resection. Front. Oncol. 2021, 11, 755920. [Google Scholar] [CrossRef]
- Takayama, T.; Hasegawa, K.; Izumi, N.; Kudo, M.; Shimada, M.; Yamanaka, N.; Inomata, M.; Kaneko, S.; Nakayama, H.; Kawaguchi, Y.; et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer 2022, 11, 209–218. [Google Scholar] [CrossRef]
- Hachiya, H.; Aoki, T.; Iso, Y.; Shimizu, T.; Tago, K.; Park, K.H.; Sakuraoka, Y.; Shiraki, T.; Mori, S.; Kubota, K. Effects of branched-chain amino acids on postoperative tumor recurrence in patients undergoing curative resection for hepatocellular carcinoma: A randomized clinical trial. J. Hepatobiliary Pancreat. Sci. 2020, 27, 819–829. [Google Scholar] [CrossRef]
- Hirokawa, F.; Komeda, K.; Taniguchi, K.; Asakuma, M.; Shimizu, T.; Inoue, Y.; Kagota, S.; Tomioka, A.; Yamamoto, K.; Uchiyama, K. Is Postoperative Adjuvant Transcatheter Arterial Infusion Therapy Effective for Patients with Hepatocellular Carcinoma who Underwent Hepatectomy? A Prospective Randomized Controlled Trial. Ann. Surg. Oncol. 2020, 27, 4143–4152. [Google Scholar] [CrossRef]
- Yoh, T.; Ishii, T.; Ogiso, S.; Nishino, H.; Nishio, T.; Koyama, Y.; Uchida, Y.; Ito, T.; Hatano, E. Long-term outcomes and salvageability in patients undergoing liver resection for intermediate- and advanced-stage hepatocellular carcinoma. Surgery 2023, 174, 858–864. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, D.; Zhuang, L.; Sun, L.; Wu, J. A meta-analysis of the efficacy and safety of adjuvant sorafenib for hepatocellular carcinoma after resection. World J. Surg. Oncol. 2021, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Fulgenzi, C.A.; D’Alessio, A.; Cheon, J.; Nishida, N.; Saeed, A.; Wietharn, B.; Cammarota, A.; Pressiani, T.; Personeni, N.; et al. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Prognostic Biomarkers in Unresectable Hepatocellular Carcinoma Treated with Atezolizumab plus Bevacizumab. Cancers 2022, 14, 5834. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. beta-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).