Understanding Local Reactions Induced by Bothrops jararaca Venom: The Role of Inflammatory Mediators in Leukocyte–Endothelium Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. BjV
2.3. Antivenom
2.4. Treatment of Mice
2.5. Intravital Microscopy of Murine Cremaster Venules
2.6. Statistical Analysis
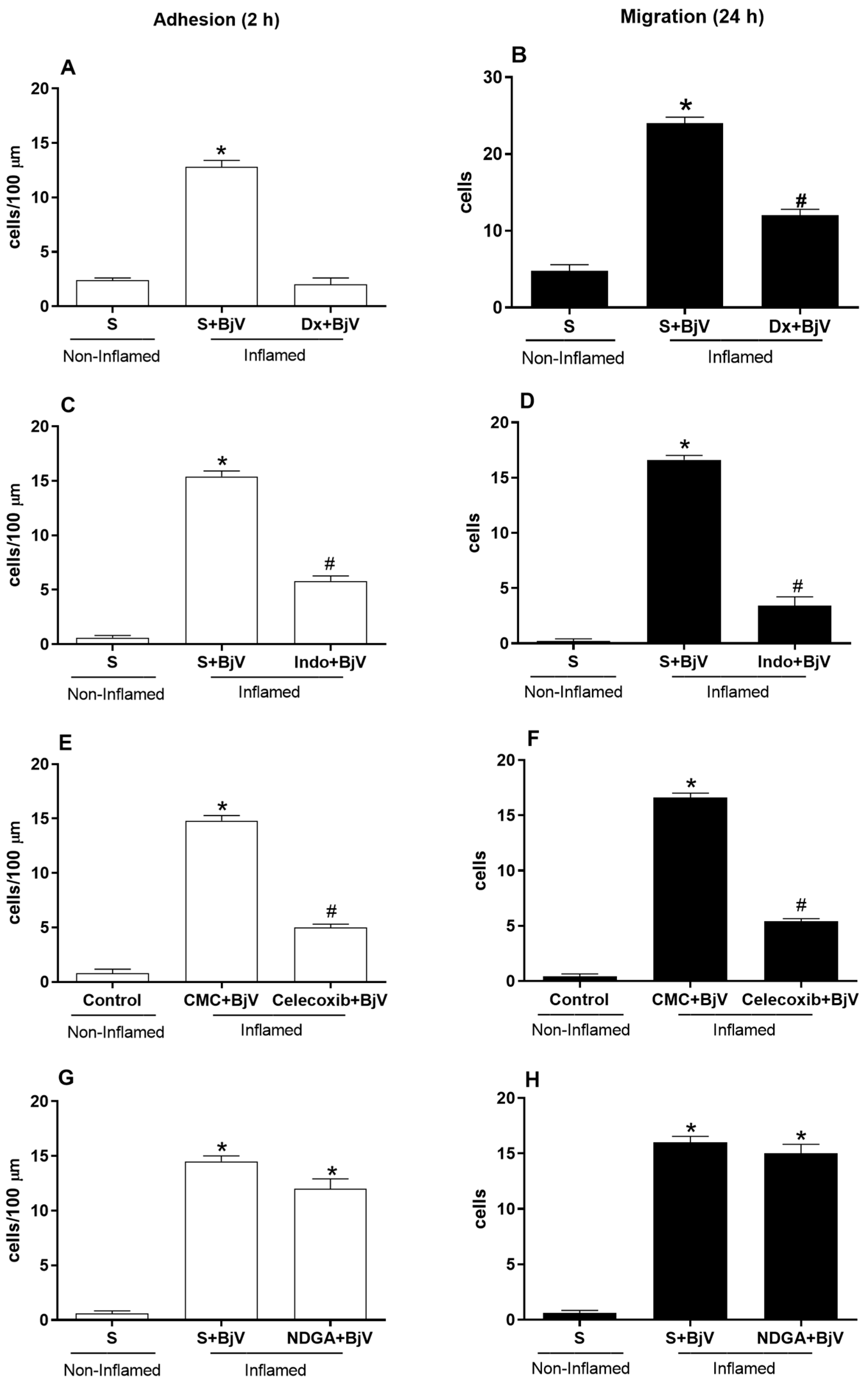
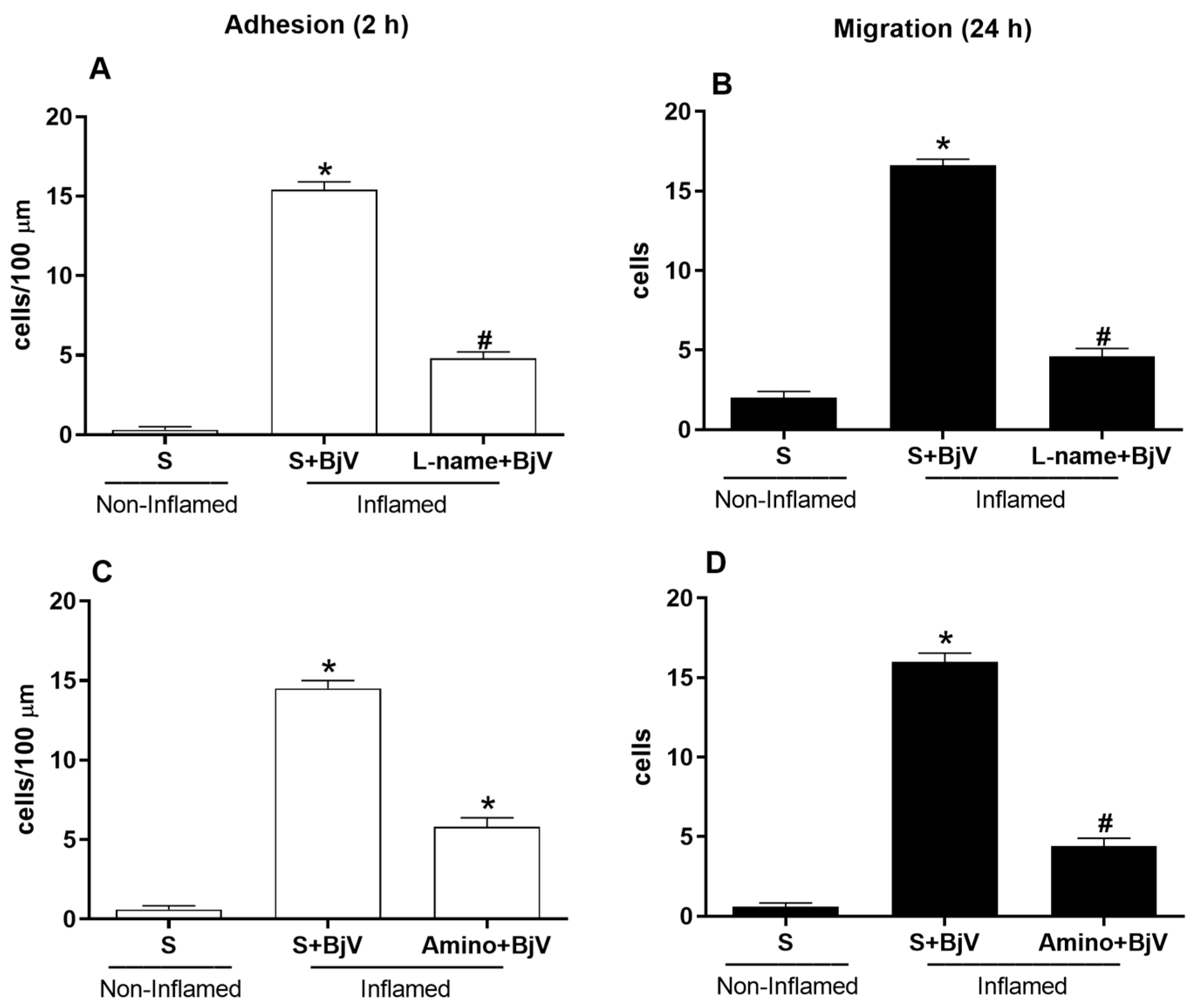
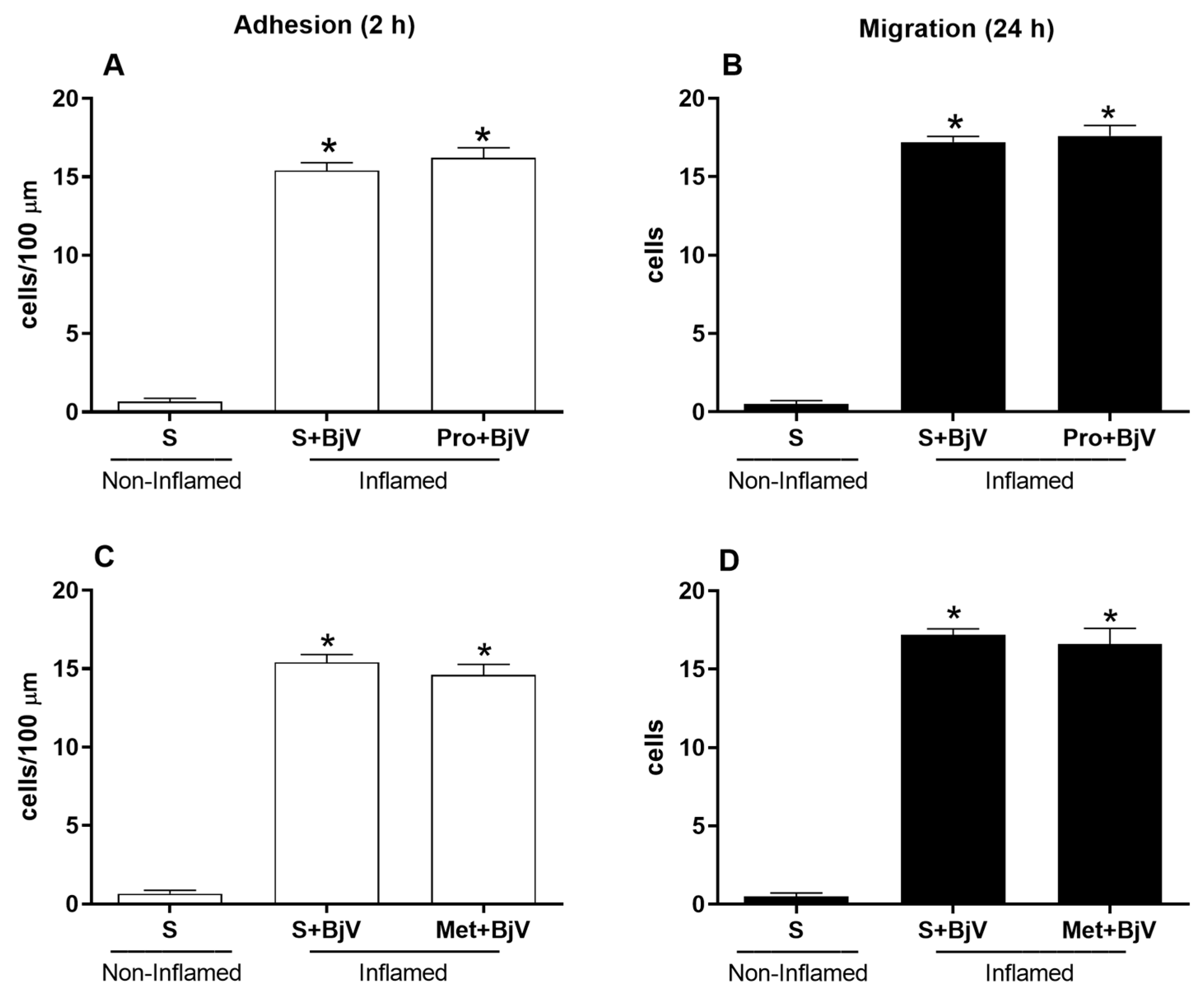
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef]
- Cruz, L.S.; Vargas, R.; Lopes, A.A. Snakebite envenomation and death in the developing world. Ethn. Dis. 2009, 19, 42–46. [Google Scholar]
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 11, e218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Seventy-First World Health Assembly Resolution Wha71.5 on Addressing the Burden of Snakebite Envenoming. Available online: http://apps.who.int/gb/ebwha/pdf_files/EB142/B142_R4-en.pdf (accessed on 4 March 2024).
- World Health Organization (WHO). Tenth Meeting of the Strategic and Technical Advisory Group on Neglected Tropical Diseases (STAG-NTD). Available online: https://www.who.int/publications/m/item/tenth-report-of-the-strategic-and-technical-advisory-group-for-neglected-tropical-diseases-(stag-ntds) (accessed on 15 March 2024).
- Souza, W.d. Doenças Negligenciadas, 1st ed.; Academia Brasileira de Ciências: Rio de Janeiro, Brazil, 2010. [Google Scholar] [CrossRef]
- Chippaux, J.P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: From obvious facts to contingencies. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.A.A.; Santalúcia, M.; Cabral, R.F. Epidemiologia dos acidentes por animais peçonhentos. In Animais Peçonhentos No Brasil: Biologia, Clínica e Terapêutica dos Acidentes, 1st ed.; Cardoso, J.L.C., França, F.O.S., Fan, H.W., Málaque, C.M.S., Haddad, V., Jr., Eds.; Sarvier: São Paulo, Brazil, 2003; pp. 6–12. [Google Scholar]
- Rosenfeld, G. Symptomatology, pathology, and treatment of snake bites in South America. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E.E., Eds.; Academic Press: New York, NY, USA, 1971; pp. 345–384. [Google Scholar]
- Cardoso, J.L.C.; Fan, H.W.; França, F.O.S.; Jorge, M.T.; Leite, R.P.; Nishioka, S.A.; Avila, A.; Sano-Martins, S.I.; Tomy, S.C.; Santoro, M.L.; et al. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q. J. Med. 1993, 86, 315–325. [Google Scholar]
- Marder, V.J.; Feinstein, D.I.; Francis, C.W.; Colman, R. Consumptive thrombohemorrhagic disorders. In Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 3rd ed.; Colman, R.W., Hirsh, J., Marder, V.J., Salzman, E.W., Eds.; J.B. Lippincott Company: Philadelphia, PA, USA, 1994; pp. 1023–1063. [Google Scholar]
- França, F.O.S.; Málaque, C.M.S. Acidente botrópico. In Animais Peçonhentos No Brasil: Biologia, Clínica e Terapêutica dos Acidentes, 1st ed.; Cardoso, J.L., França, F.O.S., Wen, F.H., Málaque, C.M.S., Haddad, V., Jr., Eds.; Sarvier: São Paulo, Brazil, 2003; pp. 72–86. [Google Scholar]
- Ministério da Saúde do Brasil. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos; FUNASA: Brasilia, Brazil, 2001; p. 112.
- Moura-da Silva, A.M.; Laing, G.D.; Paine, M.J.; Dennison, J.M.; Politi, V.; Cramptom, J.M.; Theakston, R.D. Processing of pro-tumor necrosis factor-alpha by venom metalloproteinase: A hypothesis explaining local tissue damage following snakebite. Eur. J. Immunol. 1996, 26, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T.; Herrera, C.; Fernández, J.; Lomonte, B.; Fox, J.W. Unresolved issues in the understanding of the pathogenesis of local tissue damage induced by snake venoms. Toxicon 2018, 148, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M. Comprendiendo los venenos de serpientes: 50 años de investigaciones en América Latina. Rev. Biol. Trop. 2002, 50, 377–394. [Google Scholar]
- Williams, D.; Gutiérrez, J.M.; Harrison, R.; Warrell, D.A.; White, J.; Winkel, K.D.; Gopalakrishnakone, P. The Global Snake Bite Initiative: An antidote for snake bite. Lancet 2010, 375, 89–91. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Santos Barreto, G.N.L.; de Oliveira, S.S.; dos Anjos, I.V.; Chalkidis, H.D.M.; Mourão, R.H.V.; da Silva, A.M.M.; Sano-Martins, I.S.; de Camargo Gonçalves, L.R. Experimental Bothrops atrox envenomation: Efficacy of antivenom therapy and the combination of Bothrops antivenom with dexamethasone. PLoS Negl. Trop. Dis. 2017, 11, e0005458. [Google Scholar] [CrossRef] [PubMed]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Moore, P.K. Farmacologia, 5th ed.; Elsevier: Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Trebien, H.A.; Calixto, J.B. Pharmacological evaluation of rat paw oedema induced by Bothrops jararaca venom. Agents Actions 1989, 26, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Do Amaral, R.O.; Teixeira, C.F.; Hyslop, S.; Cogo, J.C. Pharmacological characterization of mouse hind paw oedema induced by Bothrops insularis (jararaca ilhoa) snake venom. Toxicon 2003, 42, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.D.; De Souza, A.; Nunes, F.P.B.; Gonçalves, L.R.C. Effect of dexamethasone associated with serum therapy on treatment of Bothrops jararaca venom-induced paw edema in mice. Inflamm. Res. 2007, 56, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Zychar, B.C.; Castro, N.C.; Marcelino, J.R.; Gonçalves, L.R. Phenol used as a preservative in Bothrops antivenom induces impairment in leukocyte-endothelial interactions. Toxicon 2008, 51, 1151–1157. [Google Scholar] [CrossRef]
- Zychar, B.C.; Dale, C.S.; Demarchi, D.S.; Gonçalves, L.R.C. Contribution of metalloproteases, serine proteases and phospholipases A2 to the inflammatory reaction induced by Bothrops jararaca crude venom in mice. Toxicon 2010, 55, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Perales, J.; Amorim, C.Z.; Rocha, S.L.G.; Domont, G.B.; Moussatché, H. Neutralization of the oedematogenic activity of Bothrops jararaca venom on the mouse paw by an antibothropic fraction isolated from opossum (Didelphis marsupialis) serum. Agents Actions 1992, 37, 250–259. [Google Scholar] [CrossRef]
- Patrão-Neto, F.C.; Tomaz, M.A.; Strauch, M.A.; Monteiro-Machado, M.; Da Silva Rocha-Junior, J.R.; Borges, P.A.; Calil-Elias, S.; Melo, P.A. Dexamethasone antagonizes the in vivo myotoxic and inflammatory effects of Bothrops venoms. Toxicon 2013, 69, 55–64. [Google Scholar] [CrossRef]
- Wallace, J.L. Distribution and expression of cyclooxygenases (COX) isoenzymes, their physiological roles, and the categorization of nonsteroidal anti-inflammatory drugs. Am. J. Med. 1999, 107, 11–16. [Google Scholar] [CrossRef]
- Gonçalves, L.R.; Mariano, M. Local haemorrhage induced by Bothrops jararaca venom: Relationship to neurogenic inflammation. Mediat. Inflamm. 2000, 9, 101–107. [Google Scholar] [CrossRef]
- Dal Secco, D.; Paron, J.A.; Oliveira, S.H.P.; Ferreira, S.H.; Silva, J.S.; Cunha, F.Q. Neutrophil migration in inflammation: Nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide 2003, 9, 153–164. [Google Scholar] [CrossRef]
- Baez, S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc. Res. 1973, 5, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Zychar, B.C.; Clissa, P.B.; Carvalho, E.; Alves, A.S.; Baldo, C.; Faquim-Mauro, E.L.; Gonçalves, L.R.C. Modulation of Adhesion Molecules Expression by Different Metalloproteases Isolated from Bothrops Snakes. Toxins 2021, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Clissa, P.B.; Laing, G.D.; Theakston, R.D.; Mota, I.; Taylor, M.J.; Moura-da-Silva, A.M. The effect of jararhagin, a metalloproteinase from Bothrops jararaca venom, on pro-inflammatory cytokines released by murine peritoneal adherent cells. Toxicon 2001, 39, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, S.R.; Zuliani, J.P.; Fernandes, C.M.; Gutiérrez, J.M.; de Fátima Pereira Teixeira, C. Inflammation induced by Bothrops asper venom: Release of proinflammatory cytokines and eicosanoids, and role of adhesion molecules in leukocyte infiltration. Toxicon 2005, 46, 806–813. [Google Scholar] [CrossRef]
- Clissa, P.B.; Lopes-Ferreira, M.; Della-Casa, M.S.; Farsky, S.H.P.; Moura-da Silva, A.M. Importance of jararhagin disintegrin-like and cysteine-rich domains in the early events of local inflammatory response. Toxicon 2006, 47, 591. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Zamuner, S.R.; Zuliani, J.P.; Rucavado, A.; Gutiérrez, J.M.; Teixeira, C.F.P. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: Leukocyte recruitment and release of cytokines. Toxicon 2006, 47, 549. [Google Scholar] [CrossRef]
- Zychar, B.C.; Clissa, P.B.; Carvalho, E.; Baldo, C.; Gonçalves, L.R.C. Leukocyte recruitment induced by snake venom metalloproteinases: Role of the catalytic domain. Biochem. Biophys. Res. Commun. 2020, 521, 402–407. [Google Scholar] [CrossRef]
- Búrigo, A.C.; Calixto, J.B.; Medeiros, Y.S. Pharmacological profile of rat pleurisy induced by Bothrops jararaca venom. J. Pharm. Pharmacol. 1996, 48, 106–111. [Google Scholar] [CrossRef]
- Ptzalis, C.; Pipitone, N.; Perretti, M. Regulation of Leukocyte-Endothelial Interactions by Glucocorticoids. Ann. N. Y. Acad. Sci. 2002, 966, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Czock, D.; Keller, F.; Rasche, F.M.; Haussler, U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 2005, 44, 61–98. [Google Scholar] [CrossRef] [PubMed]
- Tailor, A.; Tomlinson, A.; Salas, A.; Panés, J.; Granger, D.N.; Flower, R.J.; Perretti, M. Dexamethasone inhibition of leukocyte adhesion to rat mesenteric postcapillary venules: Role of intercellular adhesion molecule 1 and KC. Gut 1999, 45, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.A.; Zappellini, A.; Prado-Franceschi, J. Lipoxygenase-derived mediators may be involved in in vivo neutrophil migration induced by Bothrops erythromelas and Bothrops alternatus venoms. Toxicon 1993, 31, 1551–1559. [Google Scholar] [CrossRef]
- Zamuner, S.R.; Teixeira, C.F. Cell adhesion molecules involved in the leukocyte recruitment induced by venom of the snake Bothrops jararaca. Med. Inflamm. 2002, 11, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Rampart, M. Neutrophil-Endothelial Cell Interations. In The Handbook of Immunopharmacology. Immunopharmacology of Microcirculation; Brain, S.D., Ed.; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Olivo, R.A.; Teixeira, C.F.P.; Wallace, J.L.; Gutiérrez, J.M.; Zamuner, S.R. Role of cyclooxigenases in oedema-forming activity of bothropic venoms. Toxicon 2007, 49, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Palmblad, J.; Lindstron, P.; Lemer, R. Leukotriene B4 induced hyperadhesiveness of endothelial cells for neutrophils. Biochem. Biophys. Res. Commun. 1990, 166, 848–851. [Google Scholar] [CrossRef]
- Kubes, P.; Suzuki, M.; Granger, D.N. Nitric Oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 1991, 88, 4651–4655. [Google Scholar] [CrossRef]
- Dal Secco, D.; Moreira, A.P.; Freitas, A.; Silva, J.S.; Rossi, M.A.; Ferreira, S.H.; Cunha, F.Q. Nitric oxide neutrophil migration by a mechanism dependent on ICAM-1: Role of soluble guanylate cyclase. Nitric Oxide 2006, 15, 77–86. [Google Scholar] [CrossRef]
- Farsky, S.H.; Goncalves, L.R.C.; Cury, Y. Characterization of local tissue damage evoked by Bothrops jararaca venom in the rat connective tissue microcirculation: An intravital microscopic study. Toxicon 1999, 37, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Farsky, S.H.; Borelli, P.; Fock, R.A.; Proto, S.Z.; Ferreira, J.M.C., Jr.; Mello, S.B.V. Chronic blockade of nitric oxide biosynthasis in rats: Effect on leukocyte endothelial interaction and on leukocyte recrutaiment. Inflamm. Res. 2004, 53, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.F.; Friedlanskaia, I.; Petricevich, V.L.; Kipini, T.L. Local inflammation, lethality, and cytokine release in mice injected with Bothrops atrox venom. Med. Inflamm. 1998, 7, 339–346. [Google Scholar] [CrossRef]
- Petricevich, V.L.; Teixeira, C.F.; Tambourgi, D.V.; Gutiérrez, J.M. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon 2000, 38, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Geffner, J.R.; Analía, S.T.; D’Elia, I.; Diament, M.; Klein, D.; Giordano, M. Involvement of nitric oxide in the regulation of peripheral blood leukocyte counts. J. Leuk. Biol. 1995, 58, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Picolo, G.; Chacur, M.; Gutiérrez, J.M.; Teixeira, C.F.; Cury, Y. Evaluation of antivenoms in the neutralization of hyperalgesia and edema induced by Bothrops jararaca and Bothrops asper snake venoms. Braz. J. Med. Biol. Res. 2002, 35, 1221–1228. [Google Scholar] [CrossRef]
- Battellino, C.; Piazza, R.; Da Silva, A.M.M.; Cury, Y.; Farsky, S.H.P. Assessment of efficacy of bothropic antivenom therapy on microcirculatory effects induced by Bothrops jararaca snake venom. Toxicon 2003, 41, 583–593. [Google Scholar] [CrossRef]
- Landon, J.; Smith, D.C. Development of novel antivenoms based on specific ovine Fab. In Evenomings and Their Treatments; Bon, C., Goyffon, M., Eds.; Editions Foudation Maecel Mérieux: Lyon, France, 1995; p. 173. [Google Scholar]
- Gutiérrez, J.M.; Leon, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef]
- Chaves, F.; Loría, G.D.; Salazar, A.; Gutiérrez, J.M. Intramuscular administration of antivenoms in experimental envenomation by Bothrops asper: Comparison between Fab and IgG. Toxicon 2003, 41, 237–244. [Google Scholar] [CrossRef]
- Dorooshi, G.; Javid, Z.N.; Meamar, R.; Farjzadegan, Z.; Nasri, M.; Eizadi-Mood, N. Evaluation of the effects of Anti-Inflammatory drugs on local and systemic manifestations of snakebite: A cross-sectional study. J. Venom. Res. 2021, 11, 21–25. [Google Scholar]
- Cury, Y.; Teixeia, C.F.; Farsky, S.H. Lack of effect of endogenous corticosteroids on the acute inflammatory reaction (edema) induced by Bothrops jararaca venom (BjV) in rats. Toxicon 1997, 35, 773–776. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zychar, B.C.; Gonçalves, L.R.C. Understanding Local Reactions Induced by Bothrops jararaca Venom: The Role of Inflammatory Mediators in Leukocyte–Endothelium Interactions. Biomedicines 2024, 12, 734. https://doi.org/10.3390/biomedicines12040734
Zychar BC, Gonçalves LRC. Understanding Local Reactions Induced by Bothrops jararaca Venom: The Role of Inflammatory Mediators in Leukocyte–Endothelium Interactions. Biomedicines. 2024; 12(4):734. https://doi.org/10.3390/biomedicines12040734
Chicago/Turabian StyleZychar, Bianca Cestari, and Luís Roberto C. Gonçalves. 2024. "Understanding Local Reactions Induced by Bothrops jararaca Venom: The Role of Inflammatory Mediators in Leukocyte–Endothelium Interactions" Biomedicines 12, no. 4: 734. https://doi.org/10.3390/biomedicines12040734





