Swollen Feet: Considering the Paradoxical Roles of Interleukins in Nephrotic Syndrome
Abstract
:1. Introduction
2. Deleterious Effects of Interleukins in NS: IL-1R Superfamily
3. Deleterious Effects of Interleukins in NS: IL-Rγc Family
4. Deleterious Effects of Interleukins in NS: IL-6 Family
5. Deleterious Effects of Interleukins in NS: IL-13
6. Deleterious Effects of Interleukins in NS: IL-17
7. Deleterious Effects of Interleukins in NS: IL-20
“Good and evil are so close as to be chained together in the soul.” Robert Louis Stevenson, Dr. Jekyll and Mr. Hyde
8. Cytoprotective Effects of Interleukins in NS: IL-1R Superfamily
9. Cytoprotective Effects of Interleukins in NS: IL-Rγc Superfamily
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tapia, C.; Bashir, K. Nephrotic Syndrome. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Trautmann, A.; Vivarelli, M.; Samuel, S.; Gipson, D.; Sinha, A.; Schaefer, F.; Hui, N.K.; Boyer, O.; Saleem, M.A.; Feltran, L.; et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr. Nephrol. 2020, 35, 1529–1561. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2022.
- Hall, G. Genetic Causes of Chronic Kidney Disease. In Chronic Kidney Disease, Dialysis and Transplantation: A Companion to Brenner and Rector’s The Kidney, 4th ed.; Ikiler, H.A., Ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 105–119. [Google Scholar]
- Kim, Y.H.; Goyal, M.; Kurnit, D.; Wharram, B.; Wiggins, J.; Holzman, L.; Kershaw, D.; Wiggins, R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001, 60, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Wharram, B.L.; Goyal, M.; Wiggins, J.E.; Sanden, S.K.; Hussain, S.; Filipiak, W.E.; Saunders, T.L.; Dysko, R.C.; Kohno, K.; Holzman, L.B.; et al. Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 2005, 16, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Jones, N. Nephrin Signaling in the Podocyte: An Updated View of Signal Regulation at the Slit Diaphragm and Beyond. Front. Endocrinol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Grahammer, F.; Schell, C.; Huber, T.B. The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat. Rev. Nephrol. 2013, 9, 587–598. [Google Scholar] [CrossRef]
- Yamada, E. The fine structure of the renal glomerulus of the mouse. J. Biophys. Biochem. Cytol. 1955, 1, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Takahashi-Nakaguchi, A.; Uematsu, K.; Yamada, H.; Sato-Okamoto, M.; Chibana, H. Ultrastructural examination of mouse kidney glomerular capillary loop by sandwich freezing and freeze-substitution. Microscopy 2022, 71, 289–296. [Google Scholar] [CrossRef]
- Sadowski, C.E.; Lovric, S.; Ashraf, S.; Pabst, W.L.; Gee, H.Y.; Kohl, S.; Engelmann, S.; Vega-Warner, V.; Fang, H.; Halbritter, J.; et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol. 2015, 26, 1279–1289. [Google Scholar] [CrossRef]
- Hall, G.; Gbadegesin, R.A. Translating genetic findings in hereditary nephrotic syndrome: The missing loops. Am. J. Physiol. Renal Physiol. 2015, 309, F24–F28. [Google Scholar] [CrossRef]
- Hackl, A.; Zed, S.; Diefenhardt, P.; Binz-Lotter, J.; Ehren, R.; Weber, L.T. The role of the immune system in idiopathic nephrotic syndrome. Mol. Cell. Pediatr. 2021, 8, 18. [Google Scholar] [CrossRef]
- Campbell, R.E.; Thurman, J.M. The Immune System and Idiopathic Nephrotic Syndrome. Clin J. Am. Soc. Nephrol. 2022, 17, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Sahali, D.; Sendeyo, K.; Mangier, M.; Audard, V.; Zhang, S.Y.; Lang, P.; Ollero, M.; Pawlak, A. Immunopathogenesis of idiopathic nephrotic syndrome with relapse. Semin. Immunopathol. 2014, 36, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Pereira Wde, F.; Brito-Melo, G.E.; Guimaraes, F.T.; Carvalho, T.G.; Mateo, E.C.; Simoes e Silva, A.C. The role of the immune system in idiopathic nephrotic syndrome: A review of clinical and experimental studies. Inflamm. Res. 2014, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Konigshausen, E.; Sellin, L. Circulating Permeability Factors in Primary Focal Segmental Glomerulosclerosis: A Review of Proposed Candidates. Biomed. Res. Int. 2016, 2016, 3765608. [Google Scholar] [CrossRef] [PubMed]
- Colucci, M.; Oniszczuk, J.; Vivarelli, M.; Audard, V. B-Cell Dysregulation in Idiopathic Nephrotic Syndrome: What We Know and What We Need to Discover. Front. Immunol. 2022, 13, 823204. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guan, F. B cell phenotype, activity, and function in idiopathic nephrotic syndrome. Pediatr. Res. 2023, 93, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.; Shokeir, M.; El-Chenawi, F.; El-Husseni, F.; Abdel-Rahman, A.; El-Ashry, R. Tumor necrosis factor-alpha production from mononuclear cells in nephrotic syndrome. Pediatr. Nephrol. 2003, 18, 516–520. [Google Scholar] [CrossRef]
- Bitzan, M.; Babayeva, S.; Vasudevan, A.; Goodyer, P.; Torban, E. TNFalpha pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and beta3 integrin activation. Pediatr. Nephrol. 2012, 27, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Delville, M.; Sigdel, T.K.; Wei, C.; Li, J.; Hsieh, S.C.; Fornoni, A.; Burke, G.W.; Bruneval, P.; Naesens, M.; Jackson, A.; et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci. Transl. Med. 2014, 6, 256ra136. [Google Scholar] [CrossRef]
- McCarthy, E.T.; Sharma, M.; Savin, V.J. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2010, 5, 2115–2121. [Google Scholar] [CrossRef]
- Ren, J.; Xu, Y.; Lu, X.; Wang, L.; Ide, S.; Hall, G.; Souma, T.; Privratsky, J.R.; Spurney, R.F.; Crowley, S.D. Twist1 in podocytes ameliorates podocyte injury and proteinuria by limiting CCL2-dependent macrophage infiltration. JCI Insight 2021, 6, e148109. [Google Scholar] [CrossRef] [PubMed]
- Mertowska, P.; Mertowski, S.; Smarz-Widelska, I.; Grywalska, E. Biological Role, Mechanism of Action and the Importance of Interleukins in Kidney Diseases. Int. J. Mol. Sci. 2022, 23, 647. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Ghoreschi, K. The Interleukin-1 Family; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Niemir, Z.I.; Stein, H.; Dworacki, G.; Mundel, P.; Koehl, N.; Koch, B.; Autschbach, F.; Rassy, K.; Ritz, E.; Waldherr, R.; et al. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int. 1997, 52, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Tesch, G.H.; Yang, N.; Yu, H.; Lan, H.Y.; Foti, R.; Chadban, S.J.; Atkins, R.C.; Nikolic-Paterson, D.J. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol. Dial. Transplant. 1997, 12, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sinniah, R.; Hsu, S.I. In situ glomerular expression of activated NF-kappaB in human lupus nephritis and other non-proliferative proteinuric glomerulopathy. Virchows. Arch. 2006, 448, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Brahler, S.; Ising, C.; Hagmann, H.; Rasmus, M.; Hoehne, M.; Kurschat, C.; Kisner, T.; Goebel, H.; Shankland, S.; Addicks, K.; et al. Intrinsic proinflammatory signaling in podocytes contributes to podocyte damage and prolonged proteinuria. Am. J. Physiol. Renal Physiol. 2012, 303, F1473–F1485. [Google Scholar] [CrossRef] [PubMed]
- Brahler, S.; Ising, C.; Barrera Aranda, B.; Hohne, M.; Schermer, B.; Benzing, T.; Brinkkoetter, P.T. The NF-kappaB essential modulator (NEMO) controls podocyte cytoskeletal dynamics independently of NF-kappaB. Am. J. Physiol. Renal Physiol. 2015, 309, F617–F626. [Google Scholar] [CrossRef] [PubMed]
- Mikulak, J.; Oriolo, F.; Portale, F.; Tentorio, P.; Lan, X.; Saleem, M.A.; Skorecki, K.; Singhal, P.C.; Mavilio, D. Impact of APOL1 polymorphism and IL-1beta priming in the entry and persistence of HIV-1 in human podocytes. Retrovirology 2016, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Yuan, L.; Zhang, Y.; Wu, J.; Guo, N.; Chen, X.; Liu, J. Down-regulation of IRAK1 attenuates podocyte apoptosis in diabetic nephropathy through PI3K/Akt signaling pathway. Biochem. Biophys. Res. Commun. 2018, 506, 529–535. [Google Scholar] [CrossRef]
- Kondo, M.; Tahara, A.; Hayashi, K.; Abe, M.; Inami, H.; Ishikawa, T.; Ito, H.; Tomura, Y. Renoprotective effects of novel interleukin-1 receptor-associated kinase 4 inhibitor AS2444697 through anti-inflammatory action in 5/6 nephrectomized rats. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 909–919. [Google Scholar] [CrossRef]
- Kondo, M.; Tahara, A.; Hayashi, K.; Inami, H.; Ishikawa, T.; Tomura, Y. Therapeutic effects of interleukin-1 receptor-associated kinase 4 inhibitor AS2444697 on diabetic nephropathy in type 2 diabetic mice. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, G.; Almond, M.; Dasgupta, B. Improved renal function in diabetic patients with acute gout treated with anakinra. Kidney Int. 2015, 88, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Tuttle, K.R.; Perkovic, V.; Libby, P.; MacFadyen, J.G. Inflammation drives residual risk in chronic kidney disease: A CANTOS substudy. Eur. Heart J. 2022, 43, 4832–4844. [Google Scholar] [CrossRef] [PubMed]
- Varan, O.; Kucuk, H.; Babaoglu, H.; Guven, S.C.; Ozturk, M.A.; Haznedaroglu, S.; Goker, B.; Tufan, A. Efficacy and safety of interleukin-1 inhibitors in familial Mediterranean fever patients complicated with amyloidosis. Mod. Rheumatol. 2019, 29, 363–366. [Google Scholar] [CrossRef] [PubMed]
- El Hasbani, G.; Jawad, A.; Uthman, I. Update on the management of colchicine resistant Familial Mediterranean Fever (FMF). Orphanet J. Rare Dis. 2019, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Koh, J.H.; Kim, S.G.; Lee, S.; Kim, Y.; Cho, S.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; et al. Mendelian randomization uncovers a protective effect of interleukin-1 receptor antagonist on kidney function. Commun. Biol. 2023, 6, 722. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 receptor antagonist: Role in biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P. Interleukin-1 receptor antagonist. Adv. Immunol. 1993, 54, 167–227. [Google Scholar]
- Hirooka, Y.; Nozaki, Y. Interleukin-18 in Inflammatory Kidney Disease. Front. Med. 2021, 8, 639103. [Google Scholar] [CrossRef]
- Hewins, P.; Morgan, M.D.; Holden, N.; Neil, D.; Williams, J.M.; Savage, C.O.; Harper, L. IL-18 is upregulated in the kidney and primes neutrophil responsiveness in ANCA-associated vasculitis. Kidney Int. 2006, 69, 605–615. [Google Scholar] [CrossRef]
- Sugiyama, M.; Kinoshita, K.; Kishimoto, K.; Shimazu, H.; Nozaki, Y.; Ikoma, S.; Funauchi, M. Deletion of IL-18 receptor ameliorates renal injury in bovine serum albumin-induced glomerulonephritis. Clin. Immunol. 2008, 128, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kanmatsuse, K. Interleukin-18 and interleukin-12 synergize to stimulate the production of vascular permeability factor by T lymphocytes in normal subjects and in patients with minimal-change nephrotic syndrome. Nephron 2000, 85, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lagrue, G.; Xheneumont, S.; Branellec, A.; Hirbec, G.; Weil, B. A vascular permeability factor elaborated from lymphocytes. I. Demonstration in patients with nephrotic syndrome. Biomedicine 1975, 23, 37–40. [Google Scholar] [PubMed]
- Maas, R.J.; Deegens, J.K.; Wetzels, J.F. Permeability factors in idiopathic nephrotic syndrome: Historical perspectives and lessons for the future. Nephrol. Dial. Transplant. 2014, 29, 2207–2216. [Google Scholar] [CrossRef]
- Huang, D.; Kidd, J.M.; Zou, Y.; Wu, X.; Gehr, T.W.B.; Li, P.L.; Li, G. Regulation of NLRP3 Inflammasome Activation and Inflammatory Exosome Release in Podocytes by Acid Sphingomyelinase During Obesity. Inflammation 2023, 46, 2037–2054. [Google Scholar] [CrossRef]
- Kaverina, N.; Schweickart, R.A.; Chan, G.C.; Maggiore, J.C.; Eng, D.G.; Zeng, Y.; McKinzie, S.R.; Perry, H.S.; Ali, A.; O’Connor, C.; et al. Inhibiting NLRP3 signaling in aging podocytes improves their life- and health-span. Aging 2023, 15, 6658–6689. [Google Scholar] [CrossRef]
- Chen, F.F.; Liu, X.T.; Tao, J.; Mao, Z.M.; Wang, H.; Tan, Y.; Qu, Z.; Yu, F. Renal NLRP3 Inflammasome activation is associated with disease activity in lupus nephritis. Clin. Immunol. 2023, 247, 109221. [Google Scholar] [CrossRef]
- Bai, M.; Chen, Y.; Zhao, M.; Zhang, Y.; He, J.C.; Huang, S.; Jia, Z.; Zhang, A. NLRP3 inflammasome activation contributes to aldosterone-induced podocyte injury. Am. J. Physiol. Renal Physiol. 2017, 312, F556–F564. [Google Scholar] [CrossRef]
- Lv, D.; Jiang, S.; Zhang, M.; Zhu, X.; Yang, F.; Wang, H.; Li, S.; Liu, F.; Zeng, C.; Qin, W.; et al. Treatment of Membranous Nephropathy by Disulfiram through Inhibition of Podocyte Pyroptosis. Kidney Dis. 2022, 8, 308–318. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, P.; Tang, M.; Song, Y.; Liu, C.; Zhao, B. Dapagliflozin alleviates renal podocyte pyroptosis via regulation of the HO-1/NLRP3 axis. Mol. Med. Rep. 2023, 28, 200. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Yang, L.; Feng, L.; Zhang, S.; An, J.; Li, J.; Gao, Y.; Pan, Z.; Xu, Y.; et al. Syringaresinol protects against diabetic nephropathy by inhibiting pyroptosis via NRF2-mediated antioxidant pathway. Cell Biol. Toxicol. 2023, 39, 621–639. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, H.; Lv, S.; Liu, Q.; Li, S.; Yang, X.; Liu, G. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Diabetic Kidney Disease Through the NLRP3 Signaling Pathway. Stem Cells 2023, 41, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; Lin, J.X.; O’Shea, J.J. The gamma(c) Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Leonard, W.J. The Common Cytokine Receptor gamma Chain Family of Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028449. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, T.J.; Wadhwa, M.; Callard, R.; Barratt, T.M. Increased IL-2, IL-4 and interferon-gamma (IFN-gamma) in steroid-sensitive nephrotic syndrome. Clin. Exp. Immunol. 1995, 100, 475–479. [Google Scholar] [CrossRef]
- Hulton, S.A.; Shah, V.; Byrne, M.R.; Morgan, G.; Barratt, T.M.; Dillon, M.J. Lymphocyte subpopulations, interleukin-2 and interleukin-2 receptor expression in childhood nephrotic syndrome. Pediatr. Nephrol. 1994, 8, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Bock, G.H.; Ongkingco, J.R.; Patterson, L.T.; Ruley, J.; Schroepfer, L.R.; Nelson, D.L. Serum and urine soluble interleukin-2 receptor in idiopathic nephrotic syndrome. Pediatr. Nephrol. 1993, 7, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, R.; Saatci, U.; Arikan, M.; Canpinar, H.; Bakkaloglu, A.; Kansu, E. T-cell subsets, interleukin-2 receptor expression and production of interleukin-2 in minimal change nephrotic syndrome. Pediatr. Nephrol. 1994, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, H.; Nakajima, M.; Naka, H.; Maruhashi, Y.; Akazawa, H.; Ueda, T.; Nishiguchi, M.; Yamoto, Y.; Kamitsuji, H.; Yoshioka, A. Up-regulation of interleukin-2 mRNA in children with idiopathic nephrotic syndrome. Pediatr. Nephrol. 2004, 19, 1115–1121. [Google Scholar] [CrossRef]
- Heslan, J.M.; Branellec, A.I.; Pilatte, Y.; Lang, P.; Lagrue, G. Differentiation between vascular permeability factor and IL-2 in lymphocyte supernatants from patients with minimal-change nephrotic syndrome. Clin. Exp. Immunol. 1991, 86, 157–162. [Google Scholar] [CrossRef]
- Muhlig, A.K.; Lee, J.Y.; Kemper, M.J.; Kronbichler, A.; Yang, J.W.; Lee, J.M.; Shin, J.I.; Oh, J. Levamisole in Children with Idiopathic Nephrotic Syndrome: Clinical Efficacy and Pathophysiological Aspects. J. Clin. Med. 2019, 8, 860. [Google Scholar] [CrossRef]
- Al-Eisa, A.A.; Al-Rushood, M. Urinary Interleukin-4 Levels in Nephrotic Children with and without Concominant Asthma. Curr. Pediatr. Res. 2017, 21, 480–484. [Google Scholar]
- Kim, A.H.; Chung, J.J.; Akilesh, S.; Koziell, A.; Jain, S.; Hodgin, J.B.; Miller, M.J.; Stappenbeck, T.S.; Miner, J.H.; Shaw, A.S. B cell-derived IL-4 acts on podocytes to induce proteinuria and foot process effacement. JCI Insight 2017, 2, e81836. [Google Scholar] [CrossRef]
- Lee, J.M.; Ko, Y.; Lee, C.H.; Jeon, N.; Lee, K.H.; Oh, J.; Kronbichler, A.; Saleem, M.A.; Lim, B.J.; Shin, J.I. The Effect of Interleukin-4 and Dexamethasone on RNA-Seq-Based Transcriptomic Profiling of Human Podocytes: A Potential Role in Minimal Change Nephrotic Syndrome. J. Clin. Med. 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Shiraishi, H.; Yamagata, T.; Ito, T.; Odaka, J.; Saito, T.; Aoyagi, J.; Momoi, M.Y. Elevated serum interleukin-7 level in idiopathic steroid-sensitive nephrotic syndrome. Pediatr. Int. 2011, 53, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Brier, M.E.; Kerlin, B.A.; Smoyer, W.E.; Pediatric Nephrology Research, C. Plasma Cytokine Profiling to Predict Steroid Resistance in Pediatric Nephrotic Syndrome. Kidney Int. Rep. 2021, 6, 785–795. [Google Scholar] [CrossRef]
- Zhai, S.; Zhao, L.; Zhang, Y.; Ma, Q. Interleukin-7 stimulation inhibits nephrin activation and induces podocyte injury. Biochem. Biophys. Res. Commun. 2018, 507, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor alpha to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Moutabarrik, A.; Nakanishi, I.; Ishibashi, M. Interleukin-6 and interleukin-6 receptor are expressed by cultured glomerular epithelial cells. Scand. J. Immunol. 1994, 40, 181–186. [Google Scholar] [CrossRef]
- Jo, H.A.; Kim, J.Y.; Yang, S.H.; Han, S.S.; Joo, K.W.; Kim, Y.S.; Kim, D.K. The role of local IL6/JAK2/STAT3 signaling in high glucose-induced podocyte hypertrophy. Kidney Res. Clin. Pract. 2016, 35, 212–218. [Google Scholar] [CrossRef] [PubMed]
- He, F.F.; Bao, D.; Su, H.; Wang, Y.M.; Lei, C.T.; Zhang, C.Y.; Ye, C.; Tang, H.; Wan, C.; You, C.Q.; et al. IL-6 increases podocyte motility via MLC-mediated focal adhesion impairment and cytoskeleton disassembly. J. Cell Physiol. 2018, 233, 7173–7181. [Google Scholar] [CrossRef]
- Nagayama, Y.; Braun, G.S.; Jakobs, C.M.; Maruta, Y.; van Roeyen, C.R.; Klinkhammer, B.M.; Boor, P.; Villa, L.; Raffetseder, U.; Trautwein, C.; et al. Gp130-dependent signaling in the podocyte. Am. J. Physiol. Renal Physiol. 2014, 307, F346–F355. [Google Scholar] [CrossRef]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Bohm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Schrader, L.I.; Kinzenbaw, D.A.; Johnson, A.W.; Faraci, F.M.; Didion, S.P. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Jayakumar, C.; Ramesh, G. Proximal tubule-specific overexpression of netrin-1 suppresses acute kidney injury-induced interstitial fibrosis and glomerulosclerosis through suppression of IL-6/STAT3 signaling. Am. J. Physiol. Renal Physiol. 2013, 304, F1054–F1065. [Google Scholar] [CrossRef]
- Kielar, M.L.; John, R.; Bennett, M.; Richardson, J.A.; Shelton, J.M.; Chen, L.; Jeyarajah, D.R.; Zhou, X.J.; Zhou, H.; Chiquett, B.; et al. Maladaptive role of IL-6 in ischemic acute renal failure. J. Am. Soc. Nephrol. 2005, 16, 3315–3325. [Google Scholar] [CrossRef]
- Lai, K.W.; Wei, C.L.; Tan, L.K.; Tan, P.H.; Chiang, G.S.; Lee, C.G.; Jordan, S.C.; Yap, H.K. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J. Am. Soc. Nephrol. 2007, 18, 1476–1485. [Google Scholar] [CrossRef]
- Ha, T.S.; Nam, J.A.; Seong, S.B.; Saleem, M.A.; Park, S.J.; Shin, J.I. Montelukast improves the changes of cytoskeletal and adaptor proteins of human podocytes by interleukin-13. Inflamm. Res. 2017, 66, 793–802. [Google Scholar] [CrossRef]
- Yu, C.C.; Fornoni, A.; Weins, A.; Hakroush, S.; Maiguel, D.; Sageshima, J.; Chen, L.; Ciancio, G.; Faridi, M.H.; Behr, D.; et al. Abatacept in B7-1-positive proteinuric kidney disease. N. Engl. J. Med. 2013, 369, 2416–2423. [Google Scholar] [CrossRef]
- Reiser, J.; von Gersdorff, G.; Loos, M.; Oh, J.; Asanuma, K.; Giardino, L.; Rastaldi, M.P.; Calvaresi, N.; Watanabe, H.; Schwarz, K.; et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J. Clin. Investig. 2004, 113, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.H.; Mu, W.; Arthur, J.M.; Rivard, C.J.; Araya, C.E.; Shimada, M.; Johnson, R.J. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010, 78, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Novelli, R.; Benigni, A.; Remuzzi, G. The role of B7-1 in proteinuria of glomerular origin. Nat. Rev. Nephrol. 2018, 14, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Saleem, M.A.; Nam, J.A.; Ha, T.S.; Shin, J.I. Effects of interleukin-13 and montelukast on the expression of zonula occludens-1 in human podocytes. Yonsei Med. J. 2015, 56, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; Ng, K.H.; Chen, J.; Lu, J.; Lee, C.G.; Tan, P.H.; Jordan, S.C.; He, H.Y.; Yap, H.K. Novel role of Vav1-Rac1 pathway in actin cytoskeleton regulation in interleukin-13-induced minimal change-like nephropathy. Clin. Sci. 2016, 130, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Sun, B.; Zhang, Y.; Zhao, L.; Zhang, L. IL-17 aggravates renal injury by promoting podocyte injury in children with primary nephrotic syndrome. Exp. Ther. Med. 2020, 20, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q.; Wang, L.; Li, C.; Yang, H.; Wang, X.; Tao, H. The role of Th17/IL-17 in the pathogenesis of primary nephrotic syndrome in children. Kidney Blood Press. Res. 2013, 37, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.H.; Dong, Q.L.; Jin, G.; Zhu, Y.N.; Zhang, L.P. Effect of Interleukin-17 gene on glomerular ultrastructure and podocyte injury in adriamycin nephropathy rat models. J. Biol. Regul. Homeost. Agents 2021, 35, 1001–1010. [Google Scholar] [PubMed]
- Zhang, F.; Yin, J.; Liu, L.; Liu, S.; Zhang, G.; Kong, Y.; Wang, Y.; Wang, N.; Chen, X.; Wang, F. IL-17C neutralization protects the kidney against acute injury and chronic injury. EBioMedicine 2023, 92, 104607. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Li, H.H.; Sung, J.M.; Chen, W.Y.; Hou, Y.C.; Weng, Y.H.; Lai, W.T.; Wu, C.H.; Chang, M.S. Interleukin-20 targets podocytes and is upregulated in experimental murine diabetic nephropathy. Exp. Mol. Med. 2017, 49, e310. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.D.; Beresford, M.W. Podocytes contribute, and respond, to the inflammatory environment in lupus nephritis. Am. J. Physiol. Renal Physiol. 2018, 315, F1683–F1694. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Wang, B.; Shirvaikar, A.; Khan, S.; Kamat, S.; Schelling, J.R.; Konieczkowski, M.; Sedor, J.R. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J. Clin. Investig. 1999, 103, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Peng, Y.; Yu, H.; Yu, X.; Zhou, J.; Xiao, J. RhoA protects the podocytes against high glucose-induced apoptosis through YAP and plays critical role in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2018, 504, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Chen, Y.; Zhang, H.; Yu, C.; Zhou, F.; Zhang, Z.; Jiang, L.; Li, R.; Ma, J.; et al. RhoA deficiency disrupts podocyte cytoskeleton and induces podocyte apoptosis by inhibiting YAP/dendrin signal. BMC Nephrol. 2016, 17, 66. [Google Scholar] [CrossRef] [PubMed]
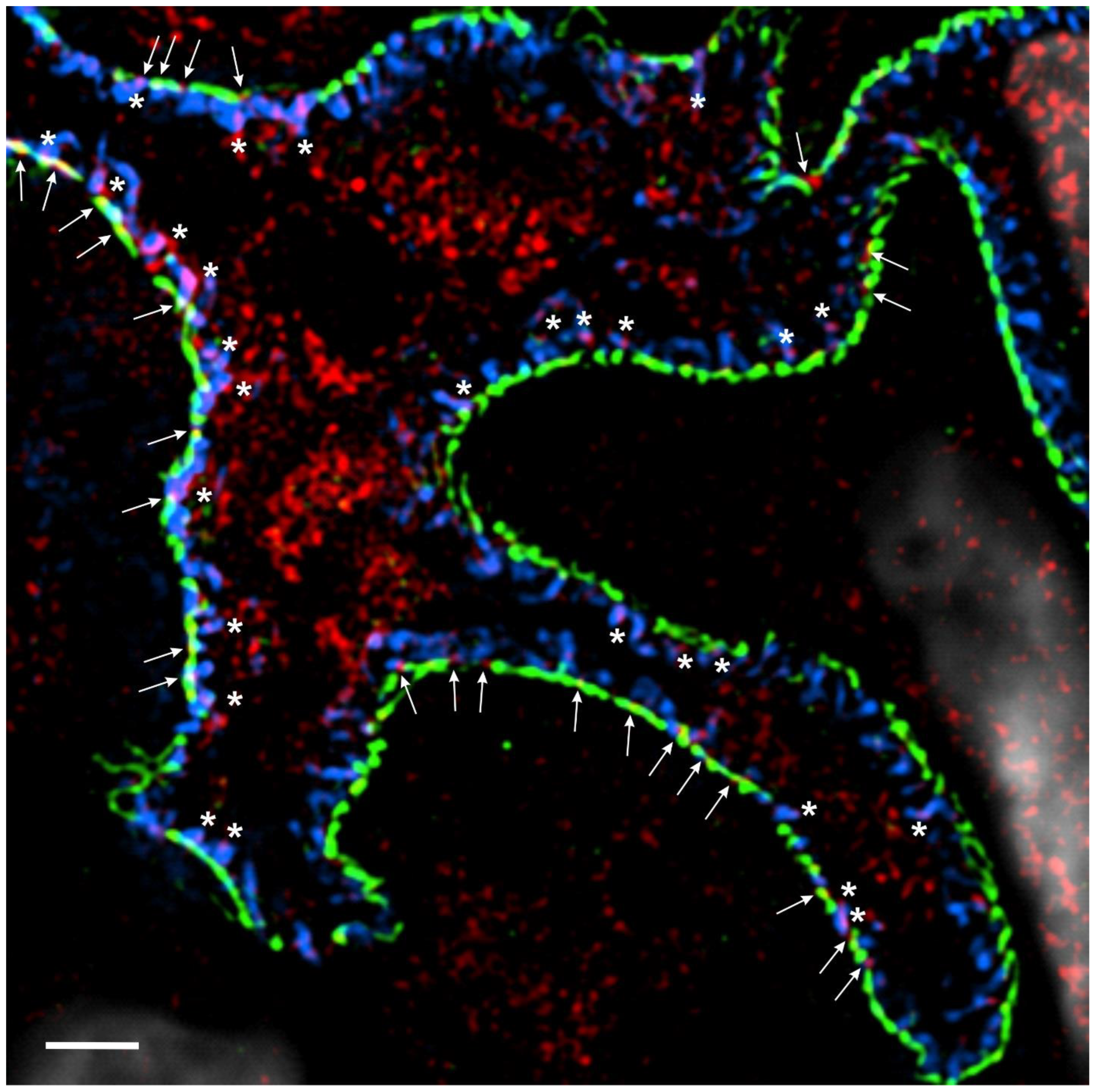
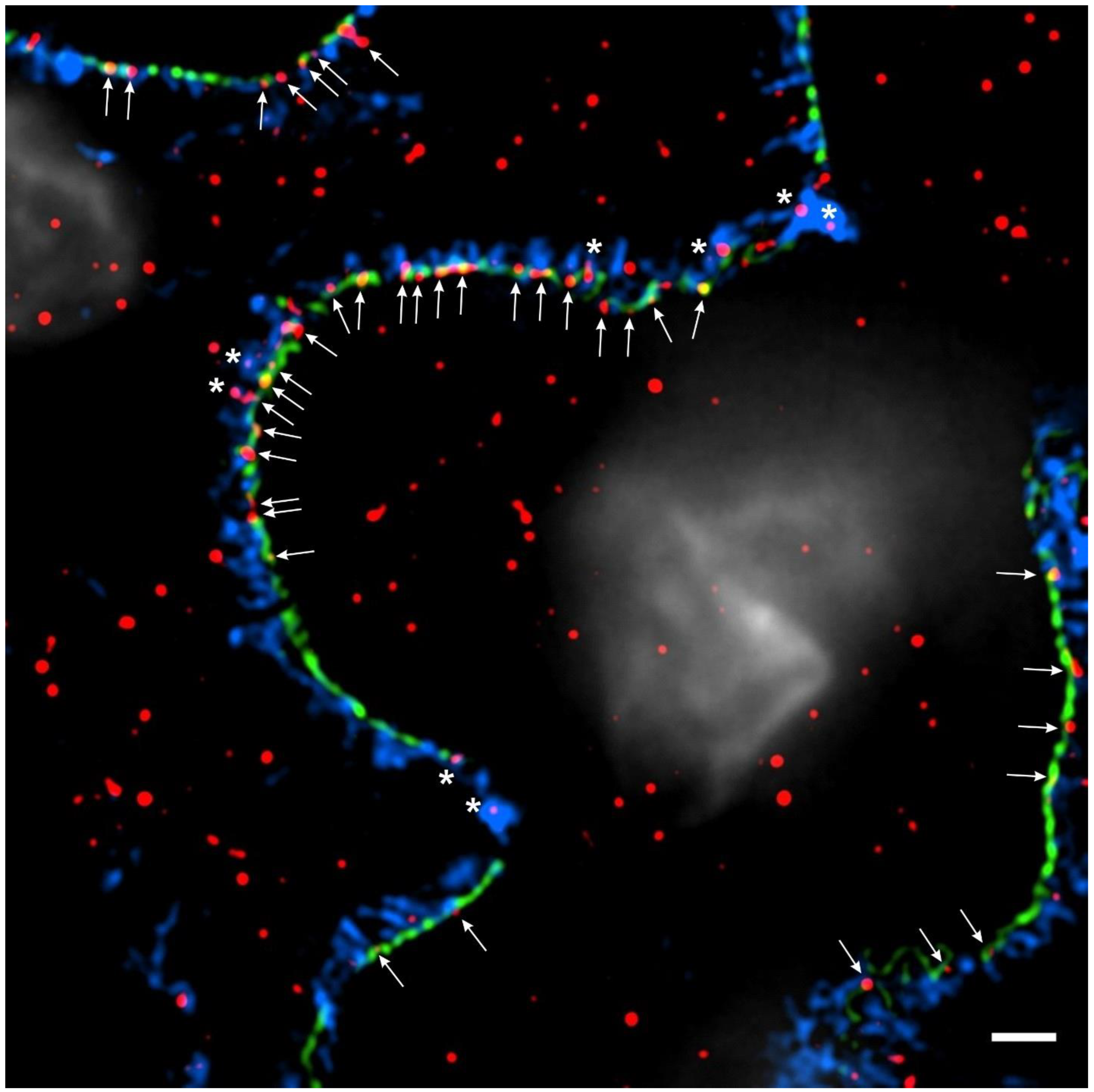
- Ren, J.; Lu, X.; Hall, G.; Privratsky, J.R.; Robson, M.J.; Blakely, R.D.; Crowley, S.D. IL-1 receptor signaling in podocytes limits susceptibility to glomerular damage. Am. J. Physiol. Renal Physiol. 2022, 322, F164–F174. [Google Scholar] [CrossRef] [PubMed]
- Babaev, V.R.; Ding, L.; Zhang, Y.; May, J.M.; Lin, P.C.; Fazio, S.; Linton, M.F. Macrophage IKKalpha Deficiency Suppresses Akt Phosphorylation, Reduces Cell Survival, and Decreases Early Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Dubois, N.; Berendsen, S.; Henry, A.; Nguyen, M.; Bours, V.; Robe, P.A. I-Kappa-B Kinase-epsilon activates nuclear factor-kappa B and STAT5B and supports glioblastoma growth but amlexanox shows little therapeutic potential in these tumors. Cancer Transl. Med. 2018, 4, 1–8. [Google Scholar]
- Yin, M.; Wang, X.; Lu, J. Advances in IKBKE as a potential target for cancer therapy. Cancer Med. 2020, 9, 247–258. [Google Scholar] [CrossRef]
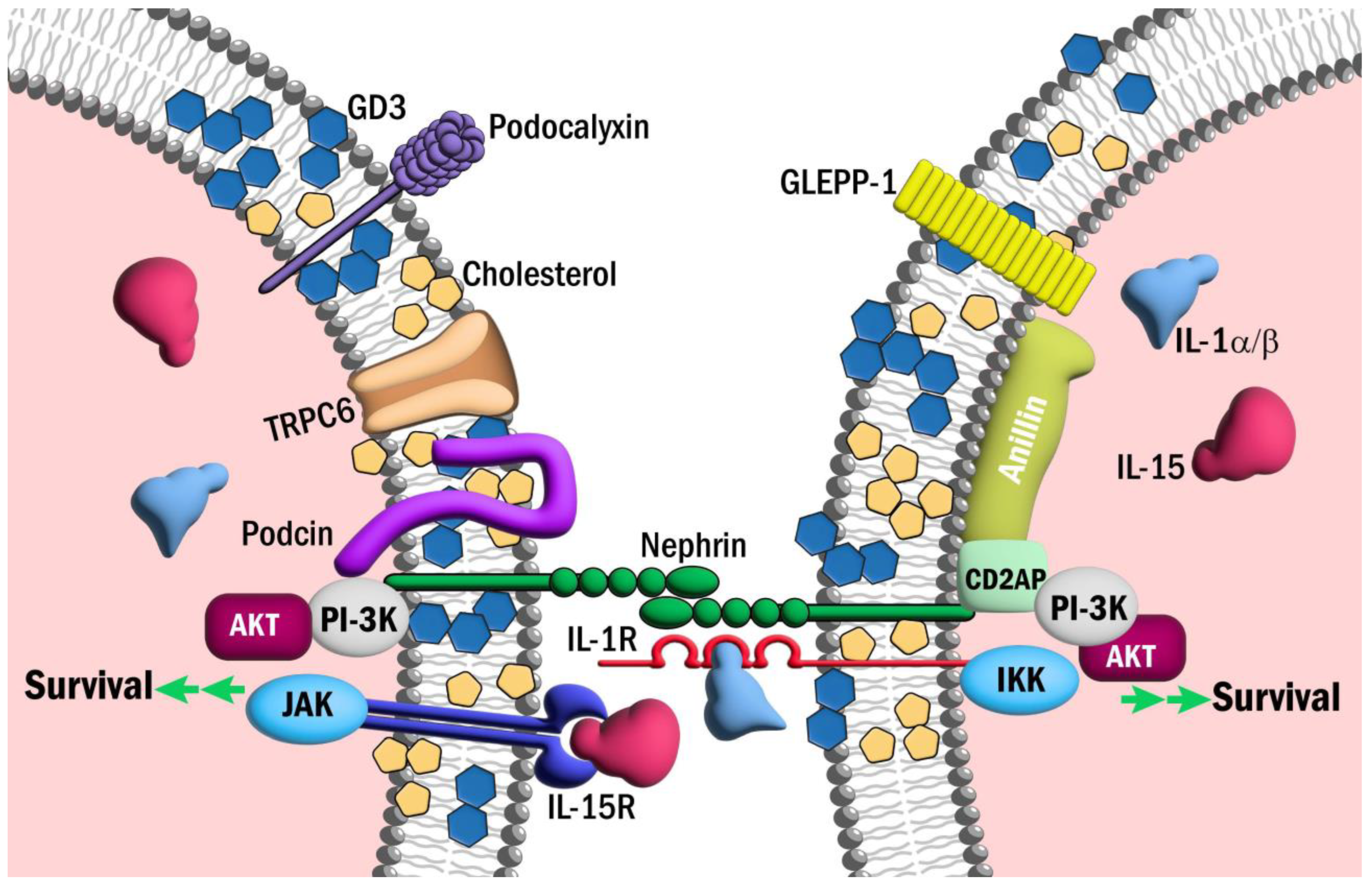
- Huber, T.B.; Hartleben, B.; Kim, J.; Schmidts, M.; Schermer, B.; Keil, A.; Egger, L.; Lecha, R.L.; Borner, C.; Pavenstadt, H.; et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell Biol. 2003, 23, 4917–4928. [Google Scholar] [CrossRef]
- Huber, T.B.; Kottgen, M.; Schilling, B.; Walz, G.; Benzing, T. Interaction with podocin facilitates nephrin signaling. J. Biol. Chem. 2001, 276, 41543–41546. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.; Lane, B.M.; Khan, K.; Pediaditakis, I.; Xiao, J.; Wu, G.; Wang, L.; Kovalik, M.E.; Chryst-Stangl, M.; Davis, E.E.; et al. The Human FSGS-Causing ANLN R431C Mutation Induces Dysregulated PI3K/AKT/mTOR/Rac1 Signaling in Podocytes. J. Am. Soc. Nephrol. 2018, 29, 2110–2122. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ye, Y.; Fu, H.; Gu, W.; Zhao, M.; Sun, J.; Cao, Z.; Huang, G.; Xie, Y.; Liu, F.; et al. Effects of a novel ANLN E841K mutation associated with SRNS on podocytes and its mechanism. Cell Commun. Signal. 2023, 21, 324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Y.; Zhou, Y.; Fei, B. Interleukin 37 (IL-37) Reduces High Glucose-Induced Inflammation, Oxidative Stress, and Apoptosis of Podocytes by Inhibiting the STAT3-Cyclophilin A (CypA) Signaling Pathway. Med. Sci. Monit. 2020, 26, e922979. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, L.; Huang, Y.; Shi, R.; Wu, Y.; Hakimah Binti Ismail, I. Contribution of IL-33/ILC2-mediated Th2 cytokines during the progression of minimal change disease. Int. Immunopharmacol. 2023, 114, 109493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.; Liu, Z. Role of IL-33-ST2 pathway in regulating inflammation: Current evidence and future perspectives. J. Transl. Med. 2023, 21, 902. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, N.; Iyoda, M.; Suzuki, T.; Tachibana, S.; Nagashima, R.; Honda, H. Exploring the significance of interleukin-33/ST2 axis in minimal change disease. Sci. Rep. 2023, 13, 18776. [Google Scholar] [CrossRef] [PubMed]
- Giron-Michel, J.; Azzi, S.; Khawam, K.; Mortier, E.; Caignard, A.; Devocelle, A.; Ferrini, S.; Croce, M.; Francois, H.; Lecru, L.; et al. Interleukin-15 plays a central role in human kidney physiology and cancer through the gammac signaling pathway. PLoS ONE 2012, 7, e31624. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Attar, M.; Gnirck, A.C.; Wunderlich, M.; Becker, M.; Rickassel, C.; Puelles, V.G.; Meyer-Schwesinger, C.; Wiech, T.; Nies, J.F.; et al. Interleukin-9 protects from early podocyte injury and progressive glomerulosclerosis in Adriamycin-induced nephropathy. Kidney Int. 2020, 98, 615–629. [Google Scholar] [CrossRef]
- Lin, Q.; Menon, M.C.; He, J.C. IL-9: A novel pro-podocyte survival cytokine in FSGS. Kidney Int. 2020, 98, 541–543. [Google Scholar] [CrossRef]
- Giron-Michel, J.; Azzi, S.; Ferrini, S.; Chouaib, S.; Camussi, G.; Eid, P.; Azzarone, B. Interleukin-15 is a major regulator of the cell-microenvironment interactions in human renal homeostasis. Cytokine Growth Factor Rev. 2013, 24, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, M.; Hirahashi, J.; Lebedeva, T.; Liew, F.Y.; Salant, D.J.; Maron, R.; Kelley, V.R. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J. Clin. Investig. 2002, 109, 951–960. [Google Scholar] [CrossRef]
- Mooslechner, A.A.; Schuller, M.; Artinger, K.; Kirsch, A.H.; Schabhuttl, C.; Eller, P.; Rosenkranz, A.R.; Eller, K. Low-Dose rIL-15 Protects from Nephrotoxic Serum Nephritis via CD8(+) T Cells. Cells 2022, 11, 3656. [Google Scholar] [CrossRef] [PubMed]
- Niasse, A.; Louis, K.; Lenoir, O.; Schwarz, C.; Xu, X.; Couturier, A.; Dobosziewicz, H.; Corchia, A.; Placier, S.; Vandermeersch, S.; et al. Protective Role of Podocytic IL-15/STAT5 Pathway in Experimental Focal and Segmental Glomerulosclerosis. BioRxiv 2022. [Google Scholar] [CrossRef]
- Shankland, S.J.; Wang, Y.; Shaw, A.S.; Vaughan, J.C.; Pippin, J.W.; Wessely, O. Podocyte Aging: Why and How Getting Old Matters. J. Am. Soc. Nephrol. 2021, 32, 2697–2713. [Google Scholar] [CrossRef] [PubMed]
- Famulski, K.S.; Halloran, P.F. Molecular events in kidney ageing. Curr. Opin. Nephrol. Hypertens. 2005, 14, 243–248. [Google Scholar] [CrossRef]
- Cianciolo, R.E.; Benali, S.L.; Aresu, L. Aging in the Canine Kidney. Vet. Pathol. 2016, 53, 299–308. [Google Scholar] [CrossRef]
- Li, Y.; Zhi, W.; Wareski, P.; Weng, N.P. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J. Immunol. 2005, 174, 4019–4024. [Google Scholar] [CrossRef]
- Watkinson, F.; Nayar, S.K.; Rani, A.; Sakellariou, C.A.; Elhage, O.; Papaevangelou, E.; Dasgupta, P.; Galustian, C. IL-15 Upregulates Telomerase Expression and Potently Increases Proliferative Capacity of NK, NKT-Like, and CD8 T Cells. Front. Immunol. 2020, 11, 594620. [Google Scholar] [CrossRef]
- Carson, W.E.; Ross, M.E.; Baiocchi, R.A.; Marien, M.J.; Boiani, N.; Grabstein, K.; Caligiuri, M.A. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J. Clin. Investig. 1995, 96, 2578–2582. [Google Scholar] [CrossRef]
- Daneshpajouhnejad, P.; Kopp, J.B.; Winkler, C.A.; Rosenberg, A.Z. The evolving story of apolipoprotein L1 nephropathy: The end of the beginning. Nat. Rev. Nephrol. 2022, 18, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, S.E.; Li, G.; Datta, S.; Soldano, K.L.; Silas, D.; Weins, A.; Hall, G.; Thomas, D.B.; Olabisi, O.A. JAK inhibitor blocks COVID-19 cytokine-induced JAK/STAT/APOL1 signaling in glomerular cells and podocytopathy in human kidney organoids. JCI Insight 2022, 7, e157432. [Google Scholar] [CrossRef]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Uscinski Knob, A.L.; et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Knudson, K.M.; Hodge, J.W.; Schlom, J.; Gameiro, S.R. Rationale for IL-15 superagonists in cancer immunotherapy. Expert Opin. Biol. Ther. 2020, 20, 705–709. [Google Scholar] [CrossRef]
- Cai, M.; Huang, X.; Huang, X.; Ju, D.; Zhu, Y.Z.; Ye, L. Research progress of interleukin-15 in cancer immunotherapy. Front. Pharmacol. 2023, 14, 1184703. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, X.; Zhao, Y.; Xu, X.; Liu, Y.; Wu, X. Excessive IL-15 promotes cytotoxic CD4 + CD28- T cell-mediated renal injury in lupus nephritis. Immun. Ageing 2022, 19, 50. [Google Scholar] [CrossRef]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.K.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalik, M.E.; Dacanay, M.A.; Crowley, S.D.; Hall, G. Swollen Feet: Considering the Paradoxical Roles of Interleukins in Nephrotic Syndrome. Biomedicines 2024, 12, 738. https://doi.org/10.3390/biomedicines12040738
Kovalik ME, Dacanay MA, Crowley SD, Hall G. Swollen Feet: Considering the Paradoxical Roles of Interleukins in Nephrotic Syndrome. Biomedicines. 2024; 12(4):738. https://doi.org/10.3390/biomedicines12040738
Chicago/Turabian StyleKovalik, Maria E., Monique A. Dacanay, Steven D. Crowley, and Gentzon Hall. 2024. "Swollen Feet: Considering the Paradoxical Roles of Interleukins in Nephrotic Syndrome" Biomedicines 12, no. 4: 738. https://doi.org/10.3390/biomedicines12040738




