Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review
Abstract
:1. Introduction
2. Pathophysiology
2.1. Hyperglycemia, Retinal Microvasculopathy and Metabolic Pathways
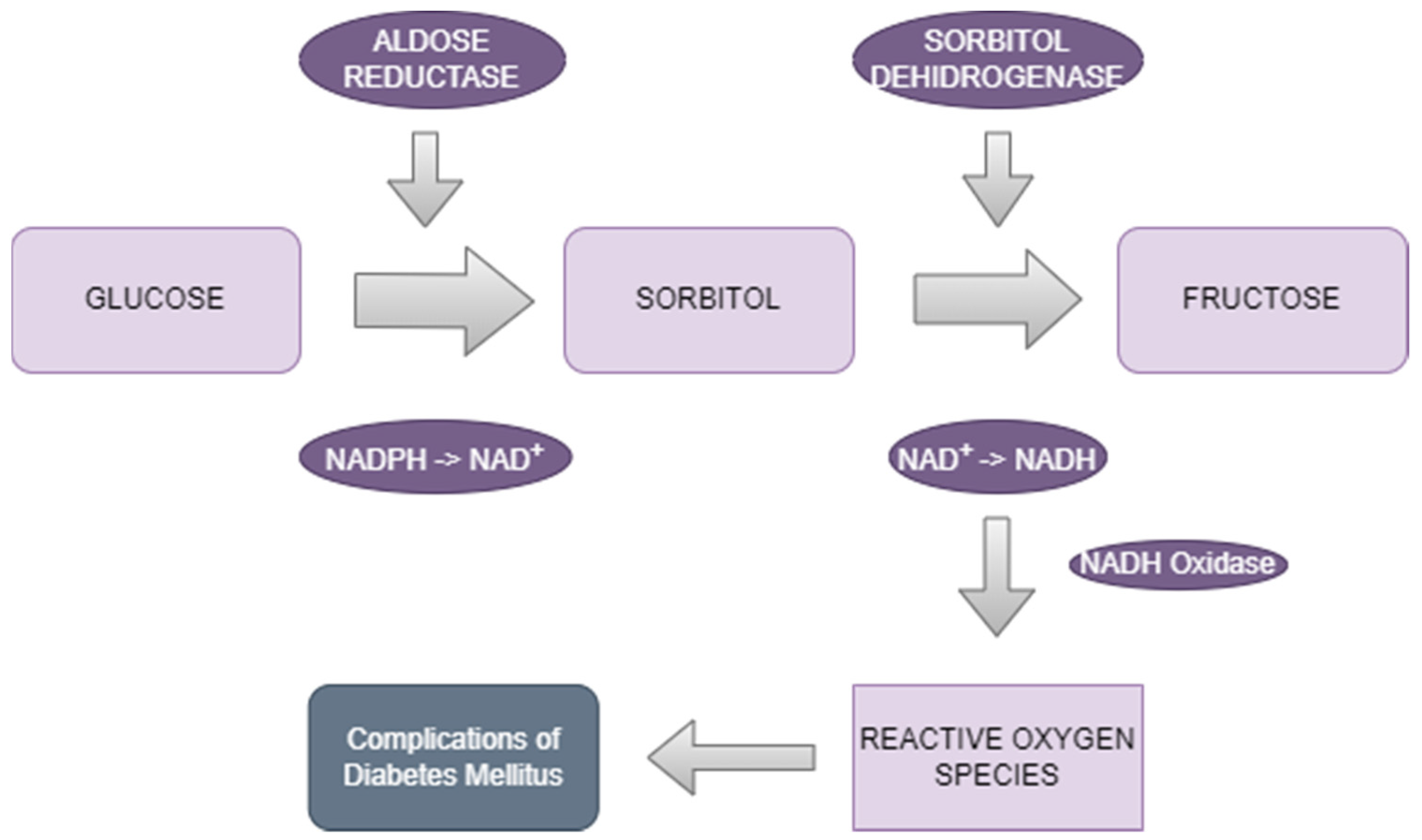
2.1.1. The Polyol Pathway
2.1.2. Advanced Glycation End-Product (AGE) Formation
2.1.3. Protein Kinase C (PKC) Activation
2.1.4. The Hexosamine Pathway
2.2. Oxidative Stress
2.3. Inflammation
2.4. Vascular Endothelial Growth Factor (VEGF)
2.5. Retinal Neurodegeneration
3. Biology and Characterization of Aldose Reductase
4. Measurement of Aldose Reductase
5. Treatment Targeting Aldose Reductase
5.1. Aldose Reductase Inhibitors
5.2. Phytocompounds
5.3. Combination Therapy
5.4. Gene Therapy
6. Benefits and Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.A.; Jonas, J.B.; Bron, A.M.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Friedman, D.S.; Keeffe, J.E.; Kempen, J.H.; Leasher, J.; et al. Prevalence and Causes of Vision Loss in High-Income Countries and in Eastern and Central Europe in 2015: Magnitude, Temporal Trends and Projections. Br. J. Ophthalmol. 2018, 102, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; Fadini, G.P. Microvascular Complications in Diabetes: A Growing Concern for Cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef]
- Hoerger, T.J.; Harris, R.; Hicks, K.A.; Donahue, K.; Sorensen, S.; Engelgau, M. Screening for Type 2 Diabetes Mellitus: A Cost-Effectiveness Analysis. Ann. Intern. Med. 2004, 140, 689. [Google Scholar] [CrossRef] [PubMed]
- Tapp, R.J.; Shaw, J.E.; Harper, C.A.; De Courten, M.P.; Balkau, B.; McCarty, D.J.; Taylor, H.R.; Welborn, T.A.; Zimmet, P.Z.; on behalf of the AusDiab Study Group. The Prevalence of and Factors Associated with Diabetic Retinopathy in the Australian Population. Diabetes Care 2003, 26, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Bebu, I.; Lachin, J.M. Optimal screening schedules for disease progression with application to diabetic retinopathy. Biostatistics 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, A.M.; Gibson, M.V.; Kulshreshtha, A. Diabetic retinopathy. Prim. Care 2015, 42, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Wong, T.Y. Current Concepts in Diabetic Retinopathy. Diabetes Metab. J. 2014, 38, 416–425. [Google Scholar] [CrossRef]
- Borțea, C.I.; Stoica, F.; Boia, M.; Iacob, E.R.; Dinu, M.; Iacob, R.; Iacob, D. Risk Factors Associated with Retinopathy of Prematurity in Very and Extremely Preterm Infants. Medicina 2021, 57, 420. [Google Scholar] [CrossRef]
- Rosu, L.M.; Prodan-Bărbulescu, C.; Maghiari, A.L.; Bernad, E.S.; Bernad, R.L.; Iacob, R.; Stoicescu, E.R.; Borozan, F.; Ghenciu, L.A. Current Trends in Diagnosis and Treatment Approach of Diabetic Retinopathy during Pregnancy: A Narrative Review. Diagnostics 2024, 14, 369. [Google Scholar] [CrossRef]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for Diabetic Retinopathy: New Perspectives and Challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- Sharma, A.; Jaganathan, B.G. Stem Cell Therapy for Retinal Degeneration: The Evidence to Date. Biol. Targets Ther. 2021, 15, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, K.H. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Annu. Rev. Med. 1975, 26, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, J.H. A Thirty Year Journey in the Polyol Pathway. Exp. Eye Res. 1990, 50, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, P.R.; Balko, C.; Gabbay, K.H. The Sorbitol Pathway and the Complications of Diabetes. N. Engl. J. Med. 1973, 288, 831–836. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Valle, M.L.; Beveridge, C.; Liu, Y.; Sharma, S. Unraveling the Role of Genetics in the Pathogenesis of Diabetic Retinopathy. Eye 2019, 33, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Abikoye, T.M.; Oluleye, T.S.; Aribaba, O.T.; Musa, K.O.; Idowu, O.O.; Onakoya, A.O. Is Primary Open-Angle Glaucoma a Risk Factor for Diabetic Retinopathy? Int. Ophthalmol. 2020, 40, 3233–3240. [Google Scholar] [CrossRef]
- Buse, J.B. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: Design and Methods. Am. J. Cardiol. 2007, 99, S21–S33. [Google Scholar] [CrossRef]
- Klein, R.; Knudtson, M.D.; Lee, K.E.; Gangnon, R.; Klein, B.E.K. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. Ophthalmology 2008, 115, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, Y.; Yang, W.; Gao, X.; Han, X.; Ji, L. The Association of Smoking and Risk of Diabetic Retinopathy in Patients with Type 1 and Type 2 Diabetes: A Meta-Analysis. Endocrine 2018, 62, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.Z.; Gao, X.; Guo, X.; Taylor, K.D.; Klein, R.; Klein, B.E.; Ipp, E.; Chen, Y.-D.I.; Varma, R.; Rotter, J.I. A Candidate-Wide Association Study of Diabetic Retinopathy in Latinos Implicates TNF Receptor Pathophysiology: Genetics of Latino Diabetic Retinopathy (GOLDR) and Los Angeles Latino Eye (LALES) studies. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4509. [Google Scholar]
- Mathebula, S.D. Biochemical Changes in Diabetic Retinopathy Triggered by Hyperglycaemia: A Review. Afr. Vis. Eye Health 2018, 77, a439. [Google Scholar] [CrossRef]
- Shukla, U.V.; Tripathy, K. Diabetic Retinopathy; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pan, D.; Xu, L.; Guo, M. The Role of Protein Kinase C in Diabetic Microvascular Complications. Front. Endocrinol. 2022, 13, 973058. [Google Scholar] [CrossRef] [PubMed]
- Rübsam, A.; Parikh, S.; Fort, P. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.N.; Keirn, R.J.; Kennedy, A.; Frank, K.W. Galactose-Induced Retinal Capillary Basement Membrane Thickening: Prevention by Sorbinil. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1519–1524. [Google Scholar]
- Chakrabarti, S.; Sima, A.A.F.; Nakajima, T.; Yagihashi, S.; Greene, D.A. Aldose Reductase in the BB Rat: Isolation, Immunological Identification and Localization in the Retina and Peripheral Nerve. Diabetologia 1987, 30, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hohman, T.C.; Nishimura, C.; Robison, W.G. Aldose Reductase and Polyol in Cultured Pericytes of Human Retinal Capillaries. Exp. Eye Res. 1989, 48, 55–60. [Google Scholar] [CrossRef]
- Dagher, Z.; Park, Y.S.; Asnaghi, V.; Hoehn, T.; Gerhardinger, C.; Lorenzi, M. Studies of Rat and Human Retinas Predict a Role for the Polyol Pathway in Human Diabetic Retinopathy. Diabetes 2004, 53, 2404–2411. [Google Scholar] [CrossRef]
- Cheung, A.K.H.; Fung, M.K.L.; Lo, A.C.Y.; Lam, T.T.L.; So, K.F.; Chung, S.S.M.; Chung, S.K. Aldose Reductase Deficiency Prevents Diabetes-Induced Blood-Retinal Barrier Breakdown, Apoptosis, and Glial Reactivation in the Retina of Db/Db Mice. Diabetes 2005, 54, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Nakamura, J.; Hamada, Y.; Naruse, K.; Nakashima, E.; Kato, K.; Kasuya, Y.; Yasuda, Y.; Kamiya, H.; Hotta, N. The Role of Polyol Pathway in Glucose-Induced Apoptosis of Cultured Retinal Pericytes. Diabetes Res. Clin. Pract. 2003, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Berrone, E.; Buttiglieri, S.; Porta, M. Thiamine and Benfotiamine Prevent Increased Apoptosis in Endothelial Cells and Pericytes Cultured in High Glucose. Diabetes Metab. Res. 2004, 20, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Matsubara, A.; Taniguchi, K.; Ogura, Y. Aldose Reductase Inhibitor Fidarestat Attenuates Leukocyte-Endothelial Interactions in Experimental Diabetic Rat Retina In Vivo. Curr. Eye Res. 2010, 35, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Yan, S.D.; Wautier, J.-L.; Stern, D. Activation of Receptor for Advanced Glycation End Products: A Mechanism for Chronic Vascular Dysfunction in Diabetic Vasculopathy and Atherosclerosis. Circ. Res. 1999, 84, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Oshitari, T. Advanced Glycation End-Products and Diabetic Neuropathy of the Retina. Int. J. Mol. Sci. 2023, 24, 2927. [Google Scholar] [CrossRef] [PubMed]
- Strieder-Barboza, C.; Baker, N.A.; Flesher, C.G.; Karmakar, M.; Neeley, C.K.; Polsinelli, D.; Dimick, J.B.; Finks, J.F.; Ghaferi, A.A.; Varban, O.A.; et al. Advanced Glycation End-Products Regulate Extracellular Matrix-Adipocyte Metabolic Crosstalk in Diabetes. Sci. Rep. 2019, 9, 19748. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Dai, H.; Jiang, S.; Yu, L. Advanced Glycation End Products in Diabetic Retinopathy and Phytochemical Therapy. Front. Nutr. 2022, 9, 1037186. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Saxena, S.; Mishra, A.; Saxena, A.; Natu, S.M. Advanced Glycation End Products and Diabetic Retinopathy. J. Ocul. Biol. Dis. Inform. 2012, 5, 63–69. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Yue, T.; Shi, Y.; Luo, S.; Weng, J.; Wu, Y.; Zheng, X. The Role of Inflammation in Immune System of Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Front. Immunol. 2022, 13, 1055087. [Google Scholar] [CrossRef]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic Retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.-S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 043603. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Saxena, S.; Khanna, V.K.; Shukla, R.K.; Meyer, C.H. Status of Serum VEGF and ICAM-1 and Its Association with External Limiting Membrane and Inner Segment-Outer Segment Junction Disruption in Type 2 Diabetes Mellitus. Mol. Vis. 2013, 19, 1760–1768. [Google Scholar] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- Wermuth, B.; Bürgisser, H.; Bohren, K.; Von Wartburg, J. Purification and Characterization of Human-Brain Aldose Reductase. Eur. J. Biochem. 1982, 127, 279–284. [Google Scholar] [CrossRef]
- Ward, W.H.J.; Cook, P.N.; Mirrlees, D.J.; Brittain, D.R.; Preston, J.; Carey, F.; Tuffin, D.P.; Howe, R. (2,6-Dimethylphenylsulphonyl)Nitromethane: A New Structural Type of Aldose Reductase Inhibitor Which Follows Biphasic Kinetics and Uses an Allosteric Binding Site. Biochem. Pharmacol. 1991, 42, 2115–2123. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Mei, Z.; Zhao, F.; Xiang, Y. Polymorphisms in Sorbitol-Aldose Reductase (Polyol) Pathway Genes and Their Influence on Risk of Diabetic Retinopathy Among Han Chinese. Med. Sci. Monit. 2019, 25, 7073–7078. [Google Scholar] [CrossRef]
- Singh Grewal, A.; Bhardwaj, S.; Pandita, D.; Lather, V.; Singh Sekhon, B. Updates on Aldose Reductase Inhibitors for Management of Diabetic Complications and Non-Diabetic Diseases. Mini-Rev. Med. Chem. 2015, 16, 120–162. [Google Scholar] [CrossRef]
- Patel, D.; Kumar, R.; Kumar, M.; Sairam, K.; Hemalatha, S. Evaluation of in Vitro Aldose Reductase Inhibitory Potential of Different Fraction of Hybanthus enneaspermus Linn F. Muell. Asian Pac. J. Trop. Biomed. 2012, 2, 134–139. [Google Scholar] [CrossRef]
- Rao, M.; Huang, Y.-K.; Liu, C.-C.; Meadows, C.; Cheng, H.-C.; Zhou, M.; Chen, Y.-C.; Xia, X.; Goldberg, J.L.; Williams, A.M.; et al. Aldose Reductase Inhibition Decelerates Optic Nerve Degeneration by Alleviating Retinal Microglia Activation. Sci. Rep. 2023, 13, 5592. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Shieh, B.; Petrash, J.M. Influence of Aldose Reductase on Epithelial-to-Mesenchymal Transition Signaling in Lens Epithelial Cells. Chem.-Biol. Interact. 2017, 276, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.; Satyanarayana, A.; Balakrishna, N.; Ayyagari, R.; Padma, M.; Viswanath, K.; Petrash, J.M. Erythrocyte Aldose Reductase Activity and Sorbitol Levels in Diabetic Retinopathy. Mol. Vis. 2008, 14, 593–601. [Google Scholar] [PubMed]
- Gerhardinger, C.; Dagher, Z.; Sebastiani, P.; Park, Y.S.; Lorenzi, M. The Transforming Growth Factor-β Pathway Is a Common Target of Drugs That Prevent Experimental Diabetic Retinopathy. Diabetes 2009, 58, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, S.R.; Magar, L.B.; Thapa, R.; Joshi, S.; Khadayat, K.; Marasini, B.P.; Parajuli, N. Biochemical Analysis and Human Aldose Reductase Inhibition Activity of Selected Medicinal Plants of Nepal. J. Chem. 2023, 2023, 9614164. [Google Scholar] [CrossRef]
- Henry, D.N.; Frank, R.N.; Hootman, S.R.; Rood, S.E.; Heilig, C.W.; Busik, J.V. Glucose-Specific Regulation of Aldose Reductase in Human Retinal Pigment Epithelial Cells in Vitro. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1554–1560. [Google Scholar]
- Sato, S.; Lin, L.-R.; Reddy, V.N.; Kador, P.F. Aldose Reductase in Human Retinal Pigment Epithelial Cells. Exp. Eye Res. 1993, 57, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ghenciu, L.A.; Faur, A.C.; Bolintineanu, S.L.; Salavat, M.C.; Maghiari, A.L. Recent Advances in Diagnosis and Treatment Approaches in Fungal Keratitis: A Narrative Review. Microorganisms 2024, 12, 161. [Google Scholar] [CrossRef]
- Petrash, J.; Huang, S.-P. Elevation of Aldose Reductase in the Diabetic Eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2029. [Google Scholar]
- Wang, Y.; Yang, X.; Zhang, Y.; Hong, L.; Xie, Z.; Jiang, W.; Chen, L.; Xiong, K.; Yang, S.; Lin, M.; et al. Single-Cell RNA Sequencing Reveals Roles of Unique Retinal Microglia Types in Early Diabetic Retinopathy. Diabetol. Metab. Syndr. 2024, 16, 49. [Google Scholar] [CrossRef]
- Nishimura, C.; Hamada, Y.; Tachikawa, T.; Ishikawa, T.; Gui, T.; Tsubouchi, J.; Hotta, N.; Tanimoto, T.; Urakami, T. Enzyme Immunoassay for Erythrocyte Aldose Reductase. Clin. Chem. 1994, 40, 889–894. [Google Scholar] [CrossRef]
- Tang, J.; Du, Y.; Petrash, J.M.; Sheibani, N.; Kern, T.S. Deletion of Aldose Reductase from Mice Inhibits Diabetes-Induced Retinal Capillary Degeneration and Superoxide Generation. PLoS ONE 2013, 8, e62081. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Friedrich, B.; Srivastava, S.; Bhatnagar, A.; Srivastava, S.K. Activation of Nulcear Factor-κB by Hyperglycemia in Vascular Smooth Muscle Cells Is Regulated by Aldose Reductase. Diabetes 2004, 53, 2910–2920. [Google Scholar] [CrossRef]
- Kakehashi, A. Aldose Reductase Inhibitor Fidarestat Prevents Diabetic Ocular Complications in Spontaneously Diabetic Torii Rats. Open Diabetes J. 2011, 4, 101–107. [Google Scholar] [CrossRef]
- Julius, A.; Renuka, R.R.; Hopper, W.; Babu Raghu, P.; Rajendran, S.; Srinivasan, S.; Dharmalingam, K.; Alanazi, A.M.; Arokiyaraj, S.; Prasath, S. Inhibition of Aldose Reductase by Novel Phytocompounds: A Heuristic Approach to Treating Diabetic Retinopathy. Evid.-Based Complement. Altern. Med. 2022, 2022, 9624118. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Kato, N. Aldose Reductase Inhibitors. J. Enzym. Inhib. 2001, 16, 465–473. [Google Scholar] [CrossRef]
- Sun, W.; Oates, P.J.; Coutcher, J.B.; Gerhardinger, C.; Lorenzi, M. A Selective Aldose Reductase Inhibitor of a New Structural Class Prevents or Reverses Early Retinal Abnormalities in Experimental Diabetic Retinopathy. Diabetes 2006, 55, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S. Novel Drugs and Their Targets in the Potential Treatment of Diabetic Retinopathy. Med. Sci. Monit. 2013, 19, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Yashima, S.; Suzuki, T.; Nakayama, Y.; Jomori, T. Long-Term Treatment with Fidarestat Suppresses the Development of Diabetic Retinopathy in STZ-Induced Diabetic Rats. J. Diabetes Its Complicat. 2003, 17, 374–379. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Minchenko, A.G.; Vasupuram, R.; White, L.; Abatan, O.I.; Kumagai, A.K.; Frank, R.N.; Stevens, M.J. Aldose Reductase Inhibitor Fidarestat Prevents Retinal Oxidative Stress and Vascular Endothelial Growth Factor Overexpression in Streptozotocin-Diabetic Rats. Diabetes 2003, 52, 864–871. [Google Scholar] [CrossRef]
- Cusick, M. Effects of Aldose Reductase Inhibitors and Galactose Withdrawal on Fluorescein Angiographic Lesions in Galactose-Fed Dogs. Arch. Ophthalmol. 2003, 121, 1745. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, K.J.; Brinkmann, E.; Wells-Knecht, M.C.; Litchfield, J.E.; Ahmed, M.U.; Reddy, S.; Zyzak, D.V.; Thorpe, S.R.; Baynes, J.W. New Biomarkers of Maillard Reaction Damage to Proteins. Nephrol. Dial. Transplant. 1996, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Chen, Y.; Hu, Y.; Mayo, A.S.; Kompella, U.B.; Longeras, R.; Ma, J. Nanoparticle-Mediated Expression of an Angiogenic Inhibitor Ameliorates Ischemia-Induced Retinal Neovascularization and Diabetes-Induced Retinal Vascular Leakage. Diabetes 2009, 58, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, J.H.; Jun, H.-O.; Yu, Y.S.; Kim, K.-W. Inhibition of Protein Kinase C δ Attenuates Blood-Retinal Barrier Breakdown in Diabetic Retinopathy. Am. J. Pathol. 2010, 176, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.; Rechthand, E.; Terubayashi, H.; Palestine, A.; Rapoport, S.; Kador, P. Permeability Changes in Blood-Retinal Barrier of Galactosemic Rats Are Prevented by Aldose Reductase Inhibitors. Diabetes 1987, 36, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Oates, P.J. Aldose Reductase, Still a Compelling Target for Diabetic Neuropathy. Curr. Drug Targets 2008, 9, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.G.; Mota, C.C.; Leite, E.C.; Abreu, J.R.; Ruas, M.A. Effect of Sorbinil on Blood-Retinal Barrier in Early Diabetic Retinopathy. Diabetes 1986, 35, 574–578. [Google Scholar] [CrossRef]
- Kakehashi, A.; Ota, A.; Toyoda, F.; Kinoshita, N.; Yamagami, H.; Obata, H.; Matsumoto, T.; Tsuji, J.; Dobashi, Y.; Fujimoto, W.Y.; et al. Prophylactic Medical Treatment of Diabetic Retinopathy. In Diabetic Retinopathy; Ola, M.S., Ed.; InTech: London, UK, 2012; ISBN 9789535100447. [Google Scholar]
- Ota, A.; Kakehashi, A.; Toyoda, F.; Kinoshita, N.; Shinmura, M.; Takano, H.; Obata, H.; Matsumoto, T.; Tsuji, J.; Dobashi, Y.; et al. Effects of Long-Term Treatment with Ranirestat, a Potent Aldose Reductase Inhibitor, on Diabetic Cataract and Neuropathy in Spontaneously Diabetic Torii Rats. J. Diabetes Res. 2013, 2013, 175901. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Zheng, G.; Alshabrmi, F.M.; Bourhia, M.; Wondmie, G.F.; Mohammad Salamatullah, A. Exploring Potent Aldose Reductase Inhibitors for Anti-Diabetic (Anti-Hyperglycemic) Therapy: Integrating Structure-Based Drug Design, and MMGBSA Approaches. Front. Mol. Biosci. 2023, 10, 1271569. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, F.; Tanaka, Y.; Ota, A.; Shimmura, M.; Kinoshita, N.; Takano, H.; Matsumoto, T.; Tsuji, J.; Kakehashi, A. Effect of Ranirestat, a New Aldose Reductase Inhibitor, on Diabetic Retinopathy in SDT Rats. J. Diabetes Res. 2014, 2014, 672590. [Google Scholar] [CrossRef]
- Hotta, N.; Akanuma, Y.; Kawamori, R.; Matsuoka, K.; Oka, Y.; Shichiri, M.; Toyota, T.; Nakashima, M.; Yoshimura, I.; Sakamoto, N.; et al. Long-Term Clinical Effects of Epalrestat, an Aldose Reductase Inhibitor, on Diabetic Peripheral Neuropathy. Diabetes Care 2006, 29, 1538–1544. [Google Scholar] [CrossRef]
- Kador, P.F.; Wyman, M.; Oates, P.J. Aldose Reductase, Ocular Diabetic Complications and the Development of Topical Kinostat®. Prog. Retin. Eye Res. 2016, 54, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lupo, G.; Cambria, M.T.; Olivieri, M.; Rocco, C.; Caporarello, N.; Longo, A.; Zanghì, G.; Salmeri, M.; Foti, M.C.; Anfuso, C.D. Anti-angiogenic Effect of Quercetin and Its 8-methyl Pentamethyl Ether Derivative in Human Microvascular Endothelial Cells. J. Cell. Mol. Med. 2019, 23, 6565–6577. [Google Scholar] [CrossRef]
- Dodda, D.; Ciddi, V. Plants Used in the Management of Diabetic Complications. Indian J. Pharm. Sci. 2014, 76, 97–106. [Google Scholar] [PubMed]
- Halim, E.M.; Mukhopadhyay, A.K. Effect of Ocimum sanctum (Tulsi) and Vitamin E on Biochemical Parameters and Retinopathy in Streptozotocin Induced Diabetic Rats. Indian J. Clin. Biochem. 2006, 21, 181–188. [Google Scholar] [CrossRef]
- Chai, G.-R.; Liu, S.; Yang, H.-W.; Chen, X.-L. Quercetin Protects against Diabetic Retinopathy in Rats by Inducing Heme Oxygenase-1 Expression. Neural Regen. Res. 2021, 16, 1344. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin Prevents Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Molecules 2012, 17, 12868–12881. [Google Scholar] [CrossRef]
- Dongare, S.H.; Rajendran, S.H.; Senthilkumari, S.; Gupta, S.K.; Mathur, R.; Saxena, R.; Srivastava, R. Genistein alleviates high glucose induced toxicity and angiogenesis in cultured human RPE cells. Int. J. Pharm. Pharm. Sci. 2015, 7, 294–298. [Google Scholar]
- Varma, S.D.; Shocket, S.S.; Richards, R.D. Implications of aldose reductase in cataracts in human diabetes. Investig. Ophthalmol. Vis. Sci. 1979, 18, 237–241. [Google Scholar]
- Stefek, M. Natural Flavonoids as Potential Multifunctional Agents in Prevention of Diabetic Cataract. Interdiscip. Toxicol. 2011, 4, 69–77. [Google Scholar] [CrossRef]
- Kumar, M.P.; Sankeshi, V.; Naik, R.R.; Thirupathi, P.; Das, B.; Raju, T.N. The Inhibitory Effect of Isoflavones Isolated from Caesalpinia Pulcherrima on Aldose Reductase in STZ Induced Diabetic Rats. Chem.-Biol. Interact. 2015, 237, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Simó, R. Neurodegeneration in Diabetic Retinopathy: Current Concepts and Therapeutic Implications. Av. Diabetol. 2014, 30, 72–79. [Google Scholar] [CrossRef]
- Sun, W.; Gerhardinger, C.; Dagher, Z.; Hoehn, T.; Lorenzi, M. Aspirin at Low-Intermediate Concentrations Protects Retinal Vessels in Experimental Diabetic Retinopathy through Non–Platelet-Mediated Effects. Diabetes 2005, 54, 3418–3426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Howell, S.J.; Hatala, D.A.; Huang, K.; Kern, T.S. Salicylate-Based Anti-Inflammatory Drugs Inhibit the Early Lesion of Diabetic Retinopathy. Diabetes 2007, 56, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Miller, C.M.; Du, Y.; Zheng, L.; Mohr, S.; Ball, S.L.; Kim, M.; Jamison, J.A.; Bingaman, D.P. Topical Administration of Nepafenac Inhibits Diabetes-Induced Retinal Microvascular Disease and Underlying Abnormalities of Retinal Metabolism and Physiology. Diabetes 2007, 56, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.A.; Bakri, S.J. Update on the Management of Diabetic Retinopathy: Anti-VEGF Agents for the Prevention of Complications and Progression of Nonproliferative and Proliferative Retinopathy. Life 2023, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Abhary, S.; Hewitt, A.W.; Burdon, K.P.; Craig, J.E. A Systematic Meta-Analysis of Genetic Association Studies for Diabetic Retinopathy. Diabetes 2009, 58, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Moravski, C.J.; Skinner, S.L.; Stubbs, A.J.; Sarlos, S.; Kelly, D.J.; Cooper, M.E.; Gilbert, R.E.; Wilkinson-Berka, J.L. The Renin-Angiotensin System Influences Ocular Endothelial Cell Proliferation in Diabetes. Am. J. Pathol. 2003, 162, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, N.; Porta, M.; Klein, R.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Sjølie, A.K. Effect of Candesartan on Prevention (DIRECT-Prevent 1) and Progression (DIRECT-Protect 1) of Retinopathy in Type 1 Diabetes: Randomised, Placebo-Controlled Trials. Lancet 2008, 372, 1394–1402. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Grigoropoulos, V.; Emfietzoglou, I.; Theodossiadis, G.; Tentolouris, N.; Delicha, E.; Katsiari, C.; Alexiadou, K.; Hatziagelaki, E.; Theodossiadis, P.G. Infliximab for Diabetic Macular Edema Refractory to Laser Photocoagulation. Diabetes Care 2010, 33, 1523–1528. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Sophie, R.; Tolentino, M.; Miller, D.M.; Browning, D.; Boyer, D.S.; Heier, J.S.; Gambino, L.; Withers, B.; Brigell, M.; et al. Treatment of Diabetic Macular Edema with an Inhibitor of Vascular Endothelial-Protein Tyrosine Phosphatase That Activates Tie2. Ophthalmology 2015, 122, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Sato, S.; Jimenez, J.; McGowan, M.; Moroni, M.; Dey, A.; Ibaraki, N.; Reddy, V.N.; Carper, D. Osmotic Response Element Is Required for the Induction of Aldose Reductase by Tumor Necrosis Factor-α. J. Biol. Chem. 1999, 274, 7993–8001. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.C.; Neu, M.B.; Grant, M.B. Cell-Based Therapies for Diabetic Retinopathy. Curr. Diabetes Rep. 2011, 11, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Guerin, E.P.; Jarajapu, Y.P.R.; Caballero, S.; Sheridan, C.; Kent, D.; Kennedy, L.; Lansang, M.C.; Ruscetti, F.W.; Pepine, C.J.; et al. Transient Inhibition of Transforming Growth Factor-Β1 in Human Diabetic CD34+ Cells Enhances Vascular Reparative Functions. Diabetes 2010, 59, 2010–2019. [Google Scholar] [CrossRef]
- Demaine, A.G. Polymorphisms of the Aldose Reductase Gene and Susceptibility to Diabetic Microvascular Complications. Curr. Med. Chem. 2003, 10, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Ereqat, S.; Abdelhafez, M.; Iriqat, S.; Ghaleb, Q.; Abu Shams, A.; Abd Aldayem, O.; Ghattas, M.; Nasereddin, A. Aldose Reductase (−106) C/T Gene Polymorphism and Associated Risk Factors with Proliferative Diabetic Retinopathy in Palestine: A Cross Sectional Study. Health Sci. Rep. 2023, 6, e1605. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A. Aldose Reductase in the Retina. Curr. Enzym. Inhib. 2007, 3, 49–60. [Google Scholar] [CrossRef]
- Costantino, L.; Rastelli, G.; Cignarella, G.; Vianello, P.; Barlocco, D. New Aldose Reductase Inhibitors as Potential Agents for the Prevention of Long-Term Diabetic Complications. Expert Opin. Ther. Pat. 1997, 7, 843–858. [Google Scholar] [CrossRef]
- Anjum, A.; Sreeja, J.; Swapna, Y.; Bolleddu, R.; Venkatesh, S. Dietary Aldose Reductase Inhibitors and Prevention of Diabetic Complications. Indian. J. Health Sci. Biomed. Res. 2021, 14, 194. [Google Scholar] [CrossRef]
- Donaldson, M.; Dodson, P.M. Medical Treatment of Diabetic Retinopathy. Eye 2003, 17, 550–562. [Google Scholar] [CrossRef]
- Balestri, F.; Moschini, R.; Mura, U.; Cappiello, M.; Del Corso, A. In Search of Differential Inhibitors of Aldose Reductase. Biomolecules 2022, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Jannapureddy, S.; Sharma, M.; Yepuri, G.; Schmidt, A.M.; Ramasamy, R. Aldose Reductase: An Emerging Target for Development of Interventions for Diabetic Cardiovascular. Front. Endocrinol. 2021, 12, 636267. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gu, Q.; Zheng, X.; Ye, J.; Liu, Z.; Li, J.; Hu, X.; Hagler, A.; Xu, J. Discovery of New Selective Human Aldose Reductase Inhibitors through Virtual Screening Multiple Binding Pocket Conformations. J. Chem. Inf. Model. 2013, 53, 2409–2422. [Google Scholar] [CrossRef] [PubMed]

| Risk Factor | Description |
|---|---|
| Duration of diabetes [17] | well-known risk factor for the development and progression of DR |
| Glycemic control [16,17] | poor glycemic control (high HbA1C) is linked to increased risk of DR |
| Intraocular/systemic blood pressure [16,19] | systemic hypertension might lead to progression of DR through increased vascular permeability and microvascular damage |
| Dyslipidemia [20] | elevated levels of triglycerides, low-density lipoprotein cholesterol (LDL-C), and reduced levels of high-density lipoprotein cholesterol (HDL-C) managed through diet modification and lipid-lowering drugs |
| Obesity [21] | elevated body mass index (BMI) and abdominal adiposity leads to the release of cytokines, adipokines and to insulin-resistance (which is thought to be an independent risk factor) weight management is essential |
| Cigarette smoking [22] | a modifiable risk factor can compromise retinal microvascular integrity and function |
| Genetic predisposition [23] | an important number of genetic variants have been studied can be managed by targeted therapy in high-risk patients |
| Decreased production of sorbitol and fructose, which reduces oxidative stress and preserves the antioxidant defense system within the retina |
| Protection against neuronal apoptosis, glial reactivity, and complement deposition, all of which contribute to retinal damage |
| Reduction in microaneurysms, basement membrane thickness, and vascular permeability |
| Blockage of the expression of various pro-inflammatory cytokines and growth factors |
| Potential prevention of initial focal laser therapy, especially in cases of DME |
| Prevention of cataract development |
| Promising outcomes with novel ARIs, which demonstrate higher selectivity and fewer side effects compared to earlier generations of ARIs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dănilă, A.-I.; Ghenciu, L.A.; Stoicescu, E.R.; Bolintineanu, S.L.; Iacob, R.; Săndesc, M.-A.; Faur, A.C. Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review. Biomedicines 2024, 12, 747. https://doi.org/10.3390/biomedicines12040747
Dănilă A-I, Ghenciu LA, Stoicescu ER, Bolintineanu SL, Iacob R, Săndesc M-A, Faur AC. Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review. Biomedicines. 2024; 12(4):747. https://doi.org/10.3390/biomedicines12040747
Chicago/Turabian StyleDănilă, Alexandra-Ioana, Laura Andreea Ghenciu, Emil Robert Stoicescu, Sorin Lucian Bolintineanu, Roxana Iacob, Mihai-Alexandru Săndesc, and Alexandra Corina Faur. 2024. "Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review" Biomedicines 12, no. 4: 747. https://doi.org/10.3390/biomedicines12040747






