Trace Amine-Associated Receptors’ Role in Immune System Functions
Abstract
:1. Introduction
2. The History of TAARs’ Discovery
3. Expression Profile of TAARs and Immune Function
4. Immune Function of TAARs in Lymphoid Cells
4.1. B-Lymphocytes
4.2. T-Lymphocytes
4.3. NK Cells
5. Immune Function of TAARs in Myeloid Cells
5.1. Monocytes and Macrophages
5.2. Polymorphonuclear Leukocytes
5.3. Microglia
6. Immunity Pathophysiology of TAARs
| Immunological Role | Receptor | Expression | Biological Function | References |
|---|---|---|---|---|
| Antibacterial immunity | TAAR1 | - | The TAAR1 agonist tyramine intensifies the adhesion and invasion of E. durans in the human large intestine epithelium. | [59] |
| TAAR8 | Astrocytes | TAAR8 transcription in astroglial cells intensifies after the effect of lipopolysaccharide. | [57] | |
| TAAR1 TAAR2 | Granulocytes | The effect of TAAR agonists stimulates the chemosensory migration of polymorphonuclear leukocytes. | [34] | |
| Antiviral immunity | TAAR1 | Peripheral mononuclear blood cells (PBMC). | HIV1 infection activates TAAR1 in PBMCs, the activation is intensified during the preliminary effect of amphetamine. | [38] |
| Bronchial asthma | TAAR6 | - | The presence of single-nucleotide polymorphisms of the TAAR6 gene affects the results of treating bronchial asthma patients. | [42] |
| Fibromyalgia | TAAR1 | - | TAAR1 gene polymorphism may be interlinked to the risk of developing fibromyalgia. | [62] |
| Inflammatory bowel diseases | TAAR2 TAAR5 TAAR9 | Large intestine epitheliocytes | Elevated TAAR expression was found in the large intestine wall cells of patients with Crohn’s disease. | [58,60,61] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef]
- Karovicová, J.; Kohajdová, Z.; Simko, P.; Lukácová, D. Using capillary isotachophoresis for the determination of biogenic amines and D-isocitric acid in food products. Nahrung 2003, 47, 188–190. [Google Scholar] [CrossRef]
- Wimbiscus, M.; Kostenko, O.; Malone, D. MAO inhibitors: Risks, benefits, and lore. Clevel. Clin. J. Med. 2010, 77, 859–882. [Google Scholar] [CrossRef]
- Berry, M.D.; Gainetdinov, R.R.; Hoener, M.C.; Shahid, M. Pharmacology of Human Trace Amine-Associated Receptors: Therapeutic Opportunities and Challenges. Pharmacol. Ther. 2017, 180, 161–180. [Google Scholar] [CrossRef]
- Grandy, D.K. Trace amine-associated receptor 1-Family archetype or iconoclast. Pharmacol. Ther. 2007, 116, 355–390. [Google Scholar] [CrossRef]
- Berry, M.D. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev. Recent Clin. Trials 2007, 2, 3–19. [Google Scholar] [CrossRef]
- Espinoza, S.; Sukhanov, I.; Efimova, E.V.; Kozlova, A.; Antonova, K.A.; Illiano, P.; Leo, D.; Merkulyeva, N.; Kalinina, D.; Musienko, P.; et al. Trace Amine-Associated Receptor 5 Provides Olfactory Input Into Limbic Brain Areas and Modulates Emotional Behaviors and Serotonin Transmission. Front. Mol. Neurosci. 2020, 13, 18. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kozlova, A.A.; Razenkova, V.; Katolikova, N.V.; Antonova, K.A.; Sotnikova, T.D.; Merkulyeva, N.S.; Veshchitskii, A.S.; Kalinina, D.S.; Korzhevskii, D.E.; et al. Increased dopamine transmission and adult neurogenesis in trace amine-associated receptor 5 (TAAR5) knockout mice. Neuropharmacology 2021, 182, 108373. [Google Scholar] [CrossRef]
- Efimova, E.V.; Kuvarzin, S.R.; Mor, M.S.; Katolikova, N.V.; Shemiakova, T.S.; Razenkova, V.; Ptukha, M.; Kozlova, A.A.; Murtazina, R.Z.; Smirnova, D.; et al. Trace Amine-Associated Receptor 2 Is Expressed in the Limbic Brain Areas and Is Involved in Dopamine Regulation and Adult Neurogenesis. Front. Behav. Neurosci. 2022, 16, 847410. [Google Scholar] [CrossRef]
- Vaganova, A.N.; Shemyakova, T.S.; Lenskaia, K.V.; Rodionov, R.N.; Steenblock, C.; Gainetdinov, R.R. Trace Amine-Associated Receptors and Monoamine-Mediated Regulation of Insulin Secretion in Pancreatic Islets. Biomolecules 2023, 13, 1618. [Google Scholar] [CrossRef]
- Nelson, D.A.; Tolbert, M.D.; Singh, S.J.; Bost, K.L. Expression of neuronal trace amine-associated receptor (Taar) mRNAs in leukocytes. J. Neuroimmunol. 2007, 192, 21–30. [Google Scholar] [CrossRef]
- Fleischer, L.M.; Somaiya, R.D.; Miller, G.M. Review and Meta-Analyses of TAAR1 Expression in the Immune System and Cancers. Front. Pharmacol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Vogelsang, T.L.R.; Vattai, A.; Schmoeckel, E.; Kaltofen, T.; Chelariu-Raicu, A.; Zheng, M.; Mahner, S.; Mayr, D.; Jeschke, U.; Trillsch, F. Trace Amine-Associated Receptor 1 (TAAR1) Is a Positive Prognosticator for Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 8479. [Google Scholar] [CrossRef]
- Vaganova, A.N.; Maslennikova, D.D.; Konstantinova, V.V.; Kanov, E.V.; Gainetdinov, R.R. The Expression of Trace Amine-Associated Receptors (TAARs) in Breast Cancer Is Coincident with the Expression of Neuroactive Ligand-Receptor Systems and Depends on Tumor Intrinsic Subtype. Biomolecules 2023, 13, 1361. [Google Scholar] [CrossRef]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef]
- Lindemann, L.; Ebeling, M.; Kratochwil, N.A.; Bunzow, J.R.; Grandy, D.K.; Hoener, M.C. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 2005, 85, 372–385. [Google Scholar] [CrossRef]
- Eyun, S.I.; Moriyama, H.; Hoffmann, F.G.; Moriyama, E.N. Molecular Evolution and Functional Divergence of Trace Amine-Associated Receptors. PLoS ONE 2016, 11, e0151023. [Google Scholar] [CrossRef]
- Gloriam, D.E.; Bjarnadóttir, T.K.; Schiöth, H.B.; Fredriksson, R. High species variation within the repertoire of trace amine receptors. Ann. N. Y. Acad. Sci. 2005, 1040, 323–327. [Google Scholar] [CrossRef]
- Lindemann, L.; Meyer, C.A.; Jeanneau, K.; Bradaia, A.; Ozmen, L.; Bluethmann, H.; Bettler, B.; Wettstein, J.G.; Borroni, E.; Moreau, J.L.; et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J. Pharmacol. Exp. Ther. 2008, 324, 948–956. [Google Scholar] [CrossRef]
- Dinter, J.; Mühlhaus, J.; Wienchol, C.L.; Yi, C.X.; Nürnberg, D.; Morin, S.; Grüters, A.; Köhrle, J.; Schöneberg, T.; Tschöp, M.; et al. Inverse agonistic action of 3-iodothyronamine at the human trace amine-associated receptor 5. PLoS ONE 2015, 10, e0117774. [Google Scholar] [CrossRef]
- Espinoza, S.; Lignani, G.; Caffino, L.; Maggi, S.; Sukhanov, I.; Leo, D.; Mus, L.; Emanuele, M.; Ronzitti, G.; Harmeier, A.; et al. TAAR1 Modulates Cortical Glutamate NMDA Receptor Function. Neuropsychopharmacology 2015, 40, 2217–2227. [Google Scholar] [CrossRef]
- Liu, J.F.; Seaman, R.; Siemian, J.N.; Bhimani, R.; Johnson, B.; Zhang, Y.; Zhu, Q.; Hoener, M.C.; Park, J.; Dietz, D.M.; et al. Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects. Neuropsychopharmacology 2018, 43, 2435–2444. [Google Scholar] [CrossRef]
- Ferragud, A.; Howell, A.D.; Moore, C.F.; Ta, T.L.; Hoener, M.C.; Sabino, V.; Cottone, P. The Trace Amine-Associated Receptor 1 Agonist RO5256390 Blocks Compulsive, Binge-like Eating in Rats. Neuropsychopharmacology 2017, 42, 1458–1470. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Bugda Gwilt, K.; González, D.; Olliffe, N.; Oller, H.; Hoffing, R.; Puzan, M.; El Aidy, S.; Miller, G. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell. Mol. Neurobiol. 2020, 40, 191–201. [Google Scholar] [CrossRef]
- Christian, S.L.; Berry, M.D. Trace Amine-Associated Receptors as Novel Therapeutic Targets for Immunomodulatory Disorders. Front. Pharmacol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Simmler, L.D.; Buchy, D.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1. J. Pharmacol. Exp. Ther. 2016, 357, 134–144. [Google Scholar] [CrossRef]
- Hu, L.A.; Zhou, T.; Ahn, J.; Wang, S.; Zhou, J.; Hu, Y.; Liu, Q. Human and mouse trace amine-associated receptor 1 have distinct pharmacology towards endogenous monoamines and imidazoline receptor ligands. Biochem. J. 2009, 424, 39–45. [Google Scholar] [CrossRef]
- Sukhanov, I.; Espinoza, S.; Yakovlev, D.S.; Hoener, M.C.; Sotnikova, T.D.; Gainetdinov, R.R. TAAR1-dependent effects of apomorphine in mice. Int. J. Neuropsychopharmacol. 2014, 17, 1683–1693. [Google Scholar] [CrossRef]
- Liu, X.; Grandy, D.K.; Janowsky, A. Ractopamine, a livestock feed additive, is a full agonist at trace amine-associated receptor 1. J. Pharmacol. Exp. Ther. 2014, 350, 124–129. [Google Scholar] [CrossRef]
- Bradaia, A.; Trube, G.; Stalder, H.; Norcross, R.D.; Ozmen, L.; Wettstein, J.G.; Pinard, A.; Buchy, D.; Gassmann, M.; Hoener, M.C.; et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc. Natl. Acad. Sci. USA 2009, 106, 20081–20086. [Google Scholar] [CrossRef]
- Stalder, H.; Hoener, M.C.; Norcross, R.D. Selective antagonists of mouse trace amine-associated receptor 1 (mTAAR1): Discovery of EPPTB (RO5212773). Bioorg. Med. Chem. Lett. 2011, 21, 1227–1231. [Google Scholar] [CrossRef]
- Babusyte, A.; Kotthoff, M.; Fiedler, J.; Krautwurst, D. Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J. Leukoc. Biol. 2013, 93, 387–394. [Google Scholar] [CrossRef]
- Andersen, G.; Krautwurst, D. Trace Amine-Associated Receptors in the Cellular Immune System. In Trace Amines and Neurological Disorders; Academic Press: Cambridge, MA, USA, 2016; pp. 97–105. [Google Scholar] [CrossRef]
- Kovács, T.; Mikó, E.; Vida, A.; Sebő, E.; Toth, J.; Csonka, T.; Boratkó, A.; Ujlaki, G.; Lente, G.; Kovács, P.; et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci. Rep. 2019, 9, 1300. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, J.T.; Si, T.M.; Su, Y.A. Research progress on the immunomodulatory effects and mechanisms of trace amine-associated receptor 1. Sheng Li Xue Bao 2023, 75, 248–254. [Google Scholar]
- Sriram, U.; Cenna, J.M.; Haldar, B.; Fernandes, N.C.; Razmpour, R.; Fan, S.; Ramirez, S.H.; Potula, R. Methamphetamine induces trace amine-associated receptor 1 (TAAR1) expression in human T lymphocytes: Role in immunomodulation. J. Leukoc. Biol. 2016, 99, 213–223. [Google Scholar] [CrossRef]
- Polini, B.; Ricardi, C.; Bertolini, A.; Carnicelli, V.; Rutigliano, G.; Saponaro, F.; Zucchi, R.; Chiellini, G. T1AM/TAAR1 System Reduces Inflammatory Response and β-Amyloid Toxicity in Human Microglial HMC3 Cell Line. Int. J. Mol. Sci. 2023, 24, 11569. [Google Scholar] [CrossRef]
- Wasik, A.M.; Millan, M.J.; Scanlan, T.; Barnes, N.M.; Gordon, J. Evidence for functional trace amine associated receptor-1 in normal and malignant B cells. Leuk. Res. 2012, 36, 245–249. [Google Scholar] [CrossRef]
- D’Andrea, G.; Terrazzino, S.; Fortin, D.; Farruggio, A.; Rinaldi, L.; Leon, A. HPLC electrochemical detection of trace amines in human plasma and platelets and expression of mRNA transcripts of trace amine receptors in circulating leukocytes. Neurosci. Lett. 2003, 346, 89–92. [Google Scholar] [CrossRef]
- Chang, H.S.; Heo, J.S.; Shin, S.W.; Bae, D.J.; Song, H.J.; Jun, J.A.; Kim, J.D.; Park, J.S.; Park, B.L.; Shin, H.D.; et al. Association between TAAR6 polymorphisms and airway responsiveness to inhaled corticosteroids in asthmatic patients. Pharmacogenet. Genom. 2015, 25, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Panas, M.W.; Xie, Z.; Panas, H.N.; Hoener, M.C.; Vallender, E.J.; Miller, G.M. Trace amine associated receptor 1 signaling in activated lymphocytes. J. Neuroimmune Pharmacol. 2012, 7, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Samanta, M.; Iwakiri, D.; Kanda, T.; Imaizumi, T.; Takada, K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 2006, 25, 4207–4214. [Google Scholar] [CrossRef] [PubMed]
- Luckey, C.J.; Bhattacharya, D.; Goldrath, A.W.; Weissman, I.L.; Benoist, C.; Mathis, D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 3304–3309. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Pathophysiology of allergic inflammation. Immunol. Rev. 2011, 242, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Potula, R.; Hawkins, B.J.; Cenna, J.M.; Fan, S.; Dykstra, H.; Ramirez, S.H.; Morsey, B.; Brodie, M.R.; Persidsky, Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J. Immunol. 2010, 185, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, H.; Deng, X.; Dewan, Z.; Fujimoto, S.; Yamamoto, N. Potential role of NK cells in the induction of immune responses: Implications for NK cell-based immunotherapy for cancers and viral infections. Int. Rev. Immunol. 2008, 27, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Barnes, D.A.; Hoener, M.C.; Moore, C.S.; Berry, M.D. TAAR1 Regulates Purinergic-induced TNF Secretion from Peripheral, But Not CNS-resident, Macrophages. J. Neuroimmune Pharmacol. 2023, 18, 100–111. [Google Scholar] [CrossRef]
- Bugda Gwilt, K.; Olliffe, N.; Hoffing, R.A.; Miller, G.M. Trace amine associated receptor 1 (TAAR1) expression and modulation of inflammatory cytokine production in mouse bone marrow-derived macrophages: A novel mechanism for inflammation in ulcerative colitis. Immunopharmacol. Immunotoxicol. 2019, 41, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Lattin, J.E.; Schroder, K.; Su, A.I.; Walker, J.R.; Zhang, J.; Wiltshire, T.; Saijo, K.; Glass, C.K.; Hume, D.A.; Kellie, S.; et al. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Geoffrey, R.; Jia, S.; Kwitek, A.E.; Woodliff, J.; Ghosh, S.; Lernmark, A.; Wang, X.; Hessner, M.J. Evidence of a functional role for mast cells in the development of type 1 diabetes mellitus in the BioBreeding rat. J. Immunol. 2006, 177, 7275–7286. [Google Scholar] [CrossRef]
- Cisneros, I.E.; Ghorpade, A. HIV-1, methamphetamine and astrocyte glutamate regulation: Combined excitotoxic implications for neuro-AIDS. Curr. HIV Res. 2012, 10, 392–406. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; D’Arrigo, A.; Facchinetti, F.; Del Giudice, E.; Colavito, D.; Bernardini, D.; Leon, A. Octopamine, unlike other trace amines, inhibits responses of astroglia-enriched cultures to lipopolysaccharide via a β-adrenoreceptor-mediated mechanism. Neurosci. Lett. 2012, 517, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Taquet, N.; Philippe, C.; Reimund, J.-M.; Muller, C.D. Inflammatory bowel disease G-protein coupled receptors (GPCRs) expression profiling with microfluidic cards. In Crohn’s Disease; Books on Demand GmbH: Norderstedt, Germany, 2012; pp. 59–86. [Google Scholar] [CrossRef]
- Fernández de Palencia, P.; Fernández, M.; Mohedano, M.L.; Ladero, V.; Quevedo, C.; Alvarez, M.A.; López, P. Role of tyramine synthesis by food-borne Enterococcus durans in adaptation to the gastrointestinal tract environment. Appl. Environ. Microbiol. 2011, 77, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS ONE 2009, 4, e6386. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, I.S.; Vaganova, A.N.; Murtazina, R.Z.; Alferova, L.S.; Ermolenko, E.I.; Gainetdinov, R.R. Gut Microbiota Alterations in Trace Amine-Associated Receptor 9 (TAAR9) Knockout Rats. Biomolecules 2022, 12, 1823. [Google Scholar] [CrossRef]
- Smith, S.B.; Maixner, D.W.; Fillingim, R.B.; Slade, G.; Gracely, R.H.; Ambrose, K.; Zaykin, D.V.; Hyde, C.; John, S.; Tan, K.; et al. Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheum. 2012, 64, 584–593. [Google Scholar] [CrossRef]
- Barnes, D.A.; Galloway, D.A.; Hoener, M.C.; Berry, M.D.; Moore, C.S. TAAR1 Expression in Human Macrophages and Brain Tissue: A Potential Novel Facet of MS Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 11576. [Google Scholar] [CrossRef] [PubMed]
| Receptor | Expression in Human Immune Cell Populations | Known Ligands | Biological Function | References |
|---|---|---|---|---|
| TAAR1 | Peripheral mononuclear cells, B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocyte, NK-cells | β-Phenylethylamine (PEA) | The joint effect of β-phenylethylamine and IL-4-stimulated IgE synthesis. The chemosensory migration of polymorphonuclear leukocytes towards TAAR agonists. Possible joint effect with TAAR2 due to heterodimerization. | [34] |
| TAAR1 | Methamphetamine (METH) | The elevated concentration of intracellular calcium, active forms of oxygen. Stimulation of the differentiation of Th0 into Th2, reduced production of IL-2, intensified production of IL-6. | [34,37,38] | |
| TAAR1 | Microglia | 3-iodothyronamine (T1AM)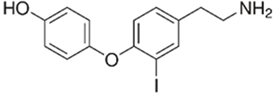 | T1AM is capable of reducing the amyloid-beta (Aβ)-stimulated TNFα and LPS’s inflammatory response on part of microglia through the inhibition of the release of pro-inflammatory factors (IL-6, TNFα, NF-kB, MCP1, and MIP1), stimulating the release of anti-inflammatory mediators (IL-10) | [39] |
| TAAR1 | Peripheral mononuclear cells, B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocyte, NK-cells | Tyramine (TYR)  3-iodothyronamine (T1AM)  | The chemosensory migration of polymorphonuclear leukocytes towards TAAR agonists. | [34,40] |
| TAAR2 | Peripheral mononuclear cells, B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocyte, NK-cells | β-Phenylethylamine (PEA) | – | [34] |
| TAAR5 | B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocytes, NK-cells | Trimethylamine (TMA) Derivative of choline  | – | [11,34] |
| TAAR6 | B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocytes, NK-cells | Potent ligands have not yet been identified Weak activity: N-methylpiperdine  | – | [36] |
| TAAR8 | mRNA expression in leucocytes is controversial | Potent ligands have not yet been identified Weak activity: N-methylpiperdine  Cadaverine 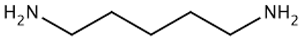 | – | [34,36,41] |
| TAAR9 | B-lymphocytes, T-lymphocytes, polymorphonuclear neutrophils, monocytes, NK-cells | Potent ligands have not yet been identified Weak activity: N-methylpiperdine  Cadaverine 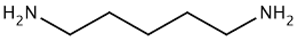 | – | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiseenko, V.I.; Apryatina, V.A.; Gainetdinov, R.R.; Apryatin, S.A. Trace Amine-Associated Receptors’ Role in Immune System Functions. Biomedicines 2024, 12, 893. https://doi.org/10.3390/biomedicines12040893
Moiseenko VI, Apryatina VA, Gainetdinov RR, Apryatin SA. Trace Amine-Associated Receptors’ Role in Immune System Functions. Biomedicines. 2024; 12(4):893. https://doi.org/10.3390/biomedicines12040893
Chicago/Turabian StyleMoiseenko, Vyacheslav I., Vera A. Apryatina, Raul R. Gainetdinov, and Sergey A. Apryatin. 2024. "Trace Amine-Associated Receptors’ Role in Immune System Functions" Biomedicines 12, no. 4: 893. https://doi.org/10.3390/biomedicines12040893





